Abstract
Context: Aldosterone has been shown to exert a central sympathoexcitatory action in multiple animal models, but evidence in humans is still lacking.
Objectives: Our objective was to determine whether hyperaldosteronism causes reversible sympathetic activation in humans.
Methods: We performed a cross-sectional comparison of muscle sympathetic nerve activity (SNA, intraneural microelectrodes) in 14 hypertensive patients with biochemically proven primary aldosteronism (PA) with 20 patients with essential hypertension (EH) and 18 age-matched normotensive (NT) controls. Seven patients with aldosterone-producing adenoma (APA) were restudied 1 month after unilateral adrenalectomy.
Results: Mean blood pressure values in patients with PA and EH and NT controls was 145 ± 4/88 ± 2, 150 ± 4/90 ± 2, and 119 ± 2/76 ± 2 mm Hg, respectively. The major new findings are 2-fold: 1) baseline SNA was significantly higher in the PA than the NT group (40 ± 3 vs. 30 ± 2 bursts/min, P = 0.014) but similar to the EH group (41 ± 3 bursts/min) and 2) after unilateral adrenalectomy for APA, SNA decreased significantly from 38 ± 5 to 27 ± 4 bursts/min (P = 0.01), plasma aldosterone levels fell from 72.4 ± 20.3 to 11.4 ± 2.3 ng/dl (P < 0.01), and blood pressure decreased from 155 ± 8/94 ± 3 to 117 ± 4/77 ± 2 mm Hg (P < 0.01).
Conclusion: These data provide the first evidence in humans that APA is accompanied by reversible sympathetic overactivity, which may contribute to the accelerated hypertensive target organ disease in this condition.
Aldosterone-producing adenoma in humans is accompanied by reversible sympathetic overactivity, which may contribute to the accelerated hypertensive target organ disease in this condition.
Although aldosterone is known to increase blood pressure (BP) by promoting renal sodium retention, an increasing body of evidence indicates that aldosterone also acts centrally to stimulate the sympathetic nervous system (1,2,3). Studies in rats and dogs demonstrated that direct infusion of aldosterone into cerebral ventricles, at the doses that were too small to raise BP when given systemically, caused a sustained increase in sympathetic nerve activity (SNA) and BP (4,5,6,7). This central pressor action of aldosterone can be blocked by intracerebroventricular infusion of mineralocorticoid receptor (MR) antagonists (4). Moreover, in mini-pigs, mineralocorticoid-induced hypertension is abrogated by chemical sympathectomy, demonstrating a major sympathetic component (8).
The question is whether these experimental animal data have any relevance to clinical hypertension. In a recent study from our lab, SNA measured directly by intraneural microelectrodes was unchanged when patients with essential hypertension (EH) were treated with an MR antagonist but significantly increased by thiazide therapy despite a similar reduction in BP (9). In another recent study, MR blockade with spironolactone was shown to reduce total body norepinephrine spillover in elderly hypertensive patients (10). However, serum aldosterone levels in these patients were normal in both studies.
Another approach to this problem is to study SNA in patients with primary aldosteronism (PA), a secondary form of hypertension characterized by adrenal aldosterone overproduction, which is surgically correctable in some cases with unilateral aldosterone-producing adenoma (APA). One group of investigators found that despite very high serum aldosterone levels, PA was accompanied by suppressed SNA, which normalized after surgical resection of the tumor (11,12). Given the limited clinical studies concerning SNA in PA and the strength of the animal data indicating the opposite, we undertook an independent microneurographic study of SNA in patients with PA before and after curative unilateral adrenalectomy.
Patients and Methods
Fourteen patients with PA, 20 patients with untreated stage 1 EH, and 18 normotensive (NT) subjects participated in the study after giving written informed consent. The study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center at Dallas. The PA subjects were recruited from the patients referred to our hypertension clinic for evaluation of resistant hypertension or suspected secondary hypertension. The EH and NT groups matched for age, gender, and ethnicity were recruited from the Dallas Heart Study (13). All subjects had no history of heart disease, diabetes mellitus, or chronic kidney diseases or evidence of target organ damage such as left ventricular hypertrophy at least by electrocardiography. Patients had not received any vasoactive medications for at least 24 h before the studies.
Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and diuretics were discontinued for at least 2 wk before biochemical evaluation. During the period of biochemical evaluation, subjects were treated with calcium channel blockers (amlodipine or diltiazem). If BP was not controlled, β-adrenergic receptor blockers (labetalol, carvedilol, or metoprolol), and/or α-adrenergic receptor blockers (doxazosin) were added to the regimen. None of these subjects had received treatment with spironolactone or central sympatholytic drugs such as clonidine or guanfacine.
The diagnosis of PA was established based on the current Endocrine Society guidelines (14). During biochemical evaluation, serum potassium levels were corrected with oral supplementation to 3.5 mEq/liter, if necessary. All PA patients had plasma aldosterone levels of more than 15 ng/dl and plasma renin activity of less than 1 ng/ml·h during screening evaluation. All subjects with PA demonstrated nonsuppressible aldosterone production after 3–5 d of high dietary sodium ingestion as evidenced by 24-h urinary aldosterone excretion of more than 12 μg and urinary sodium excretion higher than 200 mEq.
All subjects with biochemical confirmation of PA underwent adrenal vein sampling (AVS) to distinguish between unilateral vs. bilateral adrenal overproduction of aldosterone. AVS was performed during iv infusion of 0.25 mg corticotropin (Cortrosyn; Amphastar Pharmaceuticals Inc., Rancho Cucamonga, CA) in 300 ml 5% dextrose in water at a rate of 100 ml/h for the duration of the procedure. Adrenal vein cannulation was considered successful when the cortisol levels in both adrenal veins were three times (or more) higher than the cortisol level in the inferior vena cava, whereas lateralization was considered positive when the adrenal vein aldosterone to cortisol ratio in the dominant side was four times greater than the aldosterone to cortisol ratio in the contralateral side.
Microneurographic measurement of SNA
All experiments were performed with the subjects in the supine position. BP was measured by the oscillometric technique with the Welch Allyn CE00050 Monitor (Tycos Instruments, Inc., Arden, NC). Heart rate was monitored by the R wave of an electrocardiogram lead. Postganglionic efferent sympathetic nerve discharge, heart rate, and respiratory rate were recorded continuously using a multichannel digital data recorder (MacLab/8S ML780; AD Instruments Inc., Mountain View, CA).
Multiunit recordings of postganglionic SNA were obtained with tungsten microelectrodes inserted into muscle nerve fascicles of the peroneal nerves using the microneurographic technique of Valbo et al. (15). The nerve signals were amplified, filtered (bandwidth 700–2000 Hz), rectified, and integrated to obtain a mean voltage display of SNA. A recording of muscle SNA was considered acceptable when the neurograms revealed spontaneous, pulse-synchronous bursts of neural discharge, with the largest bursts showing a minimal signal-to-noise ratio of 3:1. SNA was analyzed by an investigator (Z.W.) without the knowledge of the treatment phase assigned to each subject. The interobserver and intraobserver variations in identifying bursts are less than 10% and less than 5% (16). The intra-subject variability of SNA when measured on repeated occasions without any interventions is less than 15% with the correlation coefficient of reliability in the measurement of SNA of 0.91 (17).
Assessment of SNA after adrenalectomy
In a subset of seven patients with lateralization of aldosterone production by AVS, measurement of SNA was performed before and 1 month after removal of an aldosteronoma. Pathological evidence of adrenal adenoma was confirmed from the surgical specimens in all subjects. Patients were maintained on the same type and dosage of antihypertensive medications before and after surgery. All vasoactive medications were withheld for at least 24 h before measurement of SNA.
Biochemical and hormonal assays
Blood samples were collected after fasting for 12 h with the subjects in the seated position for more than 5 min. The plasma samples were then separated and stored at −80 C until analysis. Plasma direct active renin and aldosterone were measured by RIA (Diagnostic Systems Laboratories, Webster, TX). The lower limit of the renin assay was 5 pg/ml with coefficient of variation of 1–2.6%. The lower limit of the aldosterone assay was 8 pg/ml with coefficient of variation of 3–5%.
Statistical analysis
Statistical analysis was performed with SAS version 9.2 (SAS Institute, Cary, NC). For continuous variables, one-way ANOVA models were used to compare means between the PA, ET, and NT groups. Pairwise group differences were tested using the least-squares means contrasts derived from the ANOVA models. Analysis of covariance was used to adjust for age and body mass index (BMI). Comparisons of SNA measured at two occasions in the PA and EH groups were made with two-way ANOVA with repeated measures. Results are expressed as mean and se unless otherwise indicated. A log transformation was used to reduce the skewness of direct renin and aldosterone levels.
Results
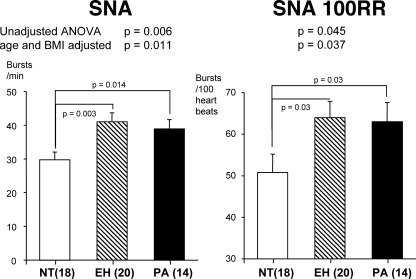
Baseline characteristics of the study subjects are shown in Table 1. The three groups were well matched on age, BMI, gender, and ethnicity. BP, as expected, was higher in patients wth PA and EH than NTs (Table 1, P < 0.0001). Also as expected, serum aldosterone levels were significantly higher in patients with PA (Table 1, P < 0.0001), whereas direct plasma renin levels were lower (P < 0.01). SNA, both in bursts per minute and bursts per 100 heartbeats, was significantly higher in the PA patients than NT controls (39.7 ± 3 vs. 29.7 ± 2 bursts/min, P = 0.014, Figs. 1 and 2) but comparable to the level seen in patients with EH (41.0 ± 3 bursts/min, P > 0.05). Within the PA group, SNA was similar between six subjects with idiopathic or bilateral hyperplasia and eight subjects with APA (40.7 ± 3 vs. 37.6 ± 4 bursts/min, P > 0.05). Because univariate analysis demonstrates correlation between age and SNA in all three groups and between BMI and SNA in the EH group, we performed SNA analysis of the whole cohort after adjustment for these two variables. Age- and BMI-adjusted analysis of SNA still yielded similar results (Fig. 2), suggesting both age- and obesity-independent sympathetic activation in PA patients.
Table 1.
Subject characteristics
| NT (n = 18) | EH (n = 20) | PA (n = 14) | ANOVA P | |
|---|---|---|---|---|
| Age (yr) | 45 ± 2 | 47 ± 2 | 47 ± 3 | 0.88 |
| Sex (male/female) | 13/5 | 14/6 | 11/3 | 1 |
| BMI (kg/m2) | 30.0 ± 1 | 31.9 ± 2 | 32.2 ± 2 | 0.64 |
| Ethnicity (AA/H/W) | 15/1/2 | 11/1/8 | 10/1/3 | 0.29 |
| Systolic BP (mm Hg) | 119 ± 2 | 150 ± 4a | 145 ± 5a | <0.001 |
| Diastolic BP (mm Hg) | 76 ± 2 | 90 ± 2a | 88 ± 2a | <0.001 |
| Heart rate (beats/min) | 61 ± 2 | 65 ± 2 | 63 ± 2 | 0.38 |
| Direct renin (pg/ml) | 32.1 ± 8.1 | 13 ± 1.9a | 10.4 ± 1.2a | 0.006 |
| Aldosterone (ng/dl) | 14.2 ± 1.8 | 16.8 ± 1.3 | 76.1 ± 14.9a | <0.0001 |
AA, African-American; H, Hispanic; W, White.
P < 0.01 vs. NT.
Figure 1.
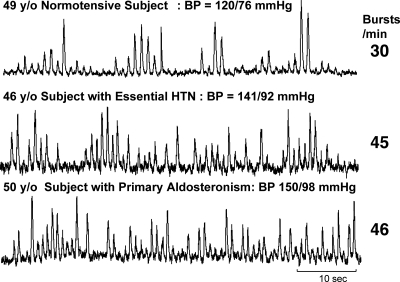
Original recordings of SNA in a 49-yr-old NT subject (top), a 46-yr-old subject with EH (middle), and a hypertensive subject with PA (bottom). HTN, Hypertension.
Figure 2.
Summary data showing SNA and SNA per 100 RR intervals (SNA 100RR) in NT, EH, and PA groups.
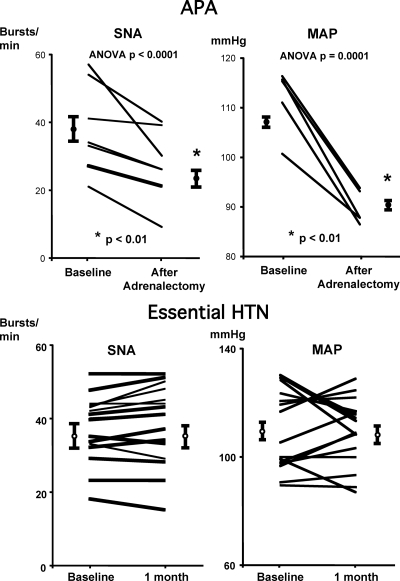
In the subset of seven subjects with surgically confirmed APA, SNA fell significantly after surgical removal of tumor (from 38.1 ± 5 to 27.3 ± 4 bursts/min, P = 0.01, and from 60.0 ± 7.8 to 42.0 ± 5.5 bursts/100 RR intervals, P = 0.003, respectively, Figs. 3 and 4). Plasma aldosterone levels and mean BP reduced significantly (from 72.4 ± 20.3 to 11.4 ± 2.3 ng/dl and from 114 ± 5 to 90 ± 2 mm Hg, respectively, P < 0.01), whereas plasma direct renin rose after surgery (from 10.4 ± 1.4 to 23.5 ± 6.8 pg/ml, P values < 0.01). There were no significant changes in heart rate or body weight after surgery (from 64 ± 4 to 61 ± 3 beats/min and from 95.5 ± 9.9 vs. 94.6 ± 11 kg, respectively, both P values > 0.05). In contrast, SNA remained unchanged in a subset of 16 patients with untreated hypertension studied on two different days, separated by a period of 1 month without any changes in medical intervention (Fig. 4), suggesting changes in SNA in the PA group were not due to random variation of SNA measurement over time.
Figure 3.
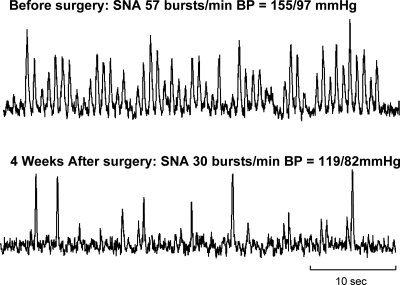
Original recordings of SNA in a 47-yr-old subject with APA before (top) and 4 wk after (bottom) surgical resection of tumor.
Figure 4.
Summary and individual data showing SNA and MAP of seven subjects with APA (top) before and after adrenalectomy and 14 subjects with untreated EH studied on two separate occasions separated by 1 month without any medical intervention. HTN, Hypertension.
Discussion
There are two major new findings in our present study. First, we found that SNA was elevated in patients with PA when compared with normal controls and comparable to those with EH. Second, sympathetic activation was normalized by laparoscopic adrenalectomy in those with a unilateral APA, further supporting a sympathoexcitatory action of aldosterone.
Our study is an important first step forward in the clinical translation of animal data showing a central pressor action of aldosterone. Circulating aldosterone is well known to penetrate the blood-brain barrier at concentrations that are parallel to plasma concentrations (18,19,20). Studies in conscious dogs demonstrate that closed intracerebroventricular infusion of aldosterone that causes no systemic spillover raises BP in both salt-depleted and salt-repleted conditions (7). The increase in BP was mainly due to an increase in total peripheral resistance rather than an increase in cardiac output or blood volume (7). Studies in NT rats produce similar results and further demonstrate an increase in sympathetic vasoconstrictor activity to the kidneys with central administration of aldosterone (4,6).
Our findings are in contrast to those of two previous studies conducted by another group of investigators, who reported that SNA was lower in patients with PA than EH patients and similar to NT controls (11,12). However, differences in patient population characteristics may have contributed to the divergent results. The study by Miyajima et al. (11) was limited to young subjects younger than 40 yr of age. Matsukawa et al. (12) studied older subjects with similar age to our subjects, but their NT controls had surprisingly high levels of SNA despite being lean with body weight within 10% of ideal range (12). SNA typically increases with both age and BMI. In the same study, the increase in SNA after adrenalectomy was limited to only a few subjects, whereas SNA in other patients displayed only minimal change (12).
Circulating levels of aldosterone in PA patients in studies by Miyajima et al. (11) and Matsukawa et al. (12) were also different from our study because they were expressed in nanograms per milliliter instead of nanograms per deciliter. However, these units appear to be incorrectly reported because plasma aldosterone levels in normal controls from both studies would have been more than 300 times higher than upper limit of normal range of 15 ng/dl (14). If the units in these two studies are expressed correctly in picograms per milliliter (rather than nanograms per milliliter), our PA patients would have aldosterone levels more than twice as high as PA patients from previous studies. This is likely to be because our PA subjects were required to demonstrate nonsuppressible aldosterone production despite increased sodium intake and volume expansion according to the current Endocrine Society guidelines (14), whereas the PA subjects in both studies by Miyajima et al. (11) and Matsukawa et al. (12) were identified by only elevated random levels of aldosterone.
Furthermore, studies by Miyajima et al. (11) and Matsukawa et al. (12) included only patients with APA that were identified primarily by imaging studies, either the computed tomography or iodomethyl-19-norcholesterol (NP-59) scintigraphy, whereas our study included both patients with APA and bilateral hyperplasia, which was confirmed the gold standard AVS according to the current guidelines (14,21,22,23). Thus, our study results are pertinent to both major subtypes of PA patients. Furthermore, we confirmed SNA results in the cross-sectional study with the prospective study in a subset of PA patients after surgical removal of aldosteronomas. Elevated SNA in PA patients remains persistent after adjustment for age and BMI. For all these reasons, we believe that our study provides the first valid evidence linking aldosterone excess to sympathetic overactivity in humans.
Both studies by Miyajima et al. (11) and Matsukawa et al. (12) were limited to the Japanese population, and therefore, we cannot exclude ethnic differences in SNA responses to aldosterone. Salt consumption in the Japanese population is generally higher than in the United States (24,25), whereas salt sensitivity in Japanese appears to be comparable to African-Americans but greater than Caucasians (26). Because volume expansion could suppress SNA via activation of cardiopulmonary baroreflexes, the net effects of aldosterone on SNA in a given subject may depend upon relative contributions of a primary central sympathoexcitatory action of aldosterone vs. a secondary sympathoinhibitory reaction of cardiopulmonary baroreflexes to aldosterone-mediated renal sodium retention with plasma volume expansion. Thus, the greater severity of aldosteronism in our patients may have allowed direct central action of aldosterone to dominate.
The mechanisms underlying elevated SNA in PA patients remain unknown, but an increasing body of evidence suggests that activation of central MRs may play an important role (5,27). MRs are abundantly expressed in many brain regions involved in cardiovascular regulation such as the hypothalamus, paraventricular nucleus, and nucleus tractus solitarius (1,28,29). Although the brain MR can also be activated by glucocorticoids, which circulate at much higher concentrations than aldosterone, Geerling and Loewy (29) recently demonstrated that coexpression of 11β-hydroxysteroid dehydrogenase enzyme type 2 allows aldosterone to gain access to these neurons by converting glucocorticoids to metabolites with lower binding affinity to MR. The central effects of MR on BP regulation may provide an explanation for the ability of spironolactone to promote natriuresis and reduce BP without causing reflex sympathetic activation in patients with primary hypertension in our recently published study (9).
Aside from its direct central action, aldosterone has also been shown to inhibit carotid baroreceptor discharge in dogs, which normally exerts an inhibitory influence on SNA (30). One study in humans, however, provided conflicting evidence because baroreflex control of heart rate indirectly assessed by spectral analysis of heart rate was shown to be augmented in patients with PA (31). Nevertheless, the relative contribution of direct central MR activation vs. secondary sympathetic activation from baroreflex inhibition on augmented SNA in patients with PA and its normalization with surgical treatment of PA remain to be further investigated.
Regardless of mechanisms mediating sympathetic activation in PA patients, our study may have potential clinical implications. The sympathetic nervous system is known to contribute to the pathogenesis of hypertension and the poor prognosis in patients with cardiovascular diseases (32,33,34). Thus, in addition to the effects of aldosterone on renal sodium absorption, aldosterone-induced sympathetic activation may constitute another potential mechanism underlying the high prevalence of resistant hypertension as well as the accelerated cardiovascular complications in PA patients (35,36,37).
Acknowledgments
We gratefully acknowledge Dr. Norman Kaplan from the Hypertension Section at the University of Texas Southwestern Medical Center at Dallas for invaluable advice regarding the critical review of the manuscript.
Footnotes
This work was supported by National Institutes of Health Grant HL-078782 (W.V.). The project was also supported by the Donald W. Reynold’s Cardiovascular Clinical Research Center (R.G.V., W.V.) and the O’Brien Kidney Center (W.V.), the Lincy Foundation (R.G.V.), the Clinical Scientist Award in Translational Research 1005954 from the Burroughs Wellcome Fund (R.A.), and Clinical and Translational Sciences Award UL1RR-024982 (B.A.-H.).
Clinical Trial Registration No. NCT00353652.
Disclosure Summary: W.V. is supported by a research grant from the National Institutes of Health (RO1HL-078782). Other authors have nothing to declare.
First Published Online July 21, 2010
Abbreviations: APA, Aldosterone-producing adenoma; AVS, adrenal vein sampling; BMI, body mass index; BP, blood pressure; EH, essential hypertension; MR, mineralocorticoid receptor; NT, normotensive; PA, primary aldosteronism; SNA, sympathetic nerve activity.
References
- de Kloet ER, Van Acker SA, Sibug RM, Oitzl MS, Meijer OC, Rahmouni K, de Jong W 2000 Brain mineralocorticoid receptors and centrally regulated functions. Kidney Int 57:1329–1336 [DOI] [PubMed] [Google Scholar]
- Felder RB 2010 Mineralocorticoid receptors, inflammation and sympathetic drive in a rat model of systolic heart failure. Exp Physiol 95:19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Sanchez EP 2010 The mammalian mineralocorticoid receptor: tying down a promiscuous receptor. Exp Physiol 95:13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Sanchez EP 1986 Intracerebroventricular infusion of aldosterone induces hypertension in rats. Endocrinology 118:819–823 [DOI] [PubMed] [Google Scholar]
- Francis J, Beltz T, Johnson AK, Felder RB 2003 Mineralocorticoids act centrally to regulate blood-borne tumor necrosis factor-α in normal rats. Am J Physiol Regul Integr Comp Physiol 285:R1402–R1409 [DOI] [PubMed] [Google Scholar]
- Wang H, Huang BS, Leenen FH 2003 Brain sodium channels and ouabainlike compounds mediate central aldosterone-induced hypertension. Am J Physiol Heart Circ Physiol 285:H2516–H2523 [DOI] [PubMed] [Google Scholar]
- Kageyama Y, Bravo EL 1988 Hypertensive mechanisms associated with centrally administered aldosterone in dogs. Hypertension 11:750–753 [DOI] [PubMed] [Google Scholar]
- Thomas GD, O'Hagan KP, Zambraski EJ 1991 Chemical sympathectomy alters the development of hypertension in miniature swine. Hypertension 17:357–362 [DOI] [PubMed] [Google Scholar]
- Menon DV, Arbique D, Wang Z, Adams-Huet B, Auchus RJ, Vongpatanasin W 2009 Differential effects of chlorthalidone versus spironolactone on muscle sympathetic nerve activity in hypertensive patients. J Clin Endocrinol Metab 94:1361–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray DW, Supiano MA 2010 Impact of aldosterone receptor blockade compared with thiazide therapy on sympathetic nervous system function in geriatric hypertension. Hypertension 55:1217–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima E, Yamada Y, Yoshida Y, Matsukawa T, Shionoiri H, Tochikubo O, Ishii M 1991 Muscle sympathetic nerve activity in renovascular hypertension and primary aldosteronism. Hypertension 17:1057–1062 [DOI] [PubMed] [Google Scholar]
- Matsukawa T, Miyamoto T 2008 Does infusion of ANG II increase muscle sympathetic nerve activity in patients with primary aldosteronism? Am J Physiol Regul Integr Comp Physiol 294:R1873–R1879 [DOI] [PubMed] [Google Scholar]
- Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH 2004 The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol 93:1473–1480 [DOI] [PubMed] [Google Scholar]
- Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, Young Jr WF, Montori VM 2008 Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 93:3266–3281 [DOI] [PubMed] [Google Scholar]
- Valbo AB, Hagbarth KE, Torebjörk HE, Wallin BG 1979 Somatosensory proprioceptive and sympathetic activity in human peripheral nerves. Physiol Rev 59:919–957 [DOI] [PubMed] [Google Scholar]
- Leimbach Jr WN, Wallin BG, Victor RG, Aylward PE, Sundlöf G, Mark AL 1986 Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation 73:913–919 [DOI] [PubMed] [Google Scholar]
- Abate NI, Mansour YH, Tuncel M, Arbique D, Chavoshan B, Kizilbash A, Howell-Stampley T, Vongpatanasin W, Victor RG 2001 Overweight and sympathetic overactivity in Black Americans. Hypertension 38:379–383 [DOI] [PubMed] [Google Scholar]
- Uhr M, Holsboer F, Müller MB 2002 Penetration of endogenous steroid hormones corticosterone, cortisol, aldosterone and progesterone into the brain is enhanced in mice deficient for both mdr1a and mdr1b P-glycoproteins. J Neuroendocrinol 14:753–759 [DOI] [PubMed] [Google Scholar]
- Yu Y, Wei SG, Zhang ZH, Gomez-Sanchez E, Weiss RM, Felder RB 2008 Does aldosterone upregulate the brain renin-angiotensin system in rats with heart failure? Hypertension 51:727–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Sanchez EP, Ahmad N, Romero DG, Gomez-Sanchez CE 2005 Is aldosterone synthesized within the rat brain? Am J Physiol Endocrinol Metab 288:E342–E346 [DOI] [PubMed] [Google Scholar]
- Nwariaku FE, Miller BS, Auchus R, Holt S, Watumull L, Dolmatch B, Nesbitt S, Vongpatanasin W, Victor R, Wians F, Livingston E, Snyder 3rd WH 2006 Primary hyperaldosteronism: effect of adrenal vein sampling on surgical outcome. Arch Surg 141:497–502; discussion 502–503 [DOI] [PubMed] [Google Scholar]
- Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR, van Heerden JA 2004 Role for adrenal venous sampling in primary aldosteronism. Surgery 136:1227–1235 [DOI] [PubMed] [Google Scholar]
- Simon DR, Palese MA 2008 Noninvasive adrenal imaging in hyperaldosteronism. Curr Urol Rep 9:80–87 [DOI] [PubMed] [Google Scholar]
- Brown IJ, Tzoulaki I, Candeias V, Elliott P 2009 Salt intakes around the world: implications for public health. Int J Epidemiol 38:791–813 [DOI] [PubMed] [Google Scholar]
- Michikawa T, Nishiwaki Y, Okamura T, Asakura K, Nakano M, Takebayashi T 2009 The taste of salt measured by a simple test and blood pressure in Japanese women and men. Hypertens Res 32:399–403 [DOI] [PubMed] [Google Scholar]
- Jurgens G, Graudal NA 2004 Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterols, and triglyceride. Cochrane Database Syst Rev CD004022 [DOI] [PubMed] [Google Scholar]
- Francis J, Weiss RM, Wei SG, Johnson AK, Beltz TG, Zimmerman K, Felder RB 2001 Central mineralocorticoid receptor blockade improves volume regulation and reduces sympathetic drive in heart failure. Am J Physiol Heart Circ Physiol 281:H2241–H2251 [DOI] [PubMed] [Google Scholar]
- Felder RB 2010 Mineralocorticoid receptors, inflammation and sympathetic drive in heart failure. Exp Physiol 95:19–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling JC, Loewy AD 2009 Aldosterone in the brain. Am J Physiol Renal Physiol 297:F559–F576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, McClain JM, Zucker IH 1992 Aldosterone reduces baroreceptor discharge in the dog. Hypertension 19:270–277 [DOI] [PubMed] [Google Scholar]
- Munakata M, Aihara A, Imai Y, Omata K, Abe K, Yoshinaga K 1995 Increased gain in baroreceptor-heart rate reflex in patients with primary aldosteronism. J Hypertens 13:1648–1653 [PubMed] [Google Scholar]
- Masuo K, Mikami H, Ogihara T, Tuck ML 1997 Sympathetic nerve hyperactivity precedes hyperinsulinemia and blood pressure elevation in a young, nonobese Japanese population. Am J Hypertens 10:77–83 [DOI] [PubMed] [Google Scholar]
- Masuo K, Kawaguchi H, Mikami H, Ogihara T, Tuck ML 2003 Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension 42:474–480 [DOI] [PubMed] [Google Scholar]
- Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T 1984 Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 311:819–823 [DOI] [PubMed] [Google Scholar]
- Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ 2005 Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol 45:1243–1248 [DOI] [PubMed] [Google Scholar]
- Catena C, Colussi G, Nadalini E, Chiuch A, Baroselli S, Lapenna R, Sechi LA 2008 Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med 168:80–85 [DOI] [PubMed] [Google Scholar]
- Rossi GP, Bernini G, Desideri G, Fabris B, Ferri C, Giacchetti G, Letizia C, Maccario M, Mannelli M, Matterello MJ, Montemurro D, Palumbo G, Rizzoni D, Rossi E, Pessina AC, Mantero F 2006 Renal damage in primary aldosteronism: results of the PAPY Study. Hypertension 48:232–238 [DOI] [PubMed] [Google Scholar]