Abstract
Context: Multiple autoimmune disorders (e.g. Addison’s disease, type 1 diabetes, celiac disease) are associated with HLA-DR3, but it is likely that alleles of additional genes in linkage disequilibrium with HLA-DRB1 contribute to disease.
Objective: The objective of the study was to characterize major histocompatability complex (MHC) haplotypes conferring extreme risk for autoimmune Addison’s disease (AD).
Design, Setting, and Participants: Eighty-six 21-hydroxylase autoantibody-positive, nonautoimmune polyendocrine syndrome type 1, Caucasian individuals collected from 1992 to 2009 with clinical AD from 68 families (12 multiplex and 56 simplex) were genotyped for HLA-DRB1, HLA-DQB1, MICA, HLA-B, and HLA-A as well as high density MHC single-nucleotide polymorphism (SNP) analysis for 34.
Main Outcome Measures: AD and genotype were measured.
Result: Ninety-seven percent of the multiplex individuals had both HLA-DR3 and HLA-B8 vs. 60% of simplex AD patients (P = 9.72 × 10−4) and 13% of general population controls (P = 3.00 × 10−19). The genotype DR3/DR4 with B8 was present in 85% of AD multiplex patients, 24% of simplex patients, and 1.5% of control individuals (P = 4.92 × 10−191). The DR3-B8 haplotype of AD patients had HLA-A1 less often (47%) than controls (81%, P = 7.00 × 10−5) and type 1 diabetes patients (73%, P = 1.93 × 10−3). Analysis of 1228 SNPs across the MHC for individuals with AD revealed a shorter conserved haplotype (3.8) with the loss of the extended conserved 3.8.1 haplotype approximately halfway between HLA-B and HLA-A.
Conclusion: Extreme risk for AD, especially in multiplex families, is associated with haplotypic DR3 variants, in particular a portion (3.8) but not all of the conserved 3.8.1 haplotype.
HLA-DR3 bearing haplotypes containing HLA-B8, but not necessarily HLA-A1, confer extremely high risk for Addison’s disease.
Autoimmune Addison’s disease is the major cause of adrenocortical destruction in countries with a low risk of tuberculosis and is present in approximately one in 10,000 individuals. Signs and symptoms include fatigue, weight loss, low blood pressure, and darkening of the skin (1). Despite such a spectrum of clinical features diagnosis is often delayed. Formation of 21-hydroxylase autoantibodies usually precedes the development of disease in the absence of symptoms. Disease onset in the form of acute adrenal failure can be sudden, severe, and life threatening. Half of Addison’s disease patients have other autoimmune diseases, indicating a related pathophysiology (2,3). Although a relatively uncommon disease, the study of Addison’s disease has greatly contributed to knowledge of many autoimmune endocrine diseases, especially type 1 diabetes in the context of patients with the autoimmune polyendocrine syndrome (APS) type 1 and APS-2 (4,5). APS-1, with its mucocutaneous candidiasis, hypoparathyroidism, and Addison’s disease, is a monogenic disorder resulting from mutations of the autoimmune regulator gene (AIRE) (6,7). In contrast, APS-2, similar to most common autoimmune disorders, is a polygenic disease with the dominant susceptibility locus on chromosome 6 within the major histocompatibility complex (MHC) (8,9,10,11,12).
In 1978 we reported that APS-2 was associated with the class 1 human leukocyte antigen (HLA) allele B8 (13). With the discovery of class II HLA alleles, and their strong association with type 1 diabetes, and linkage disequilibrium of HLA-DR3 with HLA-B8, we assumed that the association of HLA-B8 with Addison’s disease was predominantly due to its association with HLA-DR3 and DQB1*0201, as is the case for type 1 diabetes and celiac disease (10,14). In type 1 diabetes, a closely related condition, there is a considerable body of literature correlating disease risk with class II HLA alleles and the highest-risk HLA genotype DRB1*0301-DQB1*0201/DRB1*04-DQB1*0302 (or the DR3/4 genotype) (15). Further studies have shown that the extended haplotype DRB1*0301-DQA1*0501-DQB1*0201-B8-A1 (abbreviated DR3-B8-A1 or 3.8.1) confers lower risk for diabetes compared with other HLA-DR3 haplotypes (14,15). This haplotype, consisting of the nonrandom population association of alleles HLA-DR3, HLA-B8, and HLA-A1 on the same chromosome, is one of the most common extended haplotype in European populations (16).
In Addison’s disease (with and without the presence of other APS-2 associated conditions), analyses have shown several haplotypes conferring risk for disease development. The longstanding association with the DRB1*0301-DQB1*0201/DRB1*04-DQB1*0302 genotype has been previously described, as have further association with HLA-DR4 subtypes (DRB1*0403-DQB1*0305 and DRB1*0404- DQB1*0302) (10,12,17,18,19). There have also been several studies showing association with MICA 5.1 allele, which is in linkage disequilibrium with HLA-B, as well as microsatellite analysis of the class I, suggesting a common haplotype including (but not limited to) the DR-DQ region of the MHC (10,20). With this prior knowledge and lack of studies of HLA-A, we undertook further characterization of conserved ancestral haplotypes contributing to Addison’s disease risk and the characterization of the combination of class I and class II HLA regions in Addison’s disease.
With the ability to rapidly type thousands of single nucleotide polymorphisms (SNPs) across the MHC and recent documentation of the essentially invariant nature of the extended DR3-B8-A1 haplotype (21,22,23), we revisited the association between HLA-DR3, HLA-B8, HLA-A1, and Addison’s disease. We report that the highest risk for Addison’s disease is determined by a conserved region encompassing a portion of but not the full HLA-DR3-B8-A1 ancestral haplotype, making it less likely that risk is associated with the telomeric end of the conserved ancestral haplotype (that includes HLA-A1) and more likely that high-risk loci are to be found in linkage disequilibrium with HLA-DR3 and HLA-B8.
Patient Populations and Methods
We studied 86 non-APS-1 individuals with Addison’s disease from 68 families (12 multiplex families and 56 simplex families; for details on these individuals, please see Supplemental Table 1, published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). The study began in 1992 with ongoing genotype analysis through 2009. For those who volunteered race information, all individuals were Caucasian [Hispanic (n = 4) and white non-Hispanic (n = 66)].
There were 38 males and 46 females with the average age of onset 26 yr (22 yr in males and 30 yr in females). Thirty-one of 86 also had diabetes, and 36 of 86 had other autoimmune diseases including thyroiditis, celiac disease, vitiligo, and lupus. Thirty-four had no other reported autoimmune disease. Patients or their parents provided informed consent with institutional review board oversight at the University of Colorado Denver.
Addison’s disease patients were identified by several methods including referral with the diagnosis of Addison’s disease from the National Adrenal Disease Foundation, referral of 21-hydroxylase autoantibody positive or symptomatic relatives of Addison’s patients, and diabetic patients already followed at the Barbara Davis Center for Childhood Diabetes who were diagnosed after 21-hydroxylase autoantibody screening. All patients in this study were 21-hydroxylase autoantibody positive (index >99th percentile of normal). 21-Hydroxylase autoantibodies were measured with a fluid phase radioassay as previously described (12). All were interferon-α autoantibody negative as well (ruling out APS-1) (24). We performed HLA-DRB1, HLA-DQB1, HLA-B, and HLA-A typing using hybridization of linear arrays of immobilized, sequence-specific oligonucleotides with amplified exon 2 DNA similar to previously described methodology (25) and direct sequencing of amplified HLA-DRB1 exon 2 to differentiate DRB1*04 subtypes.
By analysis of inheritance of alleles in patients with family members with DNA available, chromosomal assignment (haplotypic phase) of alleles could be unambiguously defined regarding HLA-DRB1, HLA-DQB1, and HLA-B for 48 of the patients with Addison’s disease. Designation of full haplotypes in this analysis is denoted by listing alleles as HLA-DRB1-HLA-B-HLA-A. Otherwise, if phasing is inferred from linkage dysequilibrium, alleles will be listed separately in an individual (e.g. HLA-DR3+, HLA-B8+). Of note, however, in those individuals with haplotype analysis who were HLA-DR3 and HLA-B8 positive, all but three had the HLA-DR3 and HLA-B8 on the same chromosome in Addison’s individuals (47 of 50 chromosomes), making it very likely that if an individual is a carrier of both HLA-DR3 and HLA-B8, they are part of the same haplotype.
Similar HLA typing was available for families from the Type 1 Diabetes Genetics Consortium (T1DGC). The T1DGC enrolled 2300 affected sibling pairs with type 1 diabetes and their parents and completed typing for HLA alleles and SNPs across the MHC region (26). Analyses in this paper used a single individual with type 1 diabetes per family (one case per family, n = 2300).
Control individuals from the general population (healthy newborn controls followed prospectively) were available from the Diabetes Autoimmunity Study of the Young (DAISY) with HLA typing at birth of approximately 30,000 newborns. Details regarding the DAISY population are provided in the paper from Rewers et al. (27). There were 271 HLA-DR3/4 positive, autoantibody-negative, nondiabetic, unrelated individuals with HLA-B and HLA-A allele typing available. Values for the general population frequencies for DR3, DR4, and DR3/4 were derived from typing of unrelated DAISY participants (27). HLA-B8 frequency determination for HLA-DR3-positive controls used only DR3/4-positive individuals, given that this is the highest risk, most common genotype for Addison’s disease individuals.
SNP typing for 34 Addison’s disease patients was carried out using the dense Illumina MHC exon-centric standard panel (1228 SNPs typed across the MHC) with typing completed at the University of Colorado Denver microarray core. To unambiguously assign phase for SNP typing across the MHC for Addison’s disease patients, we only analyzed homozygous SNPs. A total of 17 chromosomes carried the HLA-DR3 and HLA-B8 alleles, with 10 of these having HLA-A1 and seven having a different HLA-A allele. To be sure of phase in Addison’s disease patients without family typing homozygous SNP alleles were analyzed.
The Fisher’s exact test (two sided) was used to calculate P values for association with Addison’s disease, with α = 0.05. PRISM GraphPad version 4 software (GraphPad, San Diego, CA) was used for χ2 analysis (α = 0.05).
Results
HLA-DRB1*0301 is a common allele present in 20% of newborns in Denver (Colorado) (27). Approximately half of DR3 haplotypes of the general population have both the HLA-B8 and HLA-A1 alleles due to linkage disequilibrium, and on such HLA-DR3, B8, A1 haplotypes, this region of the MHC is essentially invariant for millions of base pairs (23). This haplotype, termed 3.8.1, is the prototypic extended or ancestral MHC haplotype. By almost an order of magnitude, it is the most common conserved haplotype in North American and European individuals (28). For chromosomes with both HLA-DR3 and HLA-B8, the great majority are conserved to the HLA-A locus and have the 3.8.1 invariant haplotype.
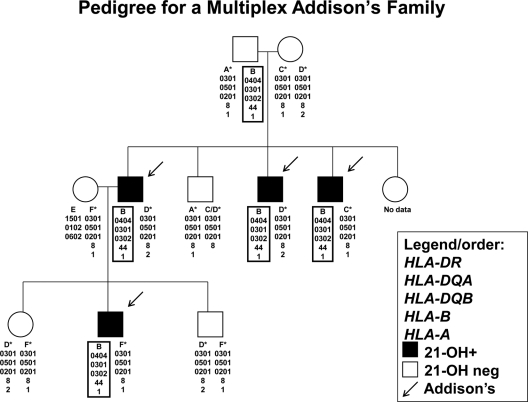
Figure 1 illustrates a multiplex Addison’s disease family in which four individuals are affected. All four with Addison’s disease have an identical HLA-DR4 haplotype (defined as HLA-DRB1*04xx-DQB1*0302) with DRB1*0404 (identical by descent inheritance). However, the four Addison’s disease patients have three different HLA-DR3 haplotypes (HLA-DRB1*0301-DQB1*0201). Two HLA-DR3 haplotypes (haplotypes C and D) are from the mother of the first generation, whereas one haplotype (haplotype F) is from an unrelated mother in the second generation. Common between these three HLA-DR3 haplotypes is the presence of HLA-DR3-B8 alleles, whereas they have different HLA-A alleles (HLA-A1 and HLA-A2).
Figure 1.
HLA haplotypes of a family with four members with Addison’s disease (arrows) in two generations. A box designates a haplotype containing the HLA-DRB1*0404 allele, and asterisk denotes the HLA-DRB1*0301 allele, and 21-hydroxylase autoantibody positivity is indicated in black.
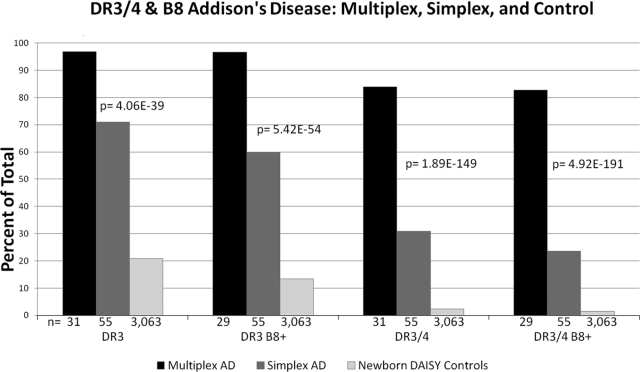
In our series of patients with Addison’s disease, 97% of the individuals typed for both HLA-DRB1 and HLA-B (26 of 27) from 12 multiplex families had both HLA-DR3 and HLA-B8 haplotypes. Of these individuals, 22 were able to be phased with HLA-typed family members. Ninety-six percent of the individuals carrying both HLA-DR3 and HLA-B8 haplotypes (21 of 22) carried at least one HLA-DR3-HLA-B8 chromosome (Fig. 1 illustrates a pedigree from one of these families). This is compared with only 13% of general population controls with HLA-DR3 and HLA-B8 haplotypes (n = 410 of 3063, P = 3.00 × 10−19) and 60% of simplex Addison’s disease patients (n = 35 of 57, P = 9.72 × 10−4) (Fig. 2). In 89% of multiplex Addison’s disease patients (n = 25 of 28) typed for HLA-DRB1, HLA-DQA1, and HLA-DQB1, the other chromosome carried an HLA-DR4 allele (HLA-DR3/4 heterozygous individuals) compared with 32% of simplex patients (n = 18 of 56). Only 2.4% of the general population have the HLA-DR3/4 genotype. Overall the genotype DR3/4 with B8 was present in 85% of Addison’s disease multiplex patients, 24% of simplex patients, and 1.5% of normal control individuals (Fig. 2).
Figure 2.
HLA-DR, HLA-DQB1, and HLA-B were evaluated in multiplex and simplex individuals with Addison’s disease as well as general population controls. P values indicate multiplex Addison’s disease individuals vs. the general population controls.
To assess whether HLA-B8 was increased in patients with Addison’s disease independent of HLA-DR3, we analyzed HLA-B allele frequency in non-HLA-DR3 Addison’s disease patients compared with two control populations (non-HLA-DR3, nontransmitted control chromosomes from T1DGC, and non-HLA-DR3 patients with diabetes). The HLA-B8 allele frequency in non-HLA-DR3 patients with Addison’s disease was 14.4% (n = 5 of 34) compared with 3.6% of nontransmitted T1DGC control chromosomes (n = 88 of 2476, P = 7.44 × 10−3) and 2.5% of non-DR3 type 1 diabetes patients (n = 46 of 1690, P = 2.70 × 10−3).
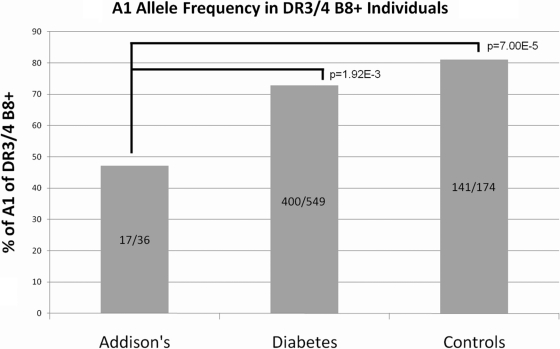
To further evaluate the HLA DR3-B8 (3.8) haplotypes, we typed HLA-A and 1228 SNPs across the MHC in a subset of patients. As shown in Fig. 3, HLA-A locus typing revealed that the DR3-B8 haplotype of Addison’s disease patients had HLA-A1 less often (47%) than HLA-DR3, HLA-B8-positive normal controls (81%, P = 7.00 × 10−5) and type 1 diabetes patients (73%, P = 1.93 × 10−3). The MICA 5.1 allele has been reported to be present on the 3.8.1 haplotype, associated with autoimmune disease (20,29), and in strong linkage disequilibrium with HLA-DR3 and HLA-B8 (10,30).
Figure 3.
HLA-DR3/DR4+ and HLA-B8+ Addison’s disease patients compared with HLA-DR3/DR4+ and HLA-B8+ general population controls from DAISY and HLA-DR3-B8/DR4 type 1 diabetes patients from the T1DGC. HLA-A1 in individuals carrying both HLA-DR3 and HLA-B8 is significantly lower in Addison’s patients vs. normal controls and diabetes patients.
Our data are consistent with this because 100% of DR3-positive, B8-positive Addison’s disease patients also have at least one MICA 5.1 allele (n = 61 of 61). Similarly looking at phased HLA-DR3-B8 chromosomes in Addison’s disease patients, 100% had MICA 5.1 (n = 38 of 38). The linkage disequilibrium of MICA5.1 and HLA-B8 can be seen in T1DGC founder chromosomes as well, in which 100% of HLA-B8-bearing haplotypes we examined (both DR3 and non-DR3) also have MICA5.1 (n = 52 of 52).
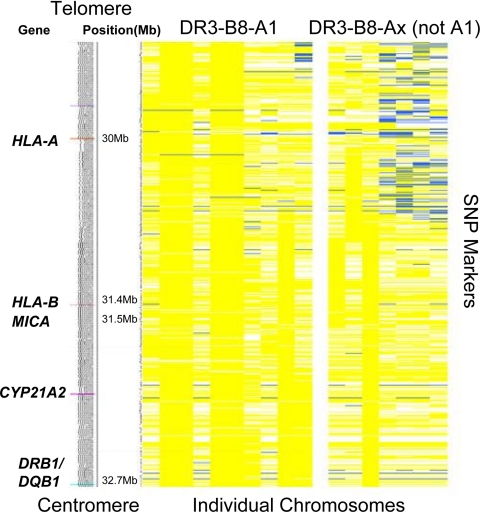
We further analyzed SNPs across the MHC in 34 Addison’s disease patients to define the region of the ancestral 3.8.1 haplotype recombination and confirm the identity of the 3.8.1 haplotype with the 3.8-non-A1 extended haplotype in the region between HLA-DRB1 and HLA-B (Fig. 4). Analysis of the series of SNPs across the MHC for individuals with Addison’s disease indicates that the expected conservation of multiple SNPs throughout the class I and class II MHC regions, namely HLA-DRB1 to HLA-B for those DR3-B8 chromosomes (3.8) and HLA-DRB1 to HLA-A for those with the full haplotype, 3.8.1 (Fig. 3). For the Addison’s disease chromosome with the shorter conserved haplotype (3.8), the loss of the conserved haplotype begins approximately halfway between HLA-B and HLA-A, with no single break point for all the chromosomes defining the start of the telomeric loss of identity to the full 3.8.1 haplotype.
Figure 4.
Schematic of the MHC region including gene position based on National Center for Biotechnology Information Human Genome build 36 (left panel) and SNP analysis of DR3 chromosomes of patients with Addison’s disease (right panel). Each column represents a single DR3 chromosome from a patient with Addison’s disease [on the left are chromosomes with HLA-B8 and A1 (10 total); on the right are chromosomes with HLA-B8 but lacking HLA-A1 (7 total)]. Each row represents a SNP with the allele in yellow if identical with the allele on the conserved DR3-B8-A1 extended haplotype and in blue if the opposite allele. White represents missing data (see Patient Populations and Methods). The common region of conservation in patients with Addison’s disease extends from HLA-DRB1 to just beyond HLA-B.
The number of males was higher for multiplex Addison’s disease than simplex [62% multiplex (n = 18 of 29), 35% simplex (n = 20 of 57), P = 0.02]. Simplex males, multiplex males, and multiplex females tended to be younger than simplex females [21 ± 3, 23 ± 2, 24 ± 4, and 30 ± 3 yr mean age of onset (±sem), respectively]. There were statistically significant differences in unpaired t test analysis between the simplex females and both simplex and multiplex males (P = 0.02 and 0.03, respectively). When looking specifically at the populations with HLA-DR3 and HLA-B8, neither age of onset nor gender was significantly different from the overall data. In families in which one Addison’s disease sibling had the HLA-DRB1*03-B8/DRB1*0404-DQB1*0302 genotype, however, eight of 12 of the other siblings with this genotype developed Addison’s disease vs. one of 15 siblings in the same families without this genotype (P = 2.7 × 10−3). This puts siblings of individuals with Addison’s disease who share a high-risk genotype (specifically HLA-DR3-B8/DR404) at a much higher risk of developing Addison’s disease themselves vs. siblings who do not share a high-risk genotype.
Discussion
Addison’s disease is rare with a prevalence of approximately one in 10,000. By far, the strongest genetic association is with alleles of genes within the MHC (HLA-DRB1, HLA-DQB1, MICA, HLA-B) (8,9,10,11,12,18,19,31,32,33), with an odds ratio as high as 36.7 for the most common DRB1*0301-DQB1*0201/DRB1*0404-DQB1* 0302 genotype (18). Similar to many complex disorders, polymorphisms of genes outside the MHC are associated as well. These include polymorphisms related to the cytotoxic T lymphocyte antigen-4 (CTLA4) gene (34), protein tyrosine phosphatase nonreceptor type 22 (PTPN22) (35), Fc receptor-like-3 (FCRL3) gene (36), the insulin gene (INS) (37), 1α-hydroxylase (CYP27B1) gene (38), the MHC transactivator (CIITA) gene (39), and the NALP1 gene (40). The odds ratios for the association of these other loci are all less than 2.
Prior reported MHC associations with Addison’s disease (and APS-2 with Addison’s disease) include the class I allele HLA-B8; the class II haplotypes HLA-DR3-DQB1*0201, HLA-DRB1*0404-DQB1*0302, and occasionally HLA-DRB1*0403-DQB1*0305; and MICA and MICB (specifically the MICA 5.1 allele) (10,12,18,19,32,33). Of note, the 21-hydroxylase gene (CYP21A2), 21-hydroxylase pseudogene (CYP21A1P), MICA, and MICB are all within the MHC between HLA-DRB1 and HLA-B. To date, there has been some characterization of haplotypic risk (specifically with microsatellite analysis of HLA-DR4 bearing haplotypes) (10), but detailed analyses of the HLA-DR3 and HLA-B8 alleles and the well-conserved 3.8.1 haplotype are lacking.
Our data document that for DR3-bearing haplotypes, Addison’s disease is strongly associated not simply with HLA-DR3 but also with a highly conserved common region between HLA-DRB1 and HLA-B, easily identifiable with typing for HLA-DR3 and HLA-B8 as well as SNPs between HLA-DRB1 and HLA-B associated with the 3.8.1 haplotype (see Fig. 4). Ninety-seven percent of the Addison’s disease patients (n = 28 of 29) from multiplex families (13 families with full genotyping) and nearly 60% of simplex patients (n = 33 of 55) had both HLA-DR3 and HLA-B8. This is compared with 37% of diabetics (n = 827 of 2210, P = 2.76 × 10−11) and 13% of general population controls (n = 410 of 3063, P = 3.00 × 10−19). The nearly absolute genetic association with multiplex Addison’s disease, we believe, is due to the requirement for highly penetrant genotype in families with multiplex disease.
The full 3.8.1 extended haplotype (HLA-DR3-B8 with HLA-A1) is less associated with Addison’s disease compared with the shorter conserved 3.8 haplotype without HLA-A1. We hypothesize that class II HLA alleles DR3 and DQB1*0201 are a major determinant of Addison’s disease risk but that other loci conserved within the HLA-DRB1 to HLA-B region greatly enhance that risk. It is possible that HLA-B itself, or loci in strong linkage disequilibrium like MICA (and its 5.1 allele), contribute to risk because HLA-B8 is significantly associated with Addison’s disease for individuals without an HLA-DR3 allele. Polymorphisms between HLA-B and HLA-A may further influence risk but less so than those between HLA-DRB1 and HLA-B. Addison’s disease DR3-B8 haplotypes less often have HLA-A1 compared with general control population controls, and SNP analysis indicates multiple sites of ancestral recombination between HLA-B and HLA-A for the 3.8 haplotypes without HLA-A1.
Seventy percent of siblings of a proband with Addison’s disease with the highest risk HLA genotype (HLA-DRB1*03-B8/DRB1*0404-DQB1*0302) had Addison’s disease themselves vs. 7% of siblings in the same family without this genotype (P = 2.7E-3). In our overall series of patients, approximately 43% of Addison’s disease patients defined for haplotype have the high-risk genotype (DR3-DQB1*0201-B8/DRB1*0404-DQB1*0302) vs. 1.5% of the general population. Based on the estimated prevalence of one in 10,000 for Addison’s disease in the general population, individuals carrying the high-risk genotype have a risk of approximately one in 300. A caveat in precisely defining the magnitude of extreme risk for such siblings is that our series of patients is enriched for families with multiple members with Addison’s disease, and population-based studies will need to assess the exact risk.
If HLA screening (e.g. HLA-DR3 and HLA-DR4) for common autoimmune disorders such as type 1 diabetes and celiac disease enters the realm of personalized medicine, those with a high risk of Addison’s disease would also be identified by HLA analysis. Such individuals would likely benefit from awareness of risk for this rare disorder, given the common long history of morbidity preceding diagnosis as well as the potential mortality associated with initial onset of disease in Addisonian crisis. In that 21-hydroxylase autoantibodies almost always precede overt disease, periodic screening for autoantibodies followed by adrenal function testing may be feasible. In addition to class II determinants of risk, identifying the actual polymorphisms within the 1 million base pairs between HLA-DRB1 and HLA-B might further improve the positive predictive value of genetic testing, depending on the number of genes of relevance. Candidate genes/regions include both the 21-hydroxylase gene and the pseudogene, and the MICA region that both lie in the HLA-DR3 Addison’s disease shared haplotype region (between HLA-DRB1 and HLA-B).
Footnotes
This research used resources provided by the Type 1 Diabetes Genetics Consortium, a collaborative clinical study sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Allergy and Infectious Diseases, National Human Genome Research Institute, National Institute of Child Health and Human Development, and Juvenile Diabetes Research Foundation International and supported by Grant U01 DK062418. This work was supported by the National Institutes of Health Grants DK32083 and DK057538, Diabetes Autoimmunity Study in the Young Grant DK32493, Autoimmunity Prevention Center Grant AI050864, Diabetes Endocrine Research Center Grant P30 DK57516, Clinical Research Centers Grants MO1 RR00069 and MO1 RR00051, the Immune Tolerance Network Grant AI15416, the American Diabetes Association, the Juvenile Diabetes Research Foundation, the Children’s Diabetes Foundation, and the Brehm Coalition. P.R.B. is a Fellow of the Pediatric Scientist Development Program. The project described was supported by Award K12-HD000850 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Disclosure Summary: We have no conflicts of interest to disclose.
First Published Online July 14, 2010
Abbreviations: APS, Autoimmune polyendocrine syndrome; DAISY, Diabetes Autoimmunity Study of the Young; HLA, human leukocyte antigen; MHC, major histocompatibility complex; SNP, single-nucleotide polymorphism; T1DGC, Type 1 Diabetes Genetics Consortium.
References
- Bornstein SR 2009 Predisposing factors for adrenal insufficiency. N Engl J Med 360:2328–2339 [DOI] [PubMed] [Google Scholar]
- De Block CE, De Leeuw IH, Vertommen JJ, Rooman RP, Du Caju MV, Van Campenhout CM, Weyler JJ, Winnock F, Van Autreve J, Gorus FK 2001 β-Cell, thyroid, gastric, adrenal and coeliac autoimmunity and HLA-DQ types in type 1 diabetes. Clin Exp Immunol 126:236–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelissen PM, Bast EJ, Croughs RJ 1995 Associated autoimmunity in Addison’s disease. J Autoimmun 8:121–130 [DOI] [PubMed] [Google Scholar]
- Bottazzo GF, Florin-Christensen A, Doniach D 1974 Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet 2:1279–1283 [DOI] [PubMed] [Google Scholar]
- Neufeld M, Maclaren NK, Blizzard RM 1981 Two types of autoimmune Addison’s disease associated with different polyglandular autoimmune (PGA) syndromes. Medicine (Baltimore) 60:355–362 [DOI] [PubMed] [Google Scholar]
- Aaltonen J, Björses P, Perheentupa J, Horelli-Kuitunen N, Palotie A PL, Lee YS, Francis F, Henning S, Thiel S, Leharach H, Yaspo ML 1997 An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet 17:399–403 [DOI] [PubMed] [Google Scholar]
- Perheentupa J 2006 Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Clin Endocrinol Metab 91:2843–2850 [DOI] [PubMed] [Google Scholar]
- Ten S, New M, Maclaren N 2001 Clinical review 130: Addison’s disease 2001. J Clin Endocrinol Metab 86:2909–2922 [DOI] [PubMed] [Google Scholar]
- Gambelunghe G, Falorni A, Ghaderi M, Laureti S, Tortoioli C, Santeusanio F, Brunetti P, Sanjeevi CB 1999 Microsatellite polymorphism of the MHC class I chain-related (MIC-A and MIC-B) genes marks the risk for autoimmune Addison’s disease. J Clin Endocrinol Metab 84:3701–3707 [DOI] [PubMed] [Google Scholar]
- Gombos Z, Hermann R, Kiviniemi M, Nejentsev S, Reimand K, Fadeyev V, Peterson P, Uibo R, Ilonen J 2007 Analysis of extended human leukocyte antigen haplotype association with Addison’s disease in three populations. Eur J Endocrinol 157:757–761 [DOI] [PubMed] [Google Scholar]
- Weetman AP, Zhang L, Tandon N, Edwards OM 1999 HLA associations with autoimmune Addison’s disease. Tissue Antigens 38:31–33 [DOI] [PubMed] [Google Scholar]
- Yu L, Brewer KW, Gates S, Wu A, Wang T, Babu SR, Gottlieb PA, Freed BM, Noble J, Erlich HA, Rewers MJ, Eisenbarth GS 1999 DRB1*04 and DQ alleles: expression of 21-hydroxylase autoantibodies and risk of progression to Addison’s disease. J Clin Endocrinol Metab 84:328–335 [DOI] [PubMed] [Google Scholar]
- Eisenbarth G, Wilson P, Ward F, Lebovitz HE 1978 HLA type and occurrence of disease in familial polyglandular failure. N Engl J Med 298:92–94 [DOI] [PubMed] [Google Scholar]
- Aly TA, Ide A, Jahromi MM, Barker JM, Fernando MS, Babu SR, Yu L, Miao D, Erlich HA, Fain PR, Barriga KJ, Norris JM, Rewers MJ, Eisenbarth GS 2006 Extreme genetic risk for type 1A diabetes. Proc Natl Acad Sci USA 103:14074–14079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, Mychaleckyj JC, Todd JA, Bonella P, Fear AL, Lavant E, Louey A, Moonsamy P 2008 HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes 57:1084–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper CA, Larsen CE, Dubey DP, Awdeh ZL, Fici DA, Yunis EJ 2006 The haplotype structure of the human major histocompatibility complex. Hum Immunol 67:73–84 [DOI] [PubMed] [Google Scholar]
- Maclaren NK, Riley WJ 1986 Inherited susceptibility to autoimmune Addison’s disease is linked to human leukocyte antigens-DR3 and/or DR4, except when associated with type 1 autoimmune polyglandular syndrome. J Clin Endocrinol Metab 62:455–459 [DOI] [PubMed] [Google Scholar]
- Myhre AG, Undlien DE, Lovås K, Uhlving S, Nedrebø BG, Fougner KJ, Trovik T, Sørheim JI, Husebye ES 2002 Autoimmune adrenocortical failure in Norway: autoantibodies and HLA class II associations related to clinical features. J Clin Endocrinol Metab 87:618–623 [DOI] [PubMed] [Google Scholar]
- Park YS, Sanjeevi CB, Robles D, Yu L, Rewers M, Gottlieb PA, Fain P, Eisenbarth GS 2002 Additional association of intra-MHC genes, MICA and D6S273, with Addison’s disease. Tissue Antigens 60:155–163 [DOI] [PubMed] [Google Scholar]
- Triolo TM, Baschal EE, Armstrong TK, Toews CS, Fain PR, Rewers MJ, Yu L, Miao D, Eisenbarth GS, Gottlieb PA, Barker JM 2009 Homozygosity of the polymorphism MICA5.1 identifies extreme risk of progression to overt adrenal insufficiency among 21-hydroxylase antibody-positive patients with type 1 diabetes. J Clin Endocrinol Metab 94:4517–4523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WP, Vu Q, Li SS, Hansen JA, Zhao LP, Geraghty DE 2006 Toward understanding MHC disease associations: partial resequencing of 46 distinct HLA haplotypes. Genomics 87:561–571 [DOI] [PubMed] [Google Scholar]
- Aly TA, Eller E, Ide A, Gowan K, Babu SR, Erlich HA, Rewers MJ, Eisenbarth GS, Fain PR 2006 Multi-SNP analysis of MHC region: remarkable conservation of HLA-A1-B8-DR3 haplotype. Diabetes 55:1265–1269 [DOI] [PubMed] [Google Scholar]
- Baschal EE, Aly TA, Jasinski JM, Steck AK, Johnson KN, Noble JA, Erlich HA, Eisenbarth GS 2009 The frequent and conserved DR3-B8-A1 extended haplotype confers less diabetes risk than other DR3 haplotypes. Diabetes Obes Metab 11(Suppl 1):25–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Barker JM, Babu S, Su M, Stenerson M, Cheng M, Shum A, Zamir E, Badolato R, Law A, Eisenbarth GS, Anderson MS 2007 A robust immunoassay for anti-interferon autoantibodies that is highly specific for patients with autoimmune polyglandular syndrome type 1. Clin Immunol 125:131–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugawan TL, Erlich HA 1991 Rapid typing of HLA-DQB1 DNA polymorphism using nonradioactive oligonucleotide probes and amplified DNA. Immunogenetics 33:163–170 [DOI] [PubMed] [Google Scholar]
- Brown WM, Pierce J, Hilner JE, Perdue LH, Lohman K, Li L, Venkatesh RB, Hunt S, Mychaleckyj JC, Deloukas P 2009 Overview of the MHC fine mapping data. Diabetes Obes Metab 11(Suppl 1):2–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewers M, Bugawan TL, Norris JM, Blair A, Beaty B, Hoffman M, McDuffie Jr RS, Hamman RF, Klingensmith G, Eisenbarth GS, Erlich HA 1996 Newborn screening for HLA markers associated with IDDM: diabetes autoimmunity study in the young (DAISY). Diabetology 39:807–812 [DOI] [PubMed] [Google Scholar]
- Baschal EE, Aly TA, Jasinski JM, Steck AK, Noble JA, Erlich HA, Eisenbarth GS 2009 Defining multiple common “completely” conserved major histocompatibility complex SNP haplotypes. Clin Immunol 132:203–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide A, Babu SR, Robles DT, Wang T, Erlich HA, Bugawan TL, Rewers M, Fain PR, Eisenbarth GS 2005 Homozygosity for premature stop codon of the MHC class I chain-related gene A (MIC-A) is associated with early activation of islet autoimmunity of DR3/4-DQ2/8 high risk DAISY relatives. J Clin Immunol 25:303–308 [DOI] [PubMed] [Google Scholar]
- Bilbao JR, Martin-Pagola A, Pérez De Nanclares G, Calvo B, Vitoria JC, Vázquez F, Castaño L 2003 HLA-DRB1 and MICA in autoimmunity: common associated alleles in autoimmune disorders. Ann NY Acad Sci 1005:314–318 [DOI] [PubMed] [Google Scholar]
- Betterle C, Scalici C, Presotto F, Pedini B, Moro L, Rigon F, Mantero F 1988 The natural history of adrenal function in autoimmune patients with adrenal autoantibodies. J Endocrinol 117:467–475 [DOI] [PubMed] [Google Scholar]
- Gambelunghe G, Gerli R, Bocci EB, Del SP, Ghaderi M, Sanjeevi CB, Bistoni O, Bini V, Falorni A 2005 Contribution of MHC class I chain-related A (MICA) gene polymorphism to genetic susceptibility for systemic lupus erythematosus. Rheumatology (Oxford) 44:287–292 [DOI] [PubMed] [Google Scholar]
- Huang W, Connor E, Rosa TD, Muir A, Schatz D, Silverstein J, Crockett S, She JX, Maclaren NK 1996 Although DR3-DQB1*0201 may be associated with multiple component diseases of the autoimmune polyglandular syndromes, the human leukocyte antigen DR4-DQB1*0302 haplotype is implicated only in β-cell autoimmunity. J Clin Endocrinol Metab 81:2559–2563 [DOI] [PubMed] [Google Scholar]
- Blomhoff A, Lie BA, Myhre AG, Kemp EH, Weetman AP, Akselsen HE, Huseby ES, Undlien DE 2004 Polymorphisms in the cytotoxic T lymphocyte antigen-4 gene region confer susceptibility to Addison’s disease. J Clin Endocrinol Metab 89:3474–3476 [DOI] [PubMed] [Google Scholar]
- Skinningsrud B, Husebye ES, Gervin K, Lovas K, Blomhoff A, Wolff AB, Kemp EH, Egeland T, Undlien DE 2008 Mutation screening of PTPN22: association of the 1858T-allele with Addison’s disease. Eur J Hum Genet 16:977–982 [DOI] [PubMed] [Google Scholar]
- Owen CJ, Kelly H, Eden JA, Merriman ME, Pearce SH, Merriman TR 2007 Analysis of the Fc receptor-like-3 (FCRL3) locus in Caucasians with autoimmune disorders suggests a complex pattern of disease association. J Clin Endocrinol Metab 92:1106–1111 [DOI] [PubMed] [Google Scholar]
- Ramos-Lopez E, Lange B, Kahles H, Willenberg HS, Meyer G, Penna-Martinez M, Reisch N, Hahner S, Seissler J, Badenhoop K 2008 Insulin gene polymorphisms in type 1 diabetes, Addison’s disease and the polyglandular autoimmune syndrome type II. BMC Med Genet 9:65:65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez ER, Zwermann O, Segni M, Meyer G, Reincke M, Seissler J, Herwig J, Usadel KH, Badenhoop K 2004 A promoter polymorphism of the CYP27B1 gene is associated with Addison’s disease, Hashimoto’s thyroiditis, Graves’ disease and type 1 diabetes mellitus in Germans. Eur J Endocrinol 151:193–197 [DOI] [PubMed] [Google Scholar]
- Skinningsrud B, Husebye ES, Pearce SH, McDonald DO, Brandal K, Wolff AB, Lovås K, Egeland T, Undlien DE 2008 Polymorphisms in CLEC16A and CIITA at 16p13 are associated with primary adrenal insufficiency. J Clin Endocrinol Metab 93:3310–3317 [DOI] [PubMed] [Google Scholar]
- Magitta NF, Bøe Wolff AS, Johansson S, Skinningsrud B, Lie BA, Myhr KM, Undlien DE, Joner G, Njølstad PR, Kvien TK, Førre Ø, Knappskog PM, Husebye ES 2009 A coding polymorphism in NALP1 confers risk for autoimmune Addison’s disease and type 1 diabetes. Genes Immun 10:120–124 [DOI] [PubMed] [Google Scholar]