Abstract
Context: Low 25-hydroxyvitamin D [25(OH)D] and high PTH may contribute to increased mortality risk in older adults.
Objective: The aim of the study was to test the association between 25(OH)D, PTH, and mortality in older men.
Design and Setting: The prospective Osteoporotic Fractures in Men (MrOS) study was conducted at six U.S. clinical centers.
Participants: We studied community-dwelling men at least 65 yr old (n = 1490).
Main Outcome Measure: Multivariate-adjusted proportional hazards models estimated the hazard ratio (HR) for mortality; cause of death was classified as cancer, cardiovascular, and other by central review of death certificates.
Results: During 7.3 yr of follow-up, 330 (22.2%) participants died: 97 from cancer, 110 from cardiovascular disease, and 106 from other causes. The adjusted HR per sd decrease in 25(OH)D for all-cause mortality was 1.01 (95% CI, 0.89, 1.14); no association between 25(OH)D and cardiovascular or other-cause mortality was seen. Unexpectedly, lower 25(OH)D levels were modestly associated with a decreased risk of cancer mortality (adjusted HR per sd decrease, 0.80; 95% CI, 0.64, 0.99). Analyzing 25(OH)D as a categorical variable did not alter these results. Higher PTH levels (log-transformed) were associated with an increased risk of all-cause mortality (adjusted HR per sd increase, 1.15; 95% CI, 1.03, 1.29) and cardiovascular mortality (adjusted HR per sd increase in PTH, 1.21; 95% CI, 1.00, 1.45).
Conclusions: In contrast to previous studies, lower 25(OH)D levels were not associated with an increased risk of all-cause or cause-specific mortality in older men. Higher PTH levels were associated with a modest increase in mortality risk.
Despite previous studies suggesting an association, we demonstrate no relation between 25(OH)D and mortality in a large cohort of community-dwelling older men.
Vitamin D deficiency, defined as serum 25-hydroxyvitamin D [25(OH)D] levels of less than 20 ng/ml (50 nmol/liter), is common in elderly U.S. and European adults. Estimates of the prevalence of deficiency range widely from 20–100% of the older population (1,2,3,4,5). Observational studies (6,7,8,9,10,11) and a meta-analysis of randomized trials (12) have suggested that individuals with low 25(OH)D levels have a higher risk of mortality, even after adjustment for potentially confounding factors such as comorbid medical conditions, although some reports suggest that no association exists (13,14,15). Few vitamin D outcome studies have considered PTH (14,16).
Individuals with excessively high levels of PTH that accompany primary or secondary hyperparathyroidism, including patients with end stage renal disease, have a high risk of cardiovascular disease and mortality (17,18). There are few reports about PTH levels and risk of mortality in older adults without kidney disease. One reported an association between higher PTH levels and increased risk of mortality among institutionalized older adults who did not have primary hyperparathyroidism (16); another demonstrated an increased risk of all-cause and cardiovascular mortality with higher PTH levels among community dwelling men (19). The relation between PTH levels and other causes of mortality, such as cancer, have not been extensively studied.
The aim of the present analyses was to test the hypothesis that lower levels of serum 25(OH)D and higher levels of PTH were independently associated with a higher risk of all-cause and cause-specific mortality in older, community-dwelling men participating in the Osteoporotic Fractures in Men (MrOS) study.
Subjects and Methods
The MrOS study was designed to understand healthy aging in older men with a particular focus on osteoporosis (20). Briefly, 5995 community-dwelling men aged 65 yr or older were recruited from six U.S. clinical centers (Birmingham, AL; Minneapolis, MN; Palo Alto, CA; the Monongahela Valley near Pittsburgh, PA; Portland, OR; and San Diego, CA) for a baseline visit between March 2000 and April 2002. Men must have been able to walk without assistance, not have had bilateral hip replacements, and have provided written informed consent. The study was approved by institutional review boards at all institutions.
Assessment of serum 25(OH)D
At the baseline examination, fasting serum was collected and stored at −70 C. Season of blood draw was recorded. Serum assays of 25(OH)D2 (derived from ergocalciferol) and 25(OH)D3 (derived from cholecalciferol) were performed at the Mayo Clinic using mass spectrometry (21). 25(OH)D2 and 25(OH)D3 were summed for total 25(OH)D level. The minimum detectable limit for 25(OH)D2 was 4 ng/ml and for 25(OH)D3 was 2 ng/ml. The interassay coefficient of variation (CV) was 4.4%, and the intraassay CV was 4.9%.
PTH assessment
Total intact PTH was assessed at Columbia University using the immunoradiometric assay (catalog no. 3KG600) from Scantibodies Laboratory Inc. (Santee, CA). This assay employs a polyclonal 1-84 PTH antibody with a tendency to bind in the N-terminal region of 1-84 PTH (label antibody) and a polyclonal 1-84 PTH antibody with a tendency to bind in the C-terminal region of 1-84 PTH (capture antibody). The use of these antibodies guarantees that both whole PTH (1-84 PTH) and truncated PTH fragments are detected; the assay detects both PTH 1-84 and some C-terminal fragments. Using pooled serum, the interassay CV was 8.4%, and the intraassay CV was 5.6%. The normal range of this assay extends to 66 ng/ml. We have also measured PTH with the Scantibodies whole PTH IRMA assay (an assay that detects only PTH 1-84). The two assays yielded results that were highly correlated (Spearman correlation coefficient = 0.88; P < 0.001), and data analyses using intact and whole assay results yielded essentially the same results; thus, only the results for total intact PTH are reported here. Serum calcium was assayed by the Oregon Veterans Administration Clinical Laboratory using a Roche COBAS Integra 800 automated analyzer (Roche Diagnostics, Indianapolis, IN) on previously thawed serum. The interassay CV was 2.6%.
Mortality
Every 4 months after the baseline exam, MrOS men were contacted to provide information about fracture status. Next-of-kin were contacted when men did not return questionnaires and could not be reached by telephone. Death certificates and discharge summaries where available and were collected for all deaths reported in MrOS. Participants were followed for vital status for an average of 7.3 yr after the baseline examination, through August 2009. Vital status information is more than 99% complete. Date and cause of death from death certificates were reviewed centrally by a physician adjudicator, with cause of death classified by ICD9 codes. Cause of death categories were broadly defined as cardiovascular (ICD9 codes 394.9, 396.9, 401.1, 401.9 to 442.0, 443.9, 785.51, 996.71), cancer (ICD9 codes 150.9 to 208.0), and other causes (other recorded ICD9 codes not in the previous categories).
Other clinical measures
Participants completed a self-administered questionnaire and clinical examination. Participants self-reported a history of cancer, stroke, heart attack, chronic obstructive pulmonary disease, hypertension, congestive heart failure, thyroid disease, Parkinson’s disease, and diabetes. Height was measured using wall-mounted stadiometers, and weight was measured with balance beam and digital scales. Body mass index (BMI) was calculated as weight (in kilograms) divided by height (in meters)2. Participants self-reported marital status (married vs. not), smoking status (current vs. never vs. past), race (Caucasian vs. non-Caucasian), education (college education vs. not), and physical activity [using the Physical Activity Scale for the Elderly (PASE)] (22). Alcohol intake was determined in a clinic interview and was classified as none/very low (<12 drinks/year), light (≥12 drinks/year to six drinks/week), and moderate/high (≥7 drinks/week). A functional status limitation was defined as having any difficulty with the following: heavy housework, shopping, or preparing meals. A mobility limitation was defined as having any difficulty walking two or three blocks or climbing 10 stairs. A modified version of the Block Food Frequency Questionnaire was used to determine total daily dietary (supplements + food) vitamin D and calcium intake (23). Glomerular filtration rate (GFR) was estimated by the Modification of Diet in Renal Disease (MDRD) study equation (24). Percentage body fat was determined from whole body dual-energy x-ray absorptiometry (DXA) scans on Hologic 4500 scanners (Hologic, Waltham, MA). Centralized quality control procedures, certification of DXA operators, repeat scanning of phantoms and standardized procedures for scanning were used to ensure reproducibility of DXA measurements. Variability across clinics was within acceptable limits, and cross-calibration correction factors were not required.
Participants included in analysis
Of the men enrolled at baseline, 5908 had at least one tube of serum available for analysis. From these men, 1594 were randomly selected to have serum 25(OH)D and PTH levels assayed. Of these, three were excluded from analyses due to assay problems [one with 25(OH)D levels >3 sd above the mean (75.6 ng/ml); one with insufficient sample for the vitamin D assay; and one with a CV for PTH assay run >25%]. Another 101 participants with missing data on covariates (GFR, serum calcium, phosphate, percentage body fat, or self-rated health) were excluded, leaving 1490 men for the present analyses. Cause of death analyses excluded 17 men for whom cause of death adjudication was pending as of August 2009.
Statistical analysis
Characteristics of the MrOS participants were compared across quartiles of total serum 25(OH)D and PTH. ANOVA was used for normally distributed continuous variables, Kruskal-Wallis for skewed continuous variables, and χ2 tests for categorical variables. The Spearman correlation coefficient was calculated between PTH and total 25(OH)D levels.
Total 25(OH)D levels were analyzed as quartiles and as clinical categories as suggested by Holick [normal (≥30 ng/ml), insufficient (>20 to <30 ng/ml), or deficient (≤20 ng/ml) (25)], and as a continuous variable with the hazard ratio (HR) reported per sd decrease. Total intact PTH was analyzed as quartiles. To investigate the influence of clinically high PTH levels on risk of mortality, PTH was also analyzed as a dichotomous variable (≥66 pg/ml vs. <66 pg/ml) (26). PTH was skewed, so PTH was log-transformed for analysis as a continuous variable; results are presented per sd increase in log-transformed PTH.
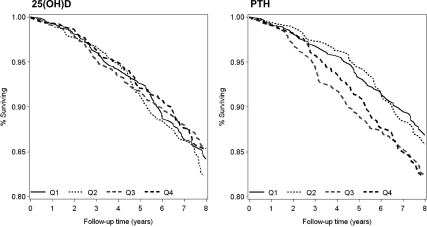
To account for seasonal and geographic variation in serum 25(OH)D levels, all models were adjusted for clinical center and season of blood draw, with seasons classified as winter (January-March), spring (April-June), summer (July-September), and fall (October-December). Cox proportional hazard models were used to estimate the HR and 95% confidence intervals (CIs) for mortality. The assumption of proportionality was tested and valid in all models. Base models that adjusted for age, season of blood draw, and clinical center were run for all-cause and cause-specific mortality for PTH and 25(OH)D levels separately. Covariates that were significantly associated with serum PTH or 25(OH)D levels at the P < 0.10 level and were associated with mortality in age-adjusted models were then included in the multivariate models. Finally, both PTH and 25(OH)D were included in a single multivariate model. Variables measuring similar characteristics (for example, percentage body fat and BMI) were selected by picking the variable or set of variables that were most strongly associated with serum PTH and 25(OH)D levels. Adjusted survival plots were also created to graphically describe the association between PTH, vitamin D, and mortality.
To test whether the association between PTH and mortality varied by 25(OH)D level (and vice versa), the significance of several interaction terms was tested. Interaction terms were evaluated for PTH∗25(OH)D as continuous variables; PTH∗25(OH)D as quartiles; and PTH∗25(OH)D as clinical categories. Analyses were also performed that excluded men with poor renal function (estimated GFR <60 ml/min/1.73 m2). Finally, to determine whether the effect of 25(OH)D or PTH varied over the course of the follow-up period, we completed several sensitivity analyses. We truncated follow-up time at 3 yr and at 5 yr, and we also excluded participants who died in the year after follow-up. The results of these sensitivity analyses for length of follow-up and GFR did not differ from the main analyses, so only the main analyses are reported.
Results
Most MrOS participants were either vitamin D deficient [25(OH)D levels <20 ng/ml; n = 376 (25.2%)] or insufficient [25(OH)D levels of 20 to <30 ng/ml; n = 737 (49.5%)]. Comparisons of characteristics of the MrOS cohort by quartile of vitamin D status are reported in Table 1. Briefly, men in the lowest quartiles of 25(OH)D were older, were more likely to be nonwhite, were more likely to report functional or mobility limitations, had greater adiposity, were more often unmarried, and were somewhat more likely to report at least one comorbid medical condition or abstention from alcohol than men with higher 25(OH)D levels.
Table 1.
Characteristics of the MrOS study population by quartile of 25(OH)D
| Characteristic | Quartile 1 (<20.0 ng/ml) | Quartile 2 (20.0 to <25.2 ng/ml) | Quartile 3 (25.2 to <30 ng/ml) | Quartile 4 (≥30 ng/ml) | P value |
|---|---|---|---|---|---|
| n | 372 | 370 | 372 | 376 | |
| Age (yr) | 74.5 ± 6.3 | 73.8 ± 5.8 | 73.9 ± 5.7 | 72.7 ± 5.4 | 0.001 |
| Weight (kg) | 84.5 ± 14.3 | 84.0 ± 13.7 | 82.4 ± 12.2 | 82.0 ± 11.1 | 0.072 |
| BMI (kg/m2) | 28.1 ± 4.2 | 27.6 ± 3.8 | 27.1 ± 3.5 | 26.8 ± 3.1 | <0.001 |
| Percentage body fat | 26.9 ± 5.5 | 26.8 ± 5.4 | 26.0 ± 4.8 | 25.7 ± 5.1 | 0.001 |
| Married | 283 (76.1) | 321 (86.8) | 314 (84.4) | 318 (84.6) | 0.001 |
| Nonwhite | 62 (16.7) | 30 (8.1) | 25 (6.7) | 22 (5.9) | <0.001 |
| Excellent/good health status | 301 (80.9) | 311 (84.1) | 321 (86.3) | 341 (90.7) | 0.002 |
| At least one functional limitation | 68 (18.3) | 40 (10.8) | 52 (14.0) | 39 (10.4) | 0.005 |
| At least one mobility limitation | 73 (19.6) | 40 (10.8) | 50 (13.4) | 37 (9.4) | <0.001 |
| Activity level (PASE score) | 137.5 ± 73.3 | 147.0 ± 67.8 | 147.8 ± 64.8 | 156.6 ± 67.7 | 0.002 |
| Current smoker | 22 (5.9) | 16 (4.3) | 10 (2.7) | 8 (2.1) | 0.029 |
| Alcohol use | 0.006 | ||||
| None/very light (<12 drinks/yr) | 135 (36.3) | 130 (35.2) | 118 (31.7) | 104 (27.7) | |
| Light (≥12 drinks/yr to 6 drinks/wk) | 103 (27.7) | 87 (23.6) | 96 (25.8) | 132 (35.1) | |
| Moderate (≥7 drinks/wk) | 134 (36.0) | 152 (41.2) | 158 (42.5) | 140 (37.2) | |
| At least one medical conditiona | 268 (72.0) | 245 (66.2) | 274 (73.7) | 252 (67.0) | 0.068 |
| Daily vitamin D intake (IU) | 272.0 ± 227.5 | 386.6 ± 239.5 | 433.9 ± 246.8 | 450.6 ± 237.5 | <0.002 |
| Serum calcium (mg/dl) | 9.3 ± 0.4 | 9.3 ± 0.4 | 9.3 ± 0.4 | 9.3 ± 0.4 | 0.014 |
| Albumin corrected calcium (mg/dl) | 9.5 ± 0.5 | 9.5 ± 0.5 | 9.5 ± 0.5 | 9.6 ± 0.5 | 0.001 |
| GFR (ml/min/1.73 m2) | 78.9 ± 19.4 | 77.5 ± 18.4 | 75.9 ± 17.8 | 73.8 ± 17.2 | 0.017 |
| Total intact PTH level (pg/ml) | 39.5 ± 45.8 | 33.2 ± 13.7 | 31.3 ± 11.8 | 29.7 ± 13.0 | <0.001 |
Data are expressed as mean ± sd or number (percentage).
At least one of the following medical conditions: stroke, heart attack, nonskin cancer, chronic obstructive pulmonary disease, hypertension, congestive heart failure, thyroid disease, diabetes, or Parkinson’s disease.
Men in the higher quartiles of PTH were older; had greater adiposity; and were more likely to report functional or mobility limitations, being unmarried, mobility limitations, and more comorbid medical conditions than men in the lower PTH quartiles (Table 2). Men with higher PTH levels also had lower vitamin D and calcium intake, lower levels of serum calcium, and worse GFR than men with lower PTH levels (P < 0.05 for all). PTH levels and vitamin D status were related, and the Spearman correlation coefficient between total 25(OH)D and PTH was −0.22 (P < 0.001).
Table 2.
Characteristics of the MrOS study population by quartile of total intact serum PTH
| Characteristic | Quartile 1 (<23.6 pg/ml) | Quartile 2 (23.6 to <29.5 pg/ml) | Quartile 3 (29.5 to <38.3 pg/ml) | Quartile 4 (≥38.3 pg/ml) | P value |
|---|---|---|---|---|---|
| n | 372 | 373 | 372 | 372 | |
| Age (yr) | 73.3 ± 5.6 | 72.8 ± 5.3 | 73.6 ± 5.8 | 75.3 ± 6.5 | <0.001 |
| Weight (kg) | 81.9 ± 11.9 | 83.3 ± 13.3 | 83.8 ± 12.7 | 83.8 ± 13.8 | 0.228 |
| BMI (kg/m2) | 26.9 ± 3.3 | 27.3 ± 3.9 | 27.6 ± 3.7 | 27.7 ± 3.9 | 0.006 |
| Percentage body fat | 25.86 ± 5.1 | 26.08 ± 5.5 | 26.64 ± 5.1 | 26.82 ± 5.1 | 0.038 |
| Married | 324 (87.1) | 314 (84.2) | 297 (79.8) | 301 (80.7) | 0.032 |
| Nonwhite | 30 (8.1) | 28 (7.5) | 39 (10.5) | 42 (11.3) | 0.222 |
| Excellent/good health status | 320 (86.0) | 319 (85.5) | 318 (85.5) | 317 (85.0) | 0.984 |
| At least one functional limitation | 36 (9.7) | 39 (10.5) | 54 (14.5) | 70 (18.8) | <0.001 |
| At least one mobility limitation | 40 (10.8) | 38 (10.2) | 61 (16.4) | 61 (16.4) | 0.011 |
| Activity level (PASE score) | 151.0 ± 69.2 | 151.6 ± 68.2 | 147.6 ± 69.2 | 138.9 ± 67.8 | 0.017 |
| Current smoker | 15 (4.0) | 15 (4.0) | 17 (4.6) | 9 (2.4) | 0.442 |
| Alcohol use | |||||
| None/very light (<12 drinks/yr) | 117 (31.5) | 109 (29.2) | 119 (32.0) | 142 (38.2) | 0.071 |
| Light (≥12 drinks/yr to 6 drinks/wk) | 117 (31.5) | 107 (28.7) | 109 (29.3) | 85 (22.9) | |
| Moderate (≥7 drinks/wk) | 138 (37.1) | 157 (42.1) | 144 (38.7) | 145 (39.0) | |
| At least one medical conditiona | 255 (68.6) | 248 (66.5) | 261 (70.2) | 275 (73.7) | 0.175 |
| Daily vitamin D intake (IU) | 425.5 ± 241.3 | 396.5 ± 253.5 | 385.6 ± 252.2 | 335.7 ± 235.8 | <0.001 |
| Daily calcium intake (mg) | 1261.2 ± 617.0 | 1166.7 ± 632.3 | 1101.1 ± 565.6 | 1047.6 ± 550.8 | <0.001 |
| Serum calcium (mg/dl) | 9.4 ± 0.3 | 9.3 ± 0.4 | 9.3 ± 0.4 | 9.3 ± 0.5 | <0.001 |
| Albumin corrected calcium (mg/dl) | 9.6 ± 0.4 | 9.6 ± 0.4 | 9.5 ± 0.5 | 9.5 ± 0.6 | <0.001 |
| GFR (ml/min/1.73 m2) | 79.1 ± 16.3 | 79.8 ± 16.9 | 77.1 ± 17.9 | 70.0 ± 20.2 | <0.001 |
| Total 25(OH)D level (ng/ml) | 27.6 ± 8.07 | 25.7 ± 7.36 | 24.7 ± 7.55 | 22.9 ± 8.22 | <0.001 |
| Vitamin D status | |||||
| Deficient (≤20 ng/ml) | 18 (4.8) | 26 (7.0) | 36 (9.7) | 67 (18.0) | <0.001 |
| Insufficient (>20 to <30 ng/ml) | 221 (59.4) | 248 (66.5) | 264 (71.0) | 238 (63.8) | |
| Sufficient (≥30 ng/ml) | 133 (35.8) | 99 (26.5) | 72 (19.4) | 68 (18.2) |
Data are expressed as mean ± sd or number (percentage).
At least one of the following medical conditions: stroke, heart attack, nonskin cancer, chronic obstructive pulmonary disease, hypertension, congestive heart failure, thyroid disease, diabetes, or Parkinson’s disease.
Over an average of 7.3 yr of follow-up (sd, 1.9 yr; range, 14 d to 9.3 yr), 330 (22.2%) participants died. Of these, 97 (29.4%) were classified as cancer deaths, 110 (33.3%) as cardiovascular deaths, and 106 (32.1%) as noncancer, noncardiovascular deaths, and 17 (5.2%) were unclassified. Of the 97 men who died from cancer, the most common type of cancer death was lung cancer (n = 33). Of the 110 men with death attributed to cardiovascular disease, the most common subtype was coronary heart disease (n = 55).
25(OH)D and all-cause mortality
There was no significant association between 25(OH)D levels and risk of all-cause mortality in this cohort (Table 3 and Fig. 1), whether 25(OH)D was analyzed as quartiles, as clinical categories, or as a continuous variable. Adjustment for potentially confounding factors did not alter this null association.
Table 3.
HRs (95% CIs) for mortality by categories of total 25(OH)D in the MrOS study
| All-cause mortality
|
Cancer
|
Cardiovascular
|
Noncancer, noncardiovascular
|
|||||
|---|---|---|---|---|---|---|---|---|
| Base modela | Multivariateb | Base modela | Multivariateb | Base modela | Multivariateb | Base modela | Multivariateb | |
| Quartiles | ||||||||
| Q1, <19.9 ng/ml (n = 372) | 1.11 (0.81, 1.52) | 0.95 (0.68, 1.34) | 0.63 (0.35, 1.14) | 0.52 (0.27, 1.00) | 1.50 (0.86, 2.63) | 1.52 (0.83, 2.80) | 1.26 (0.73, 2.17) | 0.94 (0.51, 1.72) |
| Q2, ≥19.9 to <25.2 ng/ml (n = 370) | 1.01 (0.73, 1.39) | 1.05 (0.75, 1.47) | 0.92 (0.54, 1.58) | 0.90 (0.51, 1.60) | 1.08 (0.59, 1.97) | 1.21 (0.65, 2.28) | 1.02 (0.58, 1.81) | 1.03 (0.56, 1.87) |
| Q3, ≥25.2 to <30.0 ng/ml (n = 372) | 0.94 (0.68, 1.30) | 0.89 (0.64, 1.24) | 0.82 (0.47, 1.43) | 0.80 (0.45, 1.41) | 1.18 (0.65, 2.12) | 1.12 (0.61, 2.06) | 0.88 (0.49, 1.59) | 0.80 (0.44, 1.47) |
| Q4, ≥30 ng/ml (n = 376) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| P for trend | 0.405 | 0.961 | 0.192 | 0.086 | 0.177 | 0.153 | 0.302 | 0.906 |
| Clinical categories | ||||||||
| Deficient, <20 ng/ml (n = 376) | 1.10 (0.81, 1.51) | 0.94 (0.67, 1.32) | 0.62 (0.34, 1.13) | 0.51 (0.27, 0.98) | 1.49 (0.85, 2.62) | 1.51 (0.82, 2.76) | 1.25 (0.72, 2.16) | 0.93 (0.51, 1.70) |
| Insufficient, 20 to <30 ng/ml (n = 737) | 0.98 (0.74, 1.29) | 0.97 (0.72, 1.30) | 0.88 (0.55, 1.41) | 0.85 (0.52, 1.40) | 1.13 (0.67, 1.92) | 1.17 (0.67, 2.02) | 0.95 (0.57, 1.58) | 0.91 (0.53, 1.53) |
| Sufficient, ≥30 ng/ml (n = 377) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| P for trend | 0.494 | 0.706 | 0.120 | 0.044 | 0.132 | 0.167 | 0.368 | 0.838 |
| Per sd decreasec | 1.07 (0.95, 1.20) | 1.01 (0.89, 1.14) | 0.85 (0.69, 1.04) | 0.80 (0.64, 0.99) | 1.23 (1.00, 1.52) | 1.24 (0.99, 1.55) | 1.16 (0.94, 1.43) | 1.04 (0.83, 1.30) |
Base model is adjusted for age, clinic, and season of blood draw.
Multivariate model is adjusted for age, clinic, season of blood draw, serum calcium and phosphate, GFR, percentage body fat, weight, race, health status, presence of at least one medical condition, alcohol use, education, activity level (PASE score), marital status, and presence of a functional or mobility limitation.
The sd for 25(OH)D is 7.98 ng/ml.
Figure 1.
Adjusted survival plots for participants in the MrOS study, by quartile of total intact PTH or 25(OH)D. Plots are adjusted for age, clinic, season of blood draw, serum calcium and phosphate, GFR, percentage body fat, weight, race, health status, presence of at least one medical condition, alcohol use, education, activity level (PASE score), marital status, and presence of a functional or mobility limitation.
25(OH)D and cause-specific mortality
There was a suggestion of an association between higher levels of 25(OH)D and increased risk of cancer mortality when 25(OH)D was analyzed as clinical categories, as quartiles, or as a continuous variable. No single covariate in the fully adjusted multivariate model changed the HR for mortality [expressed per sd decrease in 25(OH)D] by more than 4%, indicating that the change in the HR from the minimally adjusted to the fully adjusted model is not due to the effects of a single variable.
There was no association between 25(OH)D levels analyzed as quartiles or clinical categories and cardiovascular or noncardiovascular, noncancer mortality in either base or multivariate models. However, in base-adjusted models, when 25(OH)D was analyzed as a continuous variable, there was a modest increase in risk of CVD mortality per sd decrease in 25(OH)D (HR, 1.23; 95% CI, 1.00, 1.52). The HR was essentially unchanged after multivariate adjustment; however, this association was no longer significant.
Inclusion or exclusion of PTH from the multivariate models did not substantially alter the 25(OH)D results.
PTH and all-cause mortality
In the base-adjusted model (adjusted for age, clinical site, and season of blood draw), the risk of mortality increased as the quartile of PTH increased (Table 4 and Fig. 1). Further adjustment for numerous potentially confounding factors, including total 25(OH)D, did not substantially attenuate the association, and the association between PTH and mortality remained significant when PTH was analyzed as a continuous variable or using clinical categories (<66 pg/ml vs. ≥66 pg/ml).
Table 4.
HRs (95% CIs) for mortality by categories of total intact PTH in the MrOS study
| All-cause mortality
|
Cancer
|
Cardiovascular
|
Noncancer, noncardiovascular
|
|||||
|---|---|---|---|---|---|---|---|---|
| Base modela | Multivariateb | Base modela | Multivariateb | Base modela | Multivariateb | Base modela | Multivariateb | |
| Quartiles | ||||||||
| Q1, <23.6 pg/ml (n = 372) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Q2, 23.6 to <29.5 pg/ml (n = 373) | 0.96 (0.69, 1.36) | 1.03 (0.73, 1.45) | 0.94 (0.51, 1.73) | 1.04 (0.56, 1.93) | 1.35 (0.72, 2.52) | 1.42 (0.75, 2.67) | 0.66 (0.36, 1.23) | 0.75 (0.39, 1.41) |
| Q3, 29.5 to <38.5 pg/ml (n = 372) | 1.33 (0.97, 1.82) | 1.39 (1.00, 1.92) | 1.75 (1.03, 2.98) | 1.95 (1.12, 3.41) | 1.37 (0.74, 2.52) | 1.30 (0.69, 2.43) | 0.91 (0.52, 1.60) | 1.01 (0.56, 1.82) |
| Q4, ≥38.5 pg/ml (n = 373) | 1.34 (0.99, 1.82) | 1.32 (0.95, 1.84) | 0.77 (0.41, 1.45) | 0.94 (0.48, 1.83) | 2.04 (1.17, 3.55) | 1.51 (0.83, 2.76) | 1.25 (0.76, 2.06) | 1.33 (0.77, 2.31) |
| P for trend | 0.019 | 0.039 | 0.955 | 0.499 | 0.011 | 0.235 | 0.235 | 0.212 |
| Clinical categories | ||||||||
| <66 pg/ml (n = 51) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| ≥ 66 pg/ml (n = 1439) | 2.15 (1.42, 3.24) | 1.64 (1.06, 2.56) | 0.59 (0.15, 2.41) | 0.62 (0.15, 2.59) | 3.92 (2.26, 6.80) | 1.82 (0.96, 3.43) | 1.86 (0.86, 4.03) | 1.76 (0.78, 4.00) |
| Per sd increase in log-transformed PTHc | 1.19 (1.07, 1.32) | 1.15 (1.03, 1.29) | 0.97 (0.79, 1.19) | 1.03 (0.82, 1.29) | 1.46 (1.25, 1.71) | 1.21 (1.00, 1.45) | 1.12 (0.92, 1.35) | 1.15 (1.03, 1.29) |
Base model is adjusted for age, clinic, and season of blood draw.
Multivariate model is adjusted for age, clinic, season of blood draw, serum calcium and phosphate, GFR, percentage body fat, weight, race, health status, presence of at least one medical condition, alcohol use, education, activity level (PASE score), marital status, and presence of a functional or mobility limitation.
sd for log-transformed PTH is 0.394 log (pg/ml).
PTH and cause-specific mortality
There was a suggestion of an association between increased PTH levels and increased risk of cardiovascular mortality. In base-adjusted models, men in the highest quartile of PTH had a 2-fold increase in the risk of cardiovascular mortality compared with those in the lowest PTH quartile, but this association was no longer significant after adjustment for potentially confounding factors. In both base-adjusted and multivariate-adjusted models, each sd increase in log-transformed PTH was associated with a modestly increased risk of cardiovascular mortality. Men with clinically elevated PTH levels (≥66 ng/ml) had an elevated risk of cardiovascular mortality in base-adjusted models, but this association was attenuated and no longer significant in multivariate models.
In base-adjusted and multivariate-adjusted models, there was no general association with cancer mortality or noncancer, noncardiovascular mortality when PTH was analyzed as quartiles, as a dichotomous variable (<66 pg/nl vs. ≥66 pg/nl), or as a log-transformed continuous variable. The only exception was when PTH was analyzed as a continuous variable in multivariate-adjusted models because the HR per sd increase in log-transformed PTH reached statistical significance (HR, 1.15; 95% CI, 1.03, 1.29).
When the analyses were restricted to participants with PTH values below 66 ng/ml (n = 1439), the association between PTH and all-cause mortality was attenuated and no longer significant (HR, 1.10; 95% CI, 0.97, 1.25), suggesting that individuals with PTH values outside the normal range are at the highest risk of death.
Interaction between PTH and 25(OH)D
There was no evidence for a statistical interaction between 25(OH)D and PTH levels and risk of all-cause or cause-specific mortality. Interaction terms were tested for continuous variables of PTH and 25(OH)D, quartiles of PTH and 25(OH)D, and clinical categories of PTH and 25(OH)D. None reached the conservative P < 0.1 level of significance.
Discussion
In this prospective study of older men, there was no association between 25(OH)D levels and risk of all-cause mortality or cardiovascular mortality. In contrast, there was modest statistically significant increased risk of all-cause mortality associated with higher levels of PTH, which appears to be primarily related to cardiovascular death. The risk was highest for men with PTH values outside the normal physiological range (>66 pg/ml for the assay used in this study).
Some (16,19), but not all (14), previous reports have suggested an association between PTH and increased mortality risk in older adults without hyperparathyroidism. Our results suggest that higher PTH is associated with an elevated mortality risk due to increases in cardiovascular deaths, and other research suggests that higher PTH levels are associated with an increased prevalence of cardiovascular risk factors, such as metabolic syndrome (27). Additionally, there is evidence that individuals with primary hyperparathyroidism have an increased risk of heart disease (28), for example, arrhythmia (29) and left ventricular hypertrophy (30). These associations suggest plausible mechanisms whereby elevated PTH levels in those without hyperparathyroidism may harm the cardiovascular system.
No association between 25(OH)D and all-cause mortality was observed, although men with lower 25(OH)D tended to have worse health status than men with higher 25(OH)D levels; however, these differences tended to be small in magnitude. For example, men in the highest 25(OH)D quartile had a mean BMI that was only about 5% lower than men in the lowest 25(OH)D quartile, although this difference was highly statistically significant. How these somewhat small differences influenced mortality risk is unclear; multivariate adjustment removes the confounding effects of these imbalances.
The relation between PTH and 25(OH)D levels is complex, and most studies, including MrOS, show a modest association between these hormones. In our data, inclusion or exclusion of 25(OH)D levels in models estimating the association between PTH and mortality did not alter the effect estimate (and vice versa), suggesting that 25(OH)D and PTH may have statistically independent effects despite the biological link between them. Additionally, we did not find evidence for a statistical interaction between 25(OH)D and PTH, indicating that the association between PTH and mortality is consistent across all levels of 25(OH)D (and vice versa).
The finding that low 25(OH)D levels are not associated with increased risk of overall mortality contrasts with a meta-analysis of randomized trials (12) and large, population-based studies, such as the National Health and Nutrition Examination Survey (NHANES) (7) in which higher 25(OH)D levels were related to a lower risk of mortality. Several subtle differences between the current study and previous reports may cumulatively explain the divergent results in MrOS. First, in the MrOS study, 25(OH)D levels were assessed using liquid chromatography/mass spectroscopy, whereas most other studies have used radioimmunological or competitive binding assays. Second, most other observational studies had less well-characterized participants, which may have limited the ability to assess the role of confounding. For example, few studies have measures of adiposity (such as percentage body fat) aside from BMI. BMI is not a perfect measure of adiposity status (31,32), and vitamin D status is associated with adiposity, so studies with better assessment of adiposity might be able to better control for the confounding influence of this factor. However, in MrOS, even the minimally adjusted association between 25(OH)D and mortality was not suggestive of an association, so the lack of adjustment for potential confounders in other studies is not likely to explain the difference in the findings. Also, although one quarter of the MrOS men are vitamin D deficient [25(OH)D <20 ng/ml], very few men (<5%) had 25(OH)D levels below 10 ng/ml. If the association between vitamin D status and mortality is driven by individuals in this extremely low range, we may not have had the ability to detect an effect due to the limited number of men with very low 25(OH)D levels.
Unexpectedly, there was a suggestion of a protective association between low levels of 25(OH)D and decreased risk of cancer mortality, a result that is in the opposite direction of the many studies in the literature and our initial hypothesis. Most studies have reported either no association between 25(OH)D levels and cancer mortality (7,10,13,33) or increased cancer incidence or mortality for individuals with lower 25(OH)D levels (34,35). However, a few reports have suggested that lower levels of 25(OH)D are associated with decreased cancer risk, such as a study of pancreatic cancer in Finish smokers (36). Only two randomized studies have examined the association between vitamin D supplementation and cancer incidence with mixed results. The Women’s Health Initiative found no association between vitamin D plus calcium and incidence of colorectal cancer vs. placebo (37); a study of Nebraskan postmenopausal women found that both vitamin D plus calcium and calcium alone reduced overall cancer incidence vs. placebo (38). Additionally, meta-analyses of observational studies suggest that vitamin D status may have different relationships with different cancer types; for example, one meta-analysis found support for an inverse association between 25(OH)D levels and colorectal cancer (39), whereas another report by the same authors found no association between 25(OH)D levels and prostate cancer (40). Finally, the association we observed was only marginally significant, and we made multiple comparisons, so the cancer mortality results should be interpreted with caution. At a minimum, our results provide no support for a role of increased 25(OH)D levels in cancer mortality prevention. However, the only way to unequivocally establish a causal association between vitamin D status and cancer outcomes is through a randomized trial.
This study had a number of strengths. Both 25(OH)D and PTH were measured using state-of-the-art assays in a large cohort of well-characterized, community-dwelling older men with nearly complete follow-up. However, a number of limitations must be noted. First, ascertainment of cause of death, completed centrally, used death certificates and discharge summaries that may have led to misclassification of the cause of death for some participants. Second, the number of participants who died from each of the major causes examined in this analysis is fairly small, resulting in HRs with wide CIs. Third, the small number of cancer and cardiovascular events precluded a meaningful examination of the association between 25(OH)D and PTH and subtypes of cancer or cardiovascular mortality. This is a particularly important limitation given that vitamin D status may have different associations with different cancer types. Additionally, we cannot exclude the possibility that some of the men with the very highest PTH levels had hyperparathyroidism. Finally, this study was conducted in relatively healthy men, so generalization of these results to other populations may be limited.
In summary, and in contrast to many but not all previous reports, lower 25(OH)D levels were not associated with an increased risk of all-cause or cause-specific mortality in community-dwelling older men. We found that higher PTH levels were associated with a modest increase in mortality risk, likely due to increased cardiovascular death. Randomized trials are needed to determine the causal relation between vitamin D and mortality.
Acknowledgments
We thank Ms. Liezl Concepcion for her administrative assistance with this manuscript.
Footnotes
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health (NIH) funding. The National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institute on Aging, the National Center for Research Resources, and the NIH Roadmap for Medical Research provide support under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140. The NIH had no direct role in the design and conduction of the study; collection, management, analysis, and interpretation of data; or preparation, review, or approval of the manuscript.
Disclosure Summary: The authors have nothing to declare; all are funded by NIH grants as mentioned above in the support footnote.
First Published Online July 14, 2010
Abbreviations: BMI, Body mass index; CI, confidence interval; CV, coefficient of variation; DXA, dual-energy x-ray absorptiometry; GFR, glomerular filtration rate; HR, hazard ratio; 25(OH)D, 25-hydroxyvitamin D; PASE, Physical Activity Scale for the Elderly.
References
- Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B 2006 Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 84:18–28 [DOI] [PubMed] [Google Scholar]
- Holick MF 2006 High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 81:353–373 [DOI] [PubMed] [Google Scholar]
- Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, Chen E, de Papp AE 2005 Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab 90:3215–3224 [DOI] [PubMed] [Google Scholar]
- McKenna MJ 1992 Differences in vitamin D status between countries in young adults and the elderly. Am J Med 93:69–77 [DOI] [PubMed] [Google Scholar]
- Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC, Dick IM, Prince RL, Finkelstein JS 1998 Hypovitaminosis D in medical inpatients. N Engl J Med 338:777–783 [DOI] [PubMed] [Google Scholar]
- Kilkkinen A, Knekt P, Aro A, Rissanen H, Marniemi J, Heliövaara M, Impivaara O, Reunanen A 2009 Vitamin D status and the risk of cardiovascular disease death. Am J Epidemiol 170:1032–1039 [DOI] [PubMed] [Google Scholar]
- Melamed ML, Michos ED, Post W, Astor B 2008 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 168:1629–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz S, Dobnig H, Nijpels G, Heine RJ, Stehouwer CD, Snijder MB, van Dam RM, Dekker JM 2009 Vitamin D and mortality in older men and women. Clin Endocrinol (Oxf) 71:666–672 [DOI] [PubMed] [Google Scholar]
- Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W 2008 Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med 168:1340–1349 [DOI] [PubMed] [Google Scholar]
- Semba RD, Houston DK, Bandinelli S, Sun K, Cherubini A, Cappola AR, Guralnik JM, Ferrucci L 2010 Relationship of 25-hydroxyvitamin D with all-cause and cardiovascular disease mortality in older community-dwelling adults. Eur J Clin Nutr 64:203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba RD, Houston DK, Ferrucci L, Cappola AR, Sun K, Guralnik JM, Fried LP 2009 Low serum 25-hydroxyvitamin D concentrations are associated with greater all-cause mortality in older community-dwelling women. Nutr Res 29:525–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autier P, Gandini S 2007 Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med 167:1730–1737 [DOI] [PubMed] [Google Scholar]
- Freedman DM, Looker AC, Chang SC, Graubard BI 2007 Prospective study of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst 99:1594–1602 [DOI] [PubMed] [Google Scholar]
- Szulc P, Claustrat B, Delmas PD 2009 Serum concentrations of 17β-oestradiol and 25-hydroxycholecalciferol in relation to all-cause mortality in older men—the MINOS study. Clin Endocrinol (Oxf) 71:594–602 [DOI] [PubMed] [Google Scholar]
- Bolland MJ, Bacon CJ, Horne AM, Mason BH, Ames RW, Wang TK, Grey AB, Gamble GD, Reid IR 2010 Vitamin D insufficiency and health outcomes over 5 y in older women. Am J Clin Nutr 91:82–89 [DOI] [PubMed] [Google Scholar]
- Sambrook PN, Chen JS, March LM, Cameron ID, Cumming RG, Lord SR, Schwarz J, Seibel MJ 2004 Serum parathyroid hormone is associated with increased mortality independent of 25-hydroxy vitamin D status, bone mass, and renal function in the frail and very old: a cohort study. J Clin Endocrinol Metab 89:5477–5481 [DOI] [PubMed] [Google Scholar]
- Hedbäck G, Odén A 1998 Increased risk of death from primary hyperparathyroidism—an update. Eur J Clin Invest 28:271–276 [DOI] [PubMed] [Google Scholar]
- Nilsson IL, Yin L, Lundgren E, Rastad J, Ekbom A 2002 Clinical presentation of primary hyperparathyroidism in Europe—nationwide cohort analysis on mortality from nonmalignant causes. J Bone Miner Res 17(Suppl 2):N68–N74 [PubMed] [Google Scholar]
- Hagström E, Hellman P, Larsson TE, Ingelsson E, Berglund L, Sundström J, Melhus H, Held C, Lind L, Michaëlsson K, Arnlöv J 2009 Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation 119:2765–2771 [DOI] [PubMed] [Google Scholar]
- Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K 2005 Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials 26:569–585 [DOI] [PubMed] [Google Scholar]
- Orwoll E, Nielson CM, Marshall LM, Lambert L, Holton KF, Hoffman AR, Barrett-Connor E, Shikany JM, Dam T, Cauley JA 2009 Vitamin D deficiency in older men. J Clin Endocrinol Metab 94:1214–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn RA, Smith KW, Jette AM, Janney CA 1993 The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 46:153–162 [DOI] [PubMed] [Google Scholar]
- Block G, Woods M, Potosky A, Clifford C 1990 Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol 43:1327–1335 [DOI] [PubMed] [Google Scholar]
- Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F 2006 Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145:247–254 [DOI] [PubMed] [Google Scholar]
- Holick MF 2007 Vitamin D deficiency. N Engl J Med 357:266–281 [DOI] [PubMed] [Google Scholar]
- Boudou P, Ibrahim F, Cormier C, Chabas A, Sarfati E, Souberbielle JC 2005 Third- or second-generation parathyroid hormone assays: a remaining debate in the diagnosis of primary hyperparathyroidism. J Clin Endocrinol Metab 90:6370–6372 [DOI] [PubMed] [Google Scholar]
- Ahlström T, Hagström E, Larsson A, Rudberg C, Lind L, Hellman P 2009 Correlation between plasma calcium, parathyroid hormone (PTH) and the metabolic syndrome (MetS) in a community-based cohort of men and women. Clin Endocrinol (Oxf) 71:673–678 [DOI] [PubMed] [Google Scholar]
- Andersson P, Rydberg E, Willenheimer R 2004 Primary hyperparathyroidism and heart disease—a review. Eur Heart J 25:1776–1787 [DOI] [PubMed] [Google Scholar]
- Rayner HC, Hasking DJ 1986 Hyperparathyroidism associated with severe hypercalcaemia and myocardial calcification despite minimal bone disease. Br Med J (Clin Res Ed) 293:1277–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons C, Fortune F, Greenbaum RA, Dandona P 1985 Cardiac hypertrophy, hypertrophic cardiomyopathy, and hyperparathyroidism—an association. Br Heart J 54:539–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernán MA, Cole SR 2009 Invited commentary: causal diagrams and measurement bias. Am J Epidemiol 170:959–962; discussion 963–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahar E 2009 The association of body mass index with health outcomes: causal, inconsistent, or confounded? Am J Epidemiol 170:957–958 [DOI] [PubMed] [Google Scholar]
- Freedman DM, Fuhrman B, Graubard BI, Chang SC 2009 Vitamin D and cancer mortality. Cancer Epidemiol Biomarkers Prev 18:359; author reply 359–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz S, Dobnig H, Winklhofer-Roob B, Riedmüller G, Fischer JE, Seelhorst U, Wellnitz B, Boehm BO, März W 2008 Low serum levels of 25-hydroxyvitamin D predict fatal cancer in patients referred to coronary angiography. Cancer Epidemiol Biomarkers Prev 17:1228–1233 [DOI] [PubMed] [Google Scholar]
- Giovannucci E 2005 The epidemiology of vitamin D and cancer incidence and mortality: a review (United States). Cancer Causes Control 16:83–95 [DOI] [PubMed] [Google Scholar]
- Stolzenberg-Solomon RZ, Vieth R, Azad A, Pietinen P, Taylor PR, Virtamo J, Albanes D 2006 A prospective nested case-control study of vitamin D status and pancreatic cancer risk in male smokers. Cancer Res 66:10213–10219 [DOI] [PubMed] [Google Scholar]
- Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O'Sullivan MJ, Margolis KL, Ockene JK, Phillips L, Pottern L, Prentice RL, Robbins J, Rohan TE, Sarto GE, Sharma S, Stefanick ML, Van Horn L, Wallace RB, Whitlock E, Bassford T, Beresford SA, Black HR, Bonds DE, Brzyski RG, Caan B, Chlebowski RT, Cochrane B, Garland C, Gass M, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Judd H, Kooperberg CL, Kuller LH, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Lewis CE, Limacher MC, Manson JE 2006 Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 354:684–696 [DOI] [PubMed] [Google Scholar]
- Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP 2007 Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr 85:1586–1591 [DOI] [PubMed] [Google Scholar]
- Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H 2009 Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther 30:113–125 [DOI] [PubMed] [Google Scholar]
- Yin L, Raum E, Haug U, Arndt V, Brenner H 2009 Meta-analysis of longitudinal studies: serum vitamin D and prostate cancer risk. Cancer Epidemiol 33:435–445 [DOI] [PubMed] [Google Scholar]