Abstract
Context: Zinc transporter 8 (ZnT8) is a newly discovered islet autoantigen in human type 1A diabetes (T1D).
Objective: The objective was to document changes in ZnT8 autoantibody (ZnT8A) titer and prevalence after onset of disease in relationship to 65 kDa glutamate decarboxylase antibody (GADA) and islet cell antigen antibody (IA2A).
Design/Patients: Autoantibody radioimmunoprecipitation assays were performed on sera from three groups: 21 individuals monitored every 3 months from diagnosis for 2.5 yr; 61 individuals monitored at six monthly intervals for 5–12 yr; and a cross-sectional study of 424 patients with T1D of 20–57 yr duration. Circulating C-peptide was determined as an index of residual β-cell function.
Results: ZnT8A titers declined exponentially from clinical onset of T1D with a t1/2 ranging from 26 to 530 wk, similar to C-peptide (23–300 wk). Life-table analysis of antibody prevalence to 12 yr indicated that ZnT8A measured with either Arg325 or Trp325 probes persisted for a shorter interval than IA2A. Although prevalence of ZnT8A, IA2A, and GADA were comparable at disease onset (70.4 vs. 73.4 vs. 64%), only 6.7% of individuals remained ZnT8A positive after 25 yr compared with 19.5% for IA2A and 25.9% for GADA. Titers of ZnT8A and IA2A in seropositive individuals decreased progressively, whereas GADA remained elevated consistent with periodic reactivation of GADA humoral autoimmunity.
Conclusions: ZnT8 humoral autoreactivity declines rapidly in the first years after disease onset and is less persistent than IA2A or GADA in the longer term. ZnT8A determination may be a useful measure of therapeutic efficacy in the context of immune-based clinical interventions.
ZnT8 autoantibody titer declines rapidly post-onset of type 1 diabetes, and its prevalence falls from 70% initially to less than 10% within 20 years.
The major molecular targets of type 1 diabetic autoimmunity have been identified from investigation of circulating autoantibodies in humans, and studies of their molecular and cellular biology have provided insight into the etiology, pathogenesis, and natural history of the disease. Humoral autoreactivity typically precedes clinical disease by months or years, and progression to clinical disease (1,2) is marked by intramolecular and intermolecular epitope spreading (3,4,5,6). The number of antibody specificities rather than individual titers best predicts disease, and positivity to two or more autoantigens serves as criteria for recruitment to clinical trials. Autoantibody determinations may also be useful in assessing the immunological impact of therapeutic interventions targeting autoreactive T- and B-cells (7,8,9,10). Antibody titer, affinity, and epitope specificity in these instances also provide important metrics of the immune response (11,12,13).
Zinc transporter 8 (ZnT8) (14) is a recently discovered type 1 diabetes (T1D) autoantigen that is a major target of humoral and cell-mediated autoimmunity in humans (15,16,17). ZnT8, like insulin and IGRP (islet-specific glucose-6-phosphatase-related protein) (18), is predominantly found in the pancreatic β-cell and is more restricted in its tissue distribution than other autoantigens such as GAD2 (65 kDa glutamate decarboxylase) (19), islet cell antigen (IA2) (PTPrN) (20), and phogrin/IA2β (PTPrN2) (21). Four major antigenic determinants have been identified in ZnT8, one in the 74-amino-acid (aa) N-terminal domain and three in the 101 aa C-terminal cytosolic domain (aa 268-369), the latter characterized by dependence on the polymorphic aa325 residue (Arg, Trp, or Gln) (15).
The present study investigates ZnT8 autoantibody (ZnT8A) prevalence, titer, and epitope specificity after T1D onset, including the 2–3 yr honeymoon period in which residual endogenous insulin production is evident and in the longer term when loss of β-cell mass is extensive though at times heterogeneous (22). Of key interest was the persistence of humoral ZnT8 autoreactivity and changes in ZnT8A epitope specificity in relationship to changes in C-peptide responses and putative effects of exogenous insulin to suppress residual endogenous β-cell function or to provide a new immunogenic stimulus (23).
Patients and Methods
Study cohorts and samples
Three groups of T1D patients of predominantly Europid Caucasian origin were analyzed: 1) 21 new-onset patients (median age at diagnosis, 20.3 ± 6.2 yr; range, 12.2–34.6 yr) followed for 2.5 yr from within 6 wk of diagnosis with serum samples at intervals of 3 months (Neurocrine Diabetes Study, NBI-6024- 0101 control subjects; trial registration at www.ClinicalTrials.gov under identifier NCT00873561); 2) 61 T1D new-onset patients from the Barbara Davis Center (median age at diagnosis, 9.8 ± 5.2 yr; range, 1.6–36.7 yr) followed for an average of 7 yr (4.0–12.3 yr) from within 6 wk of diagnosis with serum samples at 6- to 12-month intervals; 3) a cross-sectional study group of 424 patients from the Barbara Davis Center with longstanding diabetes, including a group 282 of who provided single samples (median age of diagnosis, 11.4 ± 7.6 yr; range, 0.5–52.7 yr) with disease of 26.3 ± 7.6 yr duration (range, 12.0–57.1 yr) and a second group of 142 (median age of diagnosis, 8.9 ± 7.1 yr; range, 1.0–40.3 yr) with disease of 23.4 ± 7.8 yr duration (range, 6.9–48.4 yr) for whom follow-up samples (3.0–10.9 yr) were available for analysis.
Control sera were derived from the parents and children in the newborn cohort of the DAISY study (for Diabetes Autoimmunity Study in the Young) at the Barbara Davis Center (150 individuals; median age, 13.1 yr; range, 1–55 yr). The male/female gender ratio in all groups ranged from 0.8 to 1.2. Informed consent was obtained from participants and/or parents under approved Institutional Review Board oversight.
Autoantibody assays
ZnT8A assays were based on immunoprecipitation of 35S-Met-labeled, in vitro transcribed and translated probes derived from human ZnT8 cloned into the pCDNA3.1 directional TOPO vector (16). Constructs included the N-terminal cytosolic segment (aa 1-74) or the C-terminal cytosolic segment (aa 268-369), including the known aa325 codon variants CGG (Arg), TGG (Trp), and CAG (Gln) designated CR, CW, and CQ respectively (15). In addition, a series of dimeric constructs were tested that included the N-terminal (aa 1-74) ZnT8 fragment fused to the N-terminal joined to C-terminal Trp variant via an IgG heavy chain linker, designated NCW, and a dimeric construct of CW connected to CR via a hinge sequence derived from the human IgG heavy chain, designated CWCR (24). A homodimeric construct of the N terminus, designated NN, was constructed in the same manner with the human IgG heavy chain linker. The primary objective for developing these constructs were to combine known epitopes as in the case of CWCR and to increase the sensitivity of these assays through increasing the molar-specific radioactivity and molecular size of the probe to facilitate their purification by gel filtration. Procedures for 96-well filtration-based radioimmunoprecipitation assays of these constructs have been detailed previously (16,25). Samples were run alongside 16 human controls derived from first-degree relatives of T1D patients without insulin antibodies, GAD autoantibodies (GADA), or IA2 antibody (IA2A) and a series of positive controls including pooled ZnT8A-positive diabetic sera, and a 1:50 dilution of rabbit anti-ZnT8 antisera directed either at the 101 aa C terminus (BUN-E) or 74 aa N terminus (Narcissa). Autoantibody levels were calculated as the immunoprecipitation index (cpm sample − cpm control)/(cpm positive standard − cpm control), in which cpm is counts per minute. The internal cutoff index (typically 0.015–0.020) was based on mean control values + 5 sd within each assay. These corresponded to the 99th percentile determined within the Diabetes Autoantibody Standardization Program. Interassay coefficient of variation (CV) was less than 15% for samples with low antibody titer (index of 0.05–0.1). Autoantibodies to full-length GAD65 (GADA) and IA2 cytosolic domain were performed in a similar manner (25). In cases in which the IA2A or ZnT8A index exceeded 0.7, patient samples were diluted 1:25 in control patient serum and reassayed, and the resulting index was multiplied by 25.
C-peptide assays
In the 2.5 yr follow-up group stimulated C-peptide (2-h peak) was determined every 3 months with a mixed meal tolerance test (6 ml/kg Novartis Boost high protein; Novartis, Basel, Switzerland) by RIA (Diagnostic Systems Laboratories, Houston, TX) (reported interassay CV of 5.3% at 0.55 pmol/ml; detection limit of 0.03 pmol/ml). Random C-peptides were determined in the 7–12 yr follow-up study on the last sample using a two-site immunoenzymometeric assay on a Tosoh AIA-1800 autoanalyzer (Tosoh Bioscience, Tokyo, Japan). The interassay and intraassay precision analysis CV were less than 10%; assay sensitivity of 0.02 pmol/ml and normal range of 0.3–1 pmol/ml).
Unless specified, results are expressed as mean ± sd. Statistical analyses were performed with the Prism 5 software package (GraphPad Software, San Diego, CA). Uncorrected P values are documented, although the Bonferroni’s multiple comparison test was applied to interpret analysis of data involving multiple hypotheses.
Results
Changes in ZnT8A prevalence during the honeymoon period
To ascertain changes in ZnT8A levels after commencement of insulin therapy, a group of 21 newly diagnosed T1D subjects were evaluated over a 2.5 yr period at 3-month intervals. At onset, 18 individuals in this group tested positive to at least one ZnT8A probe (85.7%), 20 were positive for GADA (95.2%), and 19 were positive for IA2A (90.5%). Eighteen subjects (85.7%) showed all three autoantigen specificities, two (9.5%) showed two, and one (4.8%) showed only one. At the end of the 2.5 yr follow up, the prevalence of ZnT8A (76.2%) and GADA (85.7%) were lower, and IA2A (90.5%) was unchanged. Patients showing all three autoantigen specificities remained high (71.4%), as were those with two (14.3%) and one (14.3%), with no individuals being seronegative (Supplemental Table 1, published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org).
The ZnT8 CWCR dimeric probe detected all sera that were either CW, CR, or CQ reactive. Samples that were reactive to the NCW probe included 97.4% of CW-reactive samples and 98.1% of NN dimer reactive samples (98.1%). The CWCR dimeric probe thus integrated all of the ZnT8A responses that could be measured with the three separate C-terminal probes. The NN dimer probe detected an additional 2% of new-onset T1D patients compared with those reactive with CWCR probe and was thus not considered further in these studies (Wenzlau, J. M. and J. C. Hutton, unpublished findings).
Changes in ZnT8A titer and epitope specificity during the honeymoon period
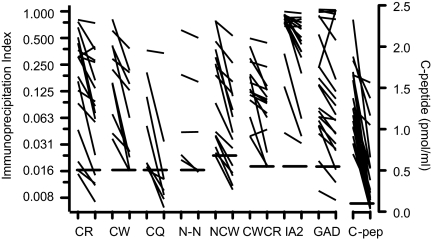
ZnT8A titers typically declined by 20–60% over the course of the 2.5 yr follow-up (Fig. 1) (Supplemental Figs. 1 and 2, Supplemental Table 1). No instances were observed of individuals developing ZnT8A after onset, but in 2 ZnT8A-positive individuals (subjects 3 and 9 in Supplemental Fig. 1), titers increased in the first 20–40 wk after onset before declining progressively. When observed, this biphasic response occurred with all ZnT8A C-terminal probes and was partly mirrored by IA2A and GADA. IA2A and GADA titers showed a downward trend of similar magnitude to ZnT8A, although there were exceptions in which titers were unchanged or even increased (Supplemental Fig. 1), particularly in the case of GADA. C-peptide levels fell by an average of 70% over the 2.5 yr follow up (Supplemental Table 1 and Supplemental Fig. 1) (Fig 1), although again there were individuals (subject 7 in Supplemental Fig. 1) in which ZnT8A declined but C-peptide levels were sustained.
Figure 1.
Initial and final autoantibody and C-peptide responses in the 2.5 yr follow-up group. The detection limit for each assay (99th percentile for antibodies) is indicated by horizontal bars. Nonparametric paired statistical analyses (see Supplemental Table 1) were significant except for the dimeric N-terminal construct (NN). To enhance resolution, antibody indices are plotted on an exponential scale.
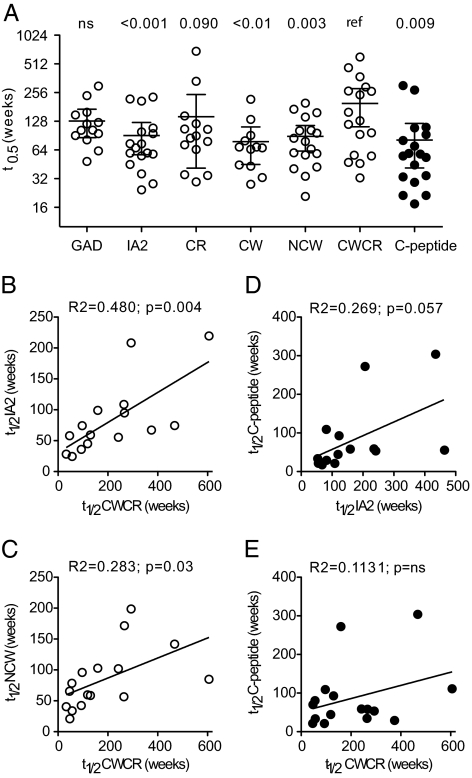
The progressive decline of ZnT8A, IA2A, and C-peptide responses (3 months to 2.5 yr after onset) in most individuals were approximated by first-order exponential decay kinetics (Supplemental Figs. 1 and 2) (Fig. 2). The median half-lives of the changes of C-peptide (25–300 wk) and antibody titers (26–530 wk) were in the same numerical range, but there was wide variation between individuals. Linear regression analysis showed that the half-life of ZnT8 CWCR reactivity was correlated with those of IA2A (Fig. 2B) and other ZnT8 constructs, including ZnT8 NCW (Fig. 2C), across the group of 12 individuals with initial antibody indices more than 0.1. However, there was no correlation between half-lives of C-peptide and antibody levels measured with ZnT8 CWCR or IA2 (Fig. 2, D and E) despite there being a correlation in the case of each individual (Supplemental Fig. 2).
Figure 2.
Half-lives of autoantibodies and C-peptide over the 2.5 yr after disease onset. The decline of antibody titer and C-peptide were modeled on first-order exponential decay kinetics (Supplemental Figs. 1 and 2) from 20 of the 21 patients followed for 2.5 yr. Data were excluded when initial autoantibody titers were low (index <0.07) and when levels were unchanged or increased (one case each for CWCR, C-peptide, and IA2, five for GAD). A, The median half-lives and 95% confidence interval are shown in each measurement. Statistical significance was determined with Wilcoxon’s matched-pairs test relative to the ZnT8 CWCR probe (ref). B, C, Linear regression analysis of autoantibody half-lives relative to ZnT8 CWCR. D, E, Linear regression analysis of autoantibody half-lives relative to C-peptide.
ZnT8A responses were further stratified in terms of restriction to epitopes involving aa325 (15,17). Four responses were restricted to Arg325 only (CR-positive, CWCR-positive, CW-negative, CQ-negative), two to Trp325 only (CR-negative, CWCR-positive, CW-positive, CQ-negative), and 10 to epitope(s) defined by equivalent reactivity to CW, CR, or CQ constructs. Each individual appeared to maintain the same pattern of reactivity throughout the study (Supplemental Fig. 2), suggesting that major epitope switching or spreading did not occur during the 2.5 yr follow-up period.
Changes in ZnT8 autoreactivity in the 4–12 yr follow-up group
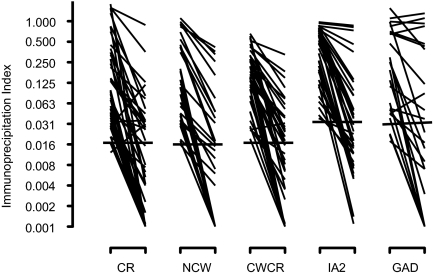
The fall in ZnT8A titer observed during the 2.5 yr follow up continued in subsequent years, reaching levels below the level of detection in many cases (Fig. 3) (Supplemental Table 2). The prevalence of ZnT8 autoreactivity (CR, NCW, or CRCW) thus fell from 80.3% at disease onset to 42.6% by the end of the follow up, values similar to those with GADA (63.0 down to 32.4%) and IA2A (73.8 down to 47.5%). ZnT8A titers determined with CR, NCW, and CWCR probes dropped by more than 80%. Random nonfasting C-peptide measurements on the last sample showed that 16 of 58 individuals (27.6%) had detectable C-peptide (>0.02 pmol/ml). The C-peptide status did not appear to be related to autoantibody prevalence whether it was measured by ZnT8 CR (81.3% with C-peptide vs. 62.8% without), ZnT8 NCW (31.3 vs. 55.8%), ZnT8 CWCR (75.0 vs. 67.4%), GADA (75.0 vs. 60.5%), or IA2A (75.0 vs.74.4%).
Figure 3.
Initial and final autoantibody responses in the first 12 yr of diabetes. The detection limit for each assay is indicated by the horizontal bars. Nonparametric paired statistical analyses were significant in all cases (see Supplemental Table 2). To enhance resolution, antibody indices are plotted on an exponential scale.
The kinetics of decline in ZnT8A and IA2A levels could be modeled by an exponential decay as in the case of the 2.5-yr study, and the corresponding half-lives were similar in magnitude (data not shown). A correlation was observed between the half-lives of ZnT8 CWCR measurements with CR (r2 = 0.291, P < 0.05) and NCW (r2 = 0.674, P < 0.001) as previously (Fig. 2).
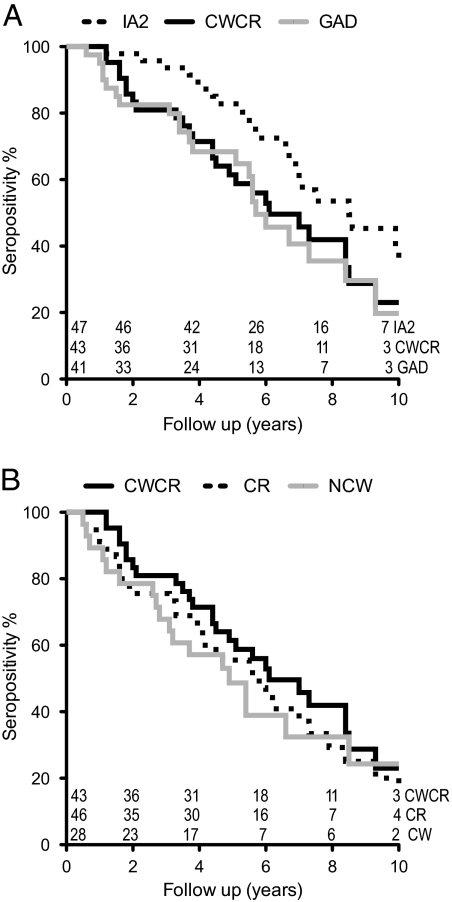
The observation that a high proportion of individuals became seronegative after 4 yr or more of follow up provided a complementary approach to titer measurements in this group of patients. Conversion over time from an initial antibody positive to negative status was thus determined by survival analysis using Kaplan-Meier statistics (Fig. 4). Autoreactivity to ZnT8 and IA2 both declined progressively, with ZnT8A falling off more rapidly than IA2 (P < 0.002) (Fig. 4) irrespective of the ZnT8 probe used.
Figure 4.
Kaplan-Meier analysis of declining autoantibody prevalence in the first 12 yr of diabetes. Statistical significance was determined by the Gehan-Breslow-Wilcoxon χ2 test, scored as reversion to antibody-negative status on two sequential samples. A, The median persistence of IA2A (dotted line) of 8.5 yr exceeded ZnT8 CWCR (black line) of 6.2 yr (P = 0.026) and GAD (gray line) of 5.7 yr (P = 0.013). B, The median persistence of CWCR (black line) did not differ from that of ZnT8 CR (dotted line) of 5.7 yr or ZnT8 NCW (gray line) of 4.9 yr.
Autoantibody persistence of ZnT8A after T1D of 20 yr or more
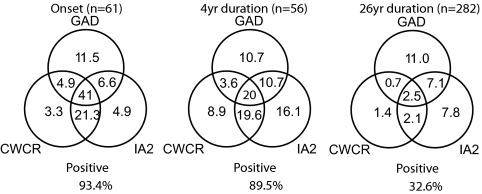
Within a group of 282 subjects with disease of 26.3 ± 7.6 yr duration (range, 12.0–57.1 yr), 60 (21.3%) were GADA positive, 55 (19.5%) were IA2A positive, but only 6.7% were reactive to the ZnT8 CWCR dimeric probe. Mean titers of ZnT8 CWCR reactivity in seropositive samples in this group were 25.9% of the 7 yr follow-up group at onset, IA2A were 40.1%, whereas GADA levels were comparable at 116%. The proportion of subjects within this group showing all three specificities was reduced compared with subjects at onset, as were the numbers showing the combination of ZnT8A and IA2A (Fig. 5). This indicated that humoral autoimmunity was not just confined to a subset of “highly autoreactive” individuals.
Figure 5.
Overlap of individual antibody responses to ZnT8 CWCR, GAD, and IA2 with diabetes progression. Data are derived from the 12 yr follow-up study at onset and at 4 yr and the cross-sectional study with T1D of more than 20 yr (median, 27.4 ± 7.1 yr). Both groups developed disease at similar ages (13.7 ± 5.4, n = 61 vs.11.3 ± 8.3, n = 282). Prevalence data are expressed as a percentage of the total number of subjects shown in parentheses.
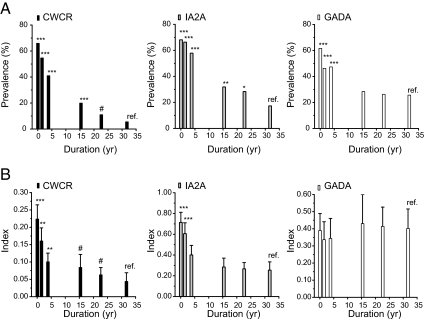
Figure 6 shows a compilation of the cross-sectional data with the first and last samples of the 2.5- and 12-yr follow-up study and stratification into six groups by duration of disease. The prevalence of GADA appeared to reach a plateau of approximately 25% by 15 yr, whereas ZnT8 CWCR and IA2 autoreactivity continued to decline up to 30 yr after onset. The titer of ZnT8 CWCR and IA2A-positive individuals dropped rapidly in the first 5 yr and then leveled off, with ZnT8 CWCR still following a downward trend for 10–30 yr. In contrast, the mean titer in GADA-positive individuals did not appear to change in this analysis, an observation that appeared to be at odds with the decline in individual titers observed in the 2.5- and 12-yr follow-up data (Figs. 1 and 3). A possible explanation could be that a significant proportion of initially GADA-negative patients seroconverted at a later time; however, analysis of 142 individuals in the cross-sectional study with multiple samples indicated only a low incidence of seroconversion (<2%) with any antibody (data not shown). A more plausible explanation lay in the observation that GADA-positive individuals frequently showed increases in titer over time (Supplemental Fig. 3), which was not seen for either IA2A or ZnT8 CWCR titers. Based on calculations of the percentage annual change in titer, ZnT8 CWCR titer changes were both more rapid and less variable than GADA, with IA2A in an intermediate range.
Figure 6.
Combined autoantibody prevalence and titer data. Data from the first and last samples assayed from the 2.5- and 12-yr follow-up studies and from the cross-sectional dataset were stratified by duration of diabetes as shown. Statistical comparison in prevalence (Fisher’s exact test) and titer (Mann-Whitney test) were made relative to the 32-wk group (ref). *, P < 0.05; **, P < 0.01; ***, P < 0.001; #, P < 0.1. Titers are shown as the mean ± 95%CI; n = 82–178.
Discussion
The major objective of these studies was to learn how ZnT8A prevalence, titer, and epitope specificity change after disease onset and how this relates to the other major autoantibodies measurable after onset, namely GADA and IA2A. Of additional interest was the relationship of these parameters of autoimmunity with evoked C-peptide response as a measure of residual β-cell function.
ZnT8A were initially evaluated using a broad range of probes representing C-terminal polymorphic variants at aa325 Arg (CR), Trp (CW), or Gln (CQ) and N-terminal epitopes. Autoreactivity to all C-terminal probes declined in parallel, although the half-lives of ZnT8A measured with probes combining multiple epitopes (CWCR) tended to be longer than with monomeric probes (CR and CW). This was not reflected in changes in ZnT8A prevalence evaluated by survival analysis in which autoreactivity determined by the dimeric CWCR construct was only marginally longer than that measured with individual monomeric probes. It is likely that dimeric probes will detect antibody molecules of lower affinity because such probes provide more sites for binding and higher probability of rebinding after dissociation. Other factors that could affect sensitivity are the lower concentration of the polymorphic variants but higher specific radioactivity of dimeric vs. monomeric probes. Combining major epitopes or multimerizing epitopes in ZnT8A assays is advantageous because it improves precision and reports on a greater proportion of subjects. A caveat here is that such assays may be less sensitive in detecting changes in reactivity that is directed to a specific epitope.
The half-lives of the ZnT8A, GADA, and IA2A after disease onset exceed the half-life of human Ig molecules in the circulation, suggesting that the decline in autoantibody titer reflects waning of autoreactivity rather than cessation of autoantibody production at disease onset. The half-lives of ZnT8A determined with different epitope-sensitive probes were correlated, indicating that no major changes in ZnT8A epitope specificity occurred after disease onset. ZnT8A half-life was correlated to that of IA2A but not GADA, which may reflect the finding that IA2A and ZnT8A in the prediabetic phase typically occur around the same time (2 yr after insulin antibodies and GADA) and that titers of ZnT8A and IA2A at disease onset are weakly correlated (15,16,17).
C-peptide responses declined alongside the titers of ZnT8A and IA2A over the first 2.5 yr, indicating a possible link between autoreactivity and a decline of β-cell mass. The latter could result from persisting cell-mediated immune attack, but other components of the metabolic dysfunction need to be considered, namely glucotoxicity and oxidative stress (26). Insulin therapy in itself is likely to suppress endogenous β-cell function and the expression of islet autoantigens, such as ZnT8, IA2, and IGRP that are associated with the regulated secretory pathway. Pancreas or islet transplantation in long-standing T1D patients can reactivate humoral autoimmunity (27), and histological studies on long-term type 1 diabetic pancreas reveal varying degrees of residual insulin immunoreactivity and lymphocytic infiltration (22,28,29), further supporting the concept that residual β-cell mass and persistent humoral autoreactivity can be linked. The variability between the half-lives of both evoked C-peptide responses and antibody titer between individuals, however, suggest a more complex scenario in which the antigen specificities, the number of antigens, and clonality of the responses may all interact. GADA is a case in point in this regard in which there is evidence of episodic elevation in antibody titer and differences in the persistence of autoantibodies depending on the targeted epitope not to mention the possibility of variation in antiidiotypic responses to the antigen (30) and epitope specificity (12). As observed previously (31,32), GADA prevalence decreased progressively yet titers remained high in seropositive individuals.
Given the high tissue specificity of ZnT8 (33,34), the question arises as to whether ZnT8A might provide a surrogate measurement of residual β-cell antigen that would reflect β-cell number, granulation, or functional mass. The lower prevalence of ZnT8A in long-term diabetic subjects and faster decline in ZnT8A in the medium term is consistent with the loss of an islet antigen source as a driver of residual autoimmunity. However, the lack of concordance with the detection of residual C-peptide and ZnT8A and the lack of correlation between the half-lives of C-peptide and ZnT8A across the patient population argues against it. The latter analysis is complicated by the absence of proportionality of antibody titer with antigen in any individual because the autoantibody index is a composite measurement dependent on antibody avidity, concentration, epitope specificity, and B-cell receptor clonality. Additional studies are clearly needed.
Intervention trials in new-onset patients typically rely on a short period of intensive treatment followed by 1–2 yr of follow up with monitoring of C-peptide production as the primary endpoint and insulin requirements and hemoglobin A1c as secondary parameters (7,8,9,10,35,36,37). Autoantibody levels have been reported in few instances (36), although it is clear that major responses can occur as in the case of anti B-lymphocyte CD20 monoclonal antibody (Rituximab) treatment (37) in which a decrease in antibody production and isotype switching to a neoantigen (phiX174) was observed (38). Considering the current observations as baseline data, then profiling of the initial trajectory of ZnT8A decline from 3 to 6 months after onset would provide the best means to evaluate response to treatment. However, this may be acceptable given that patients with higher C-peptide levels appear to respond better to therapeutic intervention (8,36), and current emphasis is to commence therapy as soon as possible after diagnosis.
Conclusion
The determination of ZnT8A at 3-month intervals after onset of T1D can provide a reliable metric of the decline of ZnT8-specific humoral autoimmunity for a period of up to 5 yr after onset of T1D. There is little evidence of changes in ZnT8 epitope specificity after disease onset, and assay of ZnT8A using a single dimeric C-terminal construct will suffice to capture the response in most individuals. ZnT8A responses were distinguishable from those of GADA and IA2A and C-peptide in both the shorter (5 yr) and longer term.
Supplementary Material
Acknowledgments
Thomas Kaupper and the NBI-6024 study group (www.ClinicalTrials.gov identifier NCT00873561) are thanked for providing samples, and Randall Wong and Jay Walters are thanked for technical support. Jerry Palmer (University of Washington Diabetes Endocrinology Research Center, Seattle, WA) performed the random C-peptide measurements.
Footnotes
This work was supported by the following: Childhood Diabetes Foundation in Denver, the University of Colorado Health Sciences Center Diabetes Endocrinology Research Center by National Institutes of Health (NIH) Grant P30 DK57516; NIH Grants R01 DK052068 and NIH R56 DK052068; and a Juvenile Diabetes Research Foundation Autoimmunity Prevention Center Grant.
Disclosure Summary: None of the authors declare a conflict of interest connected with this work.
First Published Online July 7, 2010
Abbreviations: aa, Amino acid; cpm, counts per minute; CQ, codon variant CAG; CR, codon variant CGG; CV, coefficient of variation; CW, codon variant TGG; CWCR, dimeric construct of CW connected to CR; GADA, 65 kDa glutamate decarboxylase antibody; GAD65, 65 kDa glutamate decarboxylase; IA2A, islet cell antigen antibody; NCW, N-terminal joined to C-terminal Trp variant via an IgG heavy chain linker; NN, homodimeric construct of the N terminus; T1D, type 1 diabetes; ZnT8, zinc transporter 8; ZnT8A, zinc transporter 8 autoantibody.
References
- Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, Chase HP, Eisenbarth GS 1996 Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes 45:926–933 [DOI] [PubMed] [Google Scholar]
- Greenbaum CJ, Sears KL, Kahn SE, Palmer JP 1999 Relationship of β-cell function and autoantibodies to progression and nonprogression of subclinical type 1 diabetes: follow-up of the Seattle Family Study. Diabetes 48:170–175 [DOI] [PubMed] [Google Scholar]
- Naserke HE, Ziegler AG, Lampasona V, Bonifacio E 1998 Early development and spreading of autoantibodies to epitopes of IA-2 and their association with progression to type 1 diabetes. J Immunol 161:6963–6969 [PubMed] [Google Scholar]
- Kawasaki E, Yu L, Rewers MJ, Hutton JC, Eisenbarth GS 1998 Definition of multiple ICA512/phogrin autoantibody epitopes and detection of intramolecular epitope spreading in relatives of patients with type 1 diabetes. Diabetes 47:733–742 [DOI] [PubMed] [Google Scholar]
- Pihoker C, Gilliam LK, Hampe CS, Lernmark A 2005 Autoantibodies in diabetes. Diabetes 54 Suppl 2:S52–S61 [DOI] [PubMed] [Google Scholar]
- Mayr A, Schlosser M, Grober N, Kenk H, Ziegler AG, Bonifacio E, Achenbach P 2007 GAD autoantibody affinity and epitope specificity identify distinct immunization profiles in children at risk for type 1 diabetes. Diabetes 56:1527–1533 [DOI] [PubMed] [Google Scholar]
- Chatenoud L, Thervet E, Primo J, Bach JF 1994 Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci USA 91:123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L 2005 Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 352:2598–2608 [DOI] [PubMed] [Google Scholar]
- Herold KC, Gitelman S, Greenbaum C, Puck J, Hagopian W, Gottlieb P, Sayre P, Bianchine P, Wong E, Seyfert-Margolis V, Bourcier K, Bluestone JA; Immune Tolerance Network ITN007AI Study Group 2009 Treatment of patients with new onset type 1 diabetes with a single course of anti-CD3 mAb teplizumab preserves insulin production for up to 5 years. Clin Immunol 132:166–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill SK, Liu E, Cambier JC 2009 Change you can B(cell)eive in: recent progress confirms a critical role for B cells in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes 16:293–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach P, Schlosser M, Williams AJ, Yu L, Mueller PW, Bingley PJ, Bonifacio E 2007 Combined testing of antibody titer and affinity improves insulin autoantibody measurement: Diabetes Antibody Standardization Program. Clin Immunol 122:85–90 [DOI] [PubMed] [Google Scholar]
- Hampe CS, Ortqvist E, Persson B, Schranz DB, Lernmark A 1999 Glutamate decarboxylase (GAD) autoantibody epitope shift during the first year of type 1 diabetes. Horm Metab Res 31:553–557 [DOI] [PubMed] [Google Scholar]
- Bingley PJ 2010 Clinical applications of diabetes antibody testing. J Clin Endocrinol Metab 95:25–33 [DOI] [PubMed] [Google Scholar]
- Chimienti F, Devergnas S, Favier A, Seve M 2004 Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes 53:2330–2337 [DOI] [PubMed] [Google Scholar]
- Wenzlau JM, Liu Y, Yu L, Moua O, Fowler KT, Rangasamy S, Walters J, Eisenbarth GS, Davidson HW, Hutton JC 2008 A common non-synonymous single nucleotide polymorphism in the SLC30A8 gene determines ZnT8 autoantibody specificity in type 1 diabetes. Diabetes 57:2693–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC 2007 The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA 104:17040–17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach P, Lampasona V, Landherr U, Koczwara K, Krause S, Grallert H, Winkler C, Pflüger M, Illig T, Bonifacio E, Ziegler AG 2009 Autoantibodies to zinc transporter 8 and SLC30A8 genotype stratify type 1 diabetes risk. Diabetologia 52:1881–1888 [DOI] [PubMed] [Google Scholar]
- Arden SD, Zahn T, Steegers S, Webb S, Bergman B, O'Brien RM, Hutton JC 1999 Molecular cloning of a pancreatic islet-specific glucose-6-phosphatase catalytic subunit-related protein. Diabetes 48:531–542 [DOI] [PubMed] [Google Scholar]
- Baekkeskov S, Aanstoot HJ, Christgau S, Reetz A, Solimena M, Cascalho M, Folli F, Richter-Olesen H, De Camilli P 1990 Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature 347:151–156 [DOI] [PubMed] [Google Scholar]
- Bonifacio E, Lampasona V, Genovese S, Ferrari M, Bosi E 1995 Identification of protein tyrosine phosphatase-like IA2 (islet cell antigen 512) as the insulin-dependent diabetes-related 37/40K autoantigen and a target of islet-cell antibodies. J Immunol 155:5419–5426 [PubMed] [Google Scholar]
- Wasmeier C, Hutton JC 1996 Molecular cloning of phogrin, a protein-tyrosine phosphatase homologue localized to insulin secretory granule membranes. J Biol Chem 271:18161–18170 [DOI] [PubMed] [Google Scholar]
- Gianani R, Campbell-Thompson M, Sarkar SA, Wasserfall C, Pugliese A, Solis JM, Kent SC, Hering BJ, West E, Steck A, Bonner-Weir S, Atkinson MA, Coppieters K, von Herrath M, Eisenbarth GS 2010 Dimorphic histopathology of long standing childhood-onset diabetes. Diabetologia 53:690–698 [DOI] [PubMed] [Google Scholar]
- Scheinin T, Groop L, Koskimies S, Kontianinen S 1989 Immune responses to insulin in patients with insulin-dependent diabetes mellitus. Autoimmunity 4:59–68 [DOI] [PubMed] [Google Scholar]
- Kawasaki E, Uga M, Nakamura K, Kuriya G, Satoh T, Fujishima K, Ozaki M, Abiru N, Yamasaki H, Wenzlau JM, Davidson HW, Hutton JC, Eguchi K 2008 Association between anti-ZnT8 autoantibody specificities and SLC30A8 Arg325Trp variant in Japanese patients with type 1 diabetes. Diabetologia 51:2299–2302 [DOI] [PubMed] [Google Scholar]
- Yu L, Cuthbertson DD, Maclaren N, Jackson R, Palmer JP, Orban T, Eisenbarth GS, Krischer JP 2001 Expression of GAD65 and islet cell antibody (ICA512) autoantibodies among cytoplasmic ICA+ relatives is associated with eligibility for the Diabetes Prevention Trial-Type 1. Diabetes 50:1735–1740 [DOI] [PubMed] [Google Scholar]
- Martín-Gallán P, Carrascosa A, Gussinyé M, Domínguez C 2007 Oxidative stress in childhood type 1 diabetes: results from a study covering the first 20 years of evolution. Free Radic Res 41:919–928 [DOI] [PubMed] [Google Scholar]
- Sibley RK, Sutherland DE, Goetz F, Michael AF 1985 Recurrent diabetes mellitus in the pancreas iso- and allograft. A light and electron microscopic and immunohistochemical analysis of four cases. Lab Invest 53:132–144 [PubMed] [Google Scholar]
- Foulis AK, Stewart JA 1984 The pancreas in recent-onset type 1 (insulin-dependent) diabetes mellitus: insulin content of islets, insulitis and associated changes in the exocrine acinar tissue. Diabetologia 26:456–461 [DOI] [PubMed] [Google Scholar]
- Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG 2009 Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol 155:173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oak S, Gilliam LK, Landin-Olsson M, Törn C, Kockum I, Pennington CR, Rowley MJ, Christie MR, Banga JP, Hampe CS 2008 The lack of anti-idiotypic antibodies, not the presence of the corresponding autoantibodies to glutamate decarboxylase, defines type 1 diabetes. Proc Natl Acad Sci USA 105:5471–5476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R, Gilliam L, Torn C, Landin-Olsson M, Palmer J, Akesson K, Kockum I, Lernmark B, Karlsson AF, Lynch KF, Breslow N, Lernmark A, Sundkvist G 2007 Islet cell autoantibody levels after the diagnosis of young adult diabetic patients. Diabet Med 24:1221–1228 [DOI] [PubMed] [Google Scholar]
- Borg H, Gottsäter A, Fernlund P, Sundkvist G 2002 A 12-year prospective study of the relationship between islet antibodies and β-cell function at and after the diagnosis in patients with adult-onset diabetes. Diabetes 51:1754–1762 [DOI] [PubMed] [Google Scholar]
- Chimienti F, Favier A, Seve M 2005 ZnT-8, a pancreatic β-cell-specific zinc transporter. Biometals 18:313–317 [DOI] [PubMed] [Google Scholar]
- Murgia C, Devirgiliis C, Mancini E, Donadel G, Zalewski P, Perozzi G 2009 Diabetes-linked zinc transporter ZnT8 is a homodimeric protein expressed by distinct rodent endocrine cell types in the pancreas and other glands. Nutr Metab Cardiovasc Dis 19:431–439 [DOI] [PubMed] [Google Scholar]
- Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA 2002 Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 346:1692–1698 [DOI] [PubMed] [Google Scholar]
- Ludvigsson J, Faresjö M, Hjorth M, Axelsson S, Chéramy M, Pihl M, Vaarala O, Forsander G, Ivarsson S, Johansson C, Lindh A, Nilsson NO, Aman J, Ortqvist E, Zerhouni P, Casas R 2008 GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med 359:1909–1920 [DOI] [PubMed] [Google Scholar]
- Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, McGee PF, Moran AM, Raskin P, Rodriguez H, Schatz DA, Wherrett D, Wilson DM, Lachin JM, Skyler JS 2009 Rituximab, B-lymphocyte depletion, and preservation of β-cell function. N Engl J Med 361:2143–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CM, Agarwal A, Book BK, Vieira CA, Sidner RA, Ochs HD, Young M, Pescovitz MD 2005 Rituximab inhibits the in vivo primary and secondary antibody response to a neoantigen, bacteriophage phiX174. Am J Transplant 5:50–57 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.