Abstract
Context: Abnormal placentation in human pregnancy is associated with intrauterine fetal growth restriction (IUGR). Our group has previously reported the association between severe IUGR, lower fetal circulating concentrations of thyroid hormones (THs), and altered expression of TH receptors and TH transporters within human placental villi. We postulate that altered TH bioavailability to trophoblasts may contribute to the pathogenesis of IUGR.
Design and Objective: Cytotrophoblasts were isolated from normal and IUGR human placentae to compare their responsiveness to T3 and their capability for T3 transport.
Results: Compared with normal cytotrophoblasts, the viability of IUGR cytotrophoblasts (assessed by methyltetrazoleum assay) was significantly reduced (P < 0.001), whereas apoptosis (assessed using caspase 3/7 activity and M30 immunoreactivity) was significantly increased after T3 treatment for 48 h (P < 0.001 and P < 0.01, respectively). The secretion of human chorionic gonadotropin was significantly increased by IUGR cytotrophoblasts compared with normal cytotrophoblasts (P < 0.001), independently of T3 treatment. Net transport of [125I]T3 was 20% higher by IUGR cytotrophoblasts compared with normal cytotrophoblasts (P < 0.001), and this was accompanied by a 2-fold increase in the protein expression of the TH transporter, monocarboxylate transporter 8, as assessed by Western immunoblotting (P < 0.01).
Conclusions: IUGR cytotrophoblasts demonstrate altered responsiveness to T3 with significant effects on cell survival and apoptosis compared with normal cytotrophoblasts. Increased monocarboxylate transporter 8 expression and intracellular T3 accumulation may contribute to the altered T3 responsiveness of IUGR cytotrophoblasts.
Cytotrophoblasts from growth-restricted pregnancies demonstrate increased net T3 uptake and increased T3 responsiveness, which affects cell survival and apoptosis compared with cytotrophoblasts from normal pregnancies.
Intrauterine growth restriction (IUGR) is a pregnancy complication that is characterized by failure to achieve the genetic growth potential of the fetus (1) and is associated with significant perinatal morbidity and mortality (2,3). IUGR is often a multifactorial disease process (4) but is commonly associated with abnormal placental morphology and maternal uteroplacental blood flow (5,6). The remodeling of maternal spiral arteries that facilitates unobstructed blood flow from the maternal circulation toward the placenta in normal pregnancies is abnormal in IUGR pregnancies (7,8,9,10). Histological examination of term placentae has revealed that the number of apoptotic nuclei within villous syncytiotrophoblasts is increased in human pregnancies complicated by IUGR (11,12,13). The syncytialization of cytotrophoblasts into syncytiotrophoblast within placental villi is also increased in IUGR, as demonstrated in experiments using placental explants (14) and primary cultures of term cytotrophoblasts (15).
Maternal thyroid status is one of several factors that are thought to be involved in human placental development. Untreated maternal hyperthyroidism has been associated with complications of malplacentation, including IUGR, placental abruption, and preeclampsia (16), whereas maternal subclinical hypothyroidism has been associated with increased risks of miscarriage, placental abruption, and preterm delivery (17,18), which suggests some influence of maternal thyroid hormones (THs) on human placentation. In vitro, T3, the active TH ligand, increases the invasive capability of first trimester human extravillous trophoblasts (19) and suppresses apoptosis in this cell type (20).
Although circulating concentrations of THs are the major determinants of cellular TH supply, there are several prerequisites for effective TH action, including TH transport into cells and prereceptor regulation of T3, by iodothyronine deiodinases and T3 binding to nuclear TH receptors (TRs) that regulate the expression of TH-responsive genes (21). Our group has previously reported changes in the mechanisms regulating TH action in placentae from severe IUGR pregnancies delivered in the early third trimester. The protein expression of the TR isoforms TRα1, TRα2, and TRβ1 is increased in placental villi from severe IUGR pregnancies (22) with no significant change in the activities of placental deiodinase types 2 and 3 (23). In addition, the expression of the TH transporter monocarboxylate transporter 8 (MCT8; official symbol, SLC16A2) is increased, whereas the expression of MCT10 (SLC16A10) is decreased in the villous placenta with severe IUGR (24,25). Using percutaneous in utero fetal blood sampling, we have also reported that the circulating concentrations of free T4 and free T3 are significantly reduced in severely growth restricted fetuses (22).
We hypothesize that the reported changes in the expression of TRs and TH transporters in IUGR placentae may be associated with altered trophoblast sensitivity to THs in IUGR pregnancies. In this study, primary cultures of cytotrophoblasts isolated from third trimester placentae from uncomplicated pregnancies (normal cytotrophoblasts) or from pregnancies complicated by IUGR (IUGR cytotrophoblasts) were used to assess differences in T3 responsiveness and to investigate whether changes in TH transport may account for such differences.
Subjects and Methods
Sample collection
Human placentae from normal (n = 27) and IUGR (n = 14) pregnancies were collected with informed written consent and local research ethics committee approval after elective delivery by cesarean section. All were delivered after 35 completed weeks of gestation, as determined by a first trimester ultrasound scan of crown-rump length. The IUGR cases were diagnosed prospectively using ultrasound and had at least two of the following characteristics: 1) abdominal circumference, measured by ultrasound, less than the 10th centile for gestation; 2) abdominal circumference growth velocity of less than 1.5 sd values over 14 d; 3) oligohydramnios, defined as maximum pool depth of 10th centile or less for gestation; and 4) absent or increased resistance index in the end diastolic flow velocity of the umbilical artery Doppler velocity waveform. The IUGR fetuses were not known to have abnormal karyotypes, and none of the pregnancies was complicated by maternal hypertension or thyroid disorders.
Trophoblast isolation and culture
Villous cytotrophoblasts were isolated as described previously (26,27,28). Isolated cytotrophoblasts were cultured in DMEM:F12 nutrient mixture (1:1), supplemented with 1000 U/liter penicillin, 0.001% (wt/vol) streptomycin, 0.029% (wt/vol) l-glutamine (all from Invitrogen, Paisley, UK), and 0.005% (wt/vol) gentamicin (Sigma-Aldrich, Dorset, UK). The medium was supplemented with 10% fetal calf serum (FCS; Invitrogen) or with 10% charcoal-stripped FCS (First Link, Birmingham, UK) for cells that would be subsequently treated with T3.
Assessment of cell survival and apoptosis
Cytotrophoblasts were seeded in 96-well plates (3 × 105 cells/well) and 18 h after isolation were treated with 0, 1, 10, or 100 nm T3 for 48 h. Cell survival was assessed in four replicates using the methyltetrazoleum (MTT; Sigma-Aldrich) assay as described previously (26). Cell apoptosis was assessed in triplicate using the luminescence-based caspase 3/7 activity assay (Promega, Southampton, UK) as described previously (29). The results were normalized to the values obtained with no T3 treatment (0 nm) within each experiment. In addition, apoptosis in response to T3 was assessed with immunofluorescent staining for the apoptotic marker, M30 (30). Cytotrophoblasts were seeded in duplicate in 24-well plates (7.5 × 105 cells/well) and 48 h after T3 treatment were fixed and permeabilized with 100% methanol. Cytotrophoblasts were probed with primary antibody against M30 (1:50; Roche, Burgess Hill, UK), followed by Alexa Fluor 488-conjugated secondary antibody (1:250; Invitrogen) and the nuclear stain, Hoechst 33258 (1:1000; Sigma-Aldrich). M30 staining was assessed by a researcher blinded to placental type and treatment. The number of nuclei with M30 perinuclear staining was expressed as a percentage of the total number of nuclei. The results were normalized within each experiment to the average percentage of M30-positive nuclei with no T3 treatment (0 nm).
Human chorionic gonadotropin (hCG) secretion
Cytotrophoblasts were seeded in 12-well plates (1.5 × 106 cells/well) and 18 h after isolation were treated with 0 or 10 nm T3. Culture media and protein were collected at 0, 48, and 72 h after treatment. Secretion of hCG was assessed in duplicate using an ELISA kit (DRG, Marburg, Germany) according to manufacturer’s guidelines. Results were expressed as milli-international units of hCG, per hour in culture, per milliliter of medium, per milligram of protein (27), and the rise in hCG secretion between 0 and 48 or 0 and 72 h after treatment was calculated.
T3 uptake and efflux
T3 uptake
Cytotrophoblasts were cultured in duplicate in 10% FCS-supplemented medium in 12-well plates for 66 h (1.5 × 106 cells/well). After incubation for 0, 5, 10, or 30 min with serum free medium (SFM) supplemented with 1 nm T3 containing approximately 2 × 105 cpm of [125I]T3 (Perkin-Elmer, Wellesley, MA), cytotrophoblasts were rapidly washed three times with ice-cold SFM with 0.1% BSA and were lysed with 2% sodium dodecyl sulfate (SDS). The radioactivity in the cell lysates (cellular radioactivity) was measured using a γ-counter and expressed as a percentage of the total radioactivity in the incubation media, which was added to the cells initially (31) (Supplemental Fig. 1, published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org).
T3 efflux
Cytotrophoblasts were incubated in duplicate with SFM supplemented with 1 nm T3 containing [125I]T3 (2 × 105 cpm/0.5 ml) for 10 min. Cytotrophoblasts were briefly washed with SFM with 0.1% BSA and incubated in SFM without T3 (efflux medium) for 0, 1, 2, 5, or 10 min. The medium was then removed, and cytotrophoblasts were lysed with 2% SDS. The proportion of the radioactivity that was retained in the cell lysates compared with that added to the cells initially was calculated. T3 efflux was expressed as a percentage of the cellular radioactivity normalized to time 0 (just before the addition of efflux medium) (31).
Quantitative TaqMan PCR
Cytotrophoblasts were cultured in 35-mm2 tissue culture dishes (3 × 106 cells/dish) in 10% FCS-supplemented medium. Total RNA was extracted at 18 or 66 h after culture with TRI reagent (Ambion, Warrington, UK) following the manufacturer’s guidelines. RNA (1 μg) was reverse-transcribed using avian myeloblastosis virus reverse transcriptase (Promega, Madison, WI) in a total reaction volume of 20 μl following the manufacturer’s guidelines. Expression of mRNA encoding TH transporters MCT8 and MCT10; the system L-amino acid transporters LAT1 (SLC7A5), LAT2 (SLC7A8), CD98 (SLC3A2); and the organic anion-transporting polypeptides OATP1A2 (SLCO1A2) or OATP4A1 (SLCO4A1) was determined and normalized to the expression of the housekeeping gene, 18S, as an internal control using the ABI PRISM 7500 Sequence Detection System (ABI, Foster City, CA) using validated primers and probes as previously described (25). Relative quantification of each gene was determined using the ΔCt method as previously described (25). The relative mRNA expression for each sample was compared with the mean gene expression in normal cytotrophoblasts at 18 h after culture that was assigned the arbitrary value of 1.
MCT8 and MCT10 antibody production and Western immunoblotting
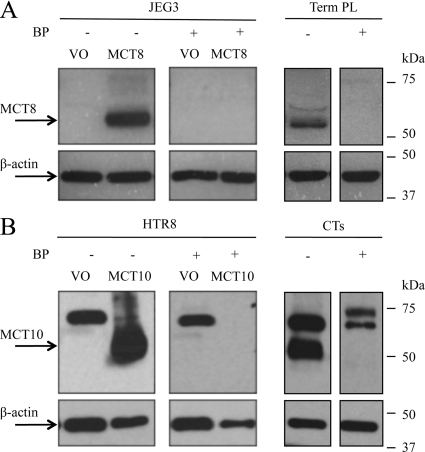
Rabbit polyclonal antisera for human MCT8 (Ab4790; Sigma-Genosys, Haverhill, UK) and MCT10 (Ab2198; Charles River Laboratories, Kisslegg, Germany) were raised against synthetic polypeptides comprising amino acids 79-92 (SQASEEAKGPWQEA) and 503-515 (SSGMFKKESDSII), respectively, each conjugated to keyhole limpet hemocyanin. Antisera from final bleeds were affinity-purified and their specificity confirmed (Fig. 1).
Figure 1.
Western immunoblotting confirming the specificity of polyclonal antibodies to MCT8 and MCT10. A, MCT8 4790 antibody. Whole cell protein extracts of MCT8-null JEG3 cells transfected with either vector only (VO) or a plasmid encoding human MCT8 and homogenates of term placenta tissue (Term PL) expressing endogenous MCT8 were probed with the MCT8 antibody (1.6 μg/ml) after preincubation with or without the blocking peptide (BP). B, MCT10 2198 antibody. Whole cell protein extracts of HTR8/SVNeo cells (with very low endogenous MCT10 mRNA expression) transfected with either VO or a plasmid encoding human MCT10 and extracts from normal cytotrophoblasts (CTs) that were cultured for 18 h were probed with the MCT10 antibody (5.2 μg/ml) after preincubation with or without the blocking peptide (BP). Bands of approximately 60 kDa for MCT8 (A) and approximately 50 kDa for MCT10 (B) are seen, consistent with their predicted molecular weights, respectively. Immunoreactivity for β-actin was used to assess protein loading.
Protein was extracted from IUGR and normal cytotrophoblasts cultured for 18 or 66 h in 10% FCS-supplemented medium using 2% SDS, and Western immunoblotting was performed as previously described (25). Briefly, protein (30 μg) was denatured (1 h at room temperature) in Laemmli buffer (Bio-Rad, Hertfordshire, UK) with 350 mm dithiothreitol, separated by electrophoresis in 8% SDS-PAGE gels, and blotted onto nitrocellulose membranes. The blots were incubated with the MCT8 (3.2 μg/ml) or the MCT10 (5.2 μg/ml) antibody followed by secondary horseradish peroxidase-conjugated antibody (1:2000; Dako, Glostrup, Denmark). Antigen-antibody complexes were visualized using the ECL+ chemiluminescence detection system (GE Healthcare, St. Giles, UK). Expression of β-actin was determined to assess protein loading.
Statistical analysis
Data were analyzed using the Minitab statistical software (version 15; Minitab Inc., State College, PA). For demographic data, the Mann-Whitney U test was used to compare continuous variables, and the Fisher’s exact test was used to analyze contingency tables. For other data sets, ANOVA was performed using the general linear model followed by Tukey all pairwise multiple comparison post hoc tests to assess differences between individual groups. Residuals (differences between the observed values and the predicted values by the general linear model) for all data sets passed the normality test as determined using the Kolmogorov-Smirnov test, except for the hCG secretion data, which, thus required logarithmic transformation before statistical analysis. For all tests, significance was taken as P < 0.05.
Results
Clinical characteristics of study groups
Comparing the demographic data of the normal and IUGR cohorts, there was no significant difference in the maternal age, proportion of cigarette smokers, parity, and fetal sex (Table 1). However, the median gestational age of delivery in the IUGR cohort was 1 wk less than in the normal (P < 0.001). As expected, the birth weights and placental weights of the IUGR group were significantly lower (P < 0.001 and P < 0.01, respectively) compared with the normal group. The birth weight percentile, which was calculated using customized growth charts and accounts for parity, ethnicity, maternal BMI, gestational age, and fetal sex (32), was also significantly lower in the IUGR group (P < 0.001), with a median of the 2nd percentile compared with the 53rd percentile for the normal group.
Table 1.
Clinical characteristics of normal and IUGR study groups
| Normal (n = 27) | IUGR (n = 14) | P value | |
|---|---|---|---|
| Maternal data | |||
| Age (yr) | 32.0 (21–41) | 31.5 (24–44) | NS |
| Smokers | 3/27 (11) | 4/14 (28) | NS |
| Nulliparous | 3/27 (11) | 5/14 (36) | NS |
| Fetal data | |||
| Males | 9/27 (33) | 5/14 (36) | NS |
| Gestational age (wk) | 39 (37–40) | 38 (35–40) | 0.0006 |
| Birth weight (g) | 3320 (2320–4400) | 2400 (1565–2960) | <0.0001 |
| Placenta weight (g) | 640 (509–883) | 464 (313–700) | 0.0012 |
| Customized birth weight percentile | 53 (23–99) | 2 (0–27) | <0.0001 |
| Abnormal umbilical artery Doppler flow | 0/27 (0) | 3/14 (21) | 0.0341 |
| Oligohydramnios | 0/27 (0) | 8/14 (57) | <0.0001 |
Values represent ratio (%) or median (range). In the IUGR group, 1) one neonate was diagnosed with Prader-Willi syndrome at 8 months of age; 2) one woman was epileptic and was treated with a low dose of lamotrigine (100 mg twice daily); and 3) another woman was a heroin addict who had been taking reducing doses of methadone during pregnancy. NS, Not statistically significant.
Survival of cytotrophoblasts in response to T3
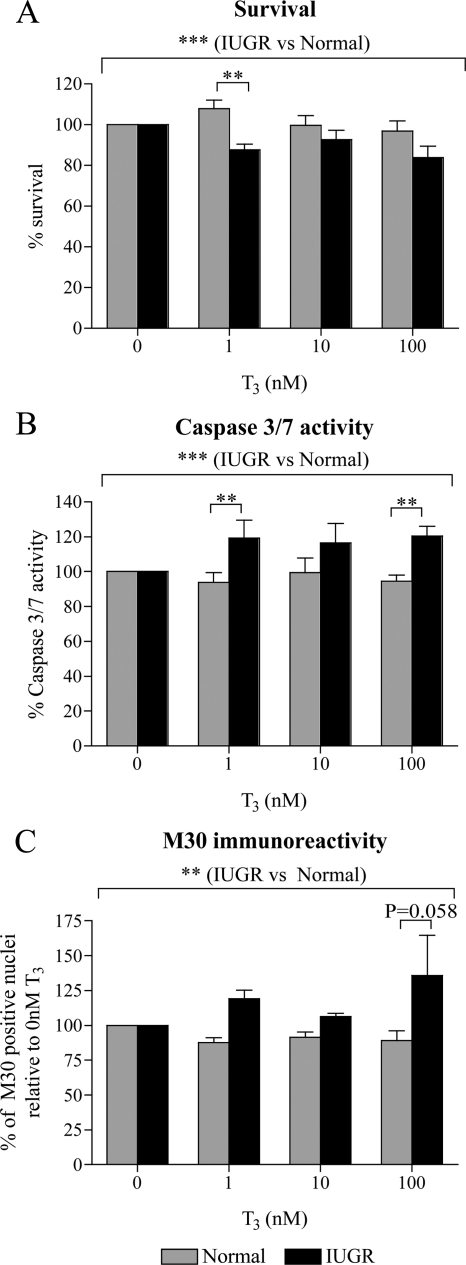
Because cytotrophoblasts do not proliferate in vitro, the MTT assay was used as a measure of cell survival. Overall, IUGR cytotrophoblasts survived less compared with normal cytotrophoblasts in response to T3 treatment (Fig. 2A; P < 0.001). Post hoc analysis revealed that this difference was significant when the cells were treated with 1 nm T3 (20% reduction; P < 0.01). There was no difference in the survival of normal and IUGR cytotrophoblasts in the absence of T3 (data not shown).
Figure 2.
Effect of T3 on the survival and apoptosis of normal and IUGR cytotrophoblasts. Survival and apoptosis were assessed after 48 h of treatment with 0, 1, 10, or 100 nm T3. Within each experiment, results were compared with that after no T3 treatment (0 nm), which was given an arbitrary value of 100%. A, Cytotrophoblast survival assessed using the MTT assay (normal, n = 9; IUGR, n = 5). B, Apoptosis assessed using the caspase 3/7 activity assay (normal, n = 9; IUGR, n = 5). C, Apoptosis assessed by immunofluorescent staining for M30 (normal, n = 4; IUGR, n = 3). **, P < 0.01; ***, P < 0.001.
Cytotrophoblast apoptosis in response to T3
We then assessed whether the effect of T3 on cytotrophoblast survival was mediated via increased apoptosis in IUGR cytotrophoblasts. Caspase 3/7 activity after T3 treatment was increased in IUGR compared with normal cytotrophoblasts (Fig. 2B; P < 0.001). Similar to what was observed with cytotrophoblast survival, the difference in caspase 3/7 activity between normal and IUGR cytotrophoblasts was particularly significant after treatment with 1 or 100 nm T3 (27% increase for both; P < 0.01). The T3-mediated increase in apoptosis of IUGR cytotrophoblasts compared with normal cytotrophoblasts was also confirmed by immunostaining for M30 (Fig. 2C), which demonstrated a highly statistically significant difference between normal and IUGR cytotrophoblasts overall (P < 0.01). The greatest increase in apoptosis of IUGR cytotrophoblasts compared with normal cytotrophoblasts occurred after treatment with 100 nm T3 (53% increase), and post hoc tests found this to be just close to significance (P = 0.058). A comparison of apoptosis in normal and IUGR cytotrophoblasts in the absence of T3 revealed no significant differences (data not shown).
hCG secretion from cytotrophoblasts in response to T3
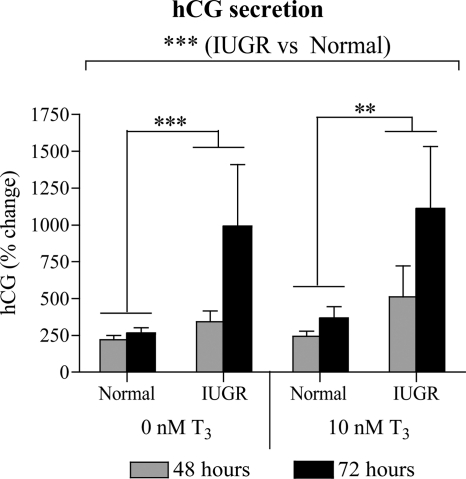
Overall, the rise in hCG secretion over time was higher in IUGR compared with normal cytotrophoblasts (P < 0.001; Fig. 3). Post hoc tests demonstrated that this effect was significant in both the absence (P < 0.001) and the presence (P < 0.01) of T3.
Figure 3.
Effect of T3 on hCG secretion by normal and IUGR cytotrophoblasts. The hCG secretion at 48 h after T3 treatment (normal, n = 9; IUGR, n = 6) and 72 h after T3 treatment (normal, n = 8; IUGR, n = 6) was assessed and normalized to the hCG secretion at 0 h of T3 treatment (18 h after culture), which was given an arbitrary value of 100%. **, P < 0.01; ***, P < 0.001.
T3 transport by cytotrophoblasts
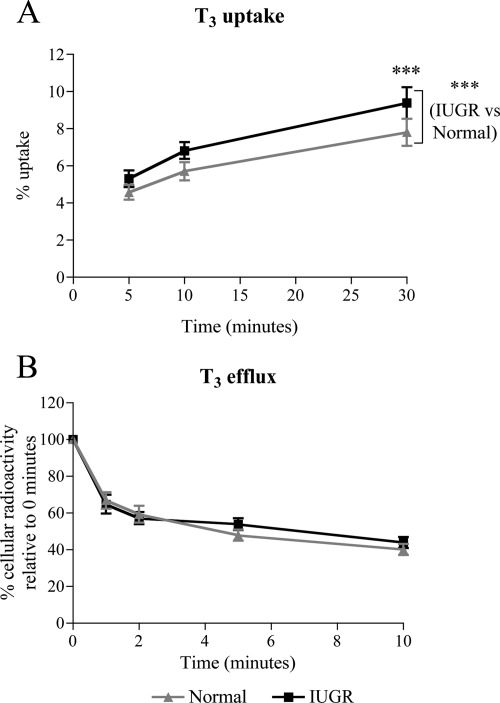
These results suggested that T3 affects the behavior of cytotrophoblasts isolated from IUGR but not from normal placentae with respect to survival and apoptosis. This effect could be mediated via differences in intracellular availability of T3. To explore this possibility, we assessed T3 transport by IUGR or normal cytotrophoblasts maintained in culture for 66 h. T3 uptake was higher by IUGR compared with normal cytotrophoblasts (P < 0.001; Fig. 4A). Post hoc tests demonstrated that this difference was only statistically significant after incubation with [125I]T3 for 30 min (23% increase; P < 0.001), indicating that there is an increase in net T3 transport by the cells rather than T3 uptake per se. In contrast, T3 efflux over a period of 10 min was similar between normal and IUGR cytotrophoblasts (Fig. 4B). These results therefore suggest that intracellular accumulation of T3 is higher in IUGR compared with normal cytotrophoblasts.
Figure 4.
T3 transport by normal and IUGR cytotrophoblasts. A, T3 uptake. Cytotrophoblasts were incubated with 1 nm T3 containing 2 × 105 cpm [125I]T3 for 5 to 30 min, and the amount of cellular radioactivity was assessed (normal, n = 7; IUGR, n = 5). B, T3 efflux. Cytotrophoblasts were incubated for 10 min with 1 nm T3 containing 2 × 105 cpm [125I]T3 and after brief washing were incubated with SFM without T3 (efflux media) for 0 to 10 min. The amount of radioactivity that was retained intracellularly was assessed and expressed as a percentage of the cellular radioactivity before the addition of efflux media (0 min) (normal, n = 6; IUGR, n = 5). ***, P < 0.001.
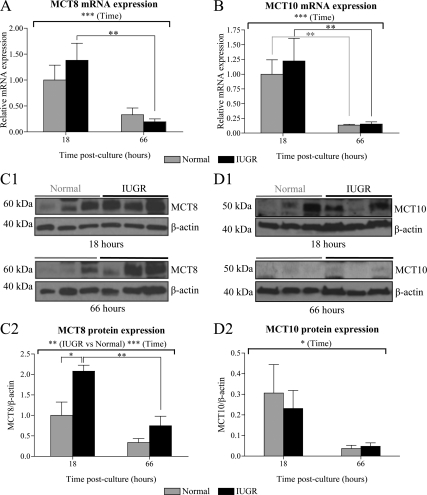
MCT8 and MCT10 expression in normal and IUGR cytotrophoblasts
Changes in the expression of TH transporters may account for the altered T3 transport in IUGR cytotrophoblasts. In previous studies, we have reported that the expression of MCT8 and MCT10 is altered in IUGR villous placental biopsies, which consist of multiple cell types (24,25). We thus sought to investigate the mRNA and protein expression of MCT8 and MCT10 in primary cultures of IUGR and normal cytotrophoblasts after 18 h of culture (presyncytialization) or 66 h (postsyncytialization). There were no differences in the expression of MCT8 or MCT10 mRNA in IUGR compared with normal cytotrophoblasts at both 18 and 66 h after culture (Fig. 5, A and B). However, Western immunoblottings and their quantification by relative densitometry demonstrated that MCT8 protein expression was higher in IUGR compared with normal cytotrophoblasts (P < 0.01) (Fig. 5C), most significantly at 18 h by 2.1-fold (P < 0.05). In contrast, there was no difference in the protein expression of MCT10 between IUGR and normal cytotrophoblasts (Fig. 5D). In addition, overall the expression of MCT8 and MCT10 in cytotrophoblasts decreased significantly between 18 and 66 h after culture at both the mRNA (P < 0.001) and protein (MCT8, P < 0.001; MCT10, P < 0.05) levels. Relative quantification of the mRNA encoding the TH transporters OATP1A2, OATP4A1, LAT1, LAT2, and CD98, which have been reported in the human placenta, revealed no significant differences between IUGR and normal cytotrophoblasts (data not shown).
Figure 5.
MCT8 and MCT10 expression in primary cultures of normal and IUGR cytotrophoblasts. Relative expression of MCT8 (A) and MCT10 (B) mRNA (mean ± sem) was assessed at 18 and 66 h after culture. The mean mRNA expression in normal samples at 18 h after culture was given an arbitrary value of 1 (normal, n = 8; IUGR, n = 8). Western immunoblotting for MCT8 (C1) and MCT10 (D1) on whole protein lysates obtained at 18 and 66 h after culture. Bands representing MCT8 and MCT10 were detected at approximately 60 and 50 kDa, respectively. Immunoblotting for β-actin on the same membranes was used to assess protein loading. The protein expression of MCT8 (C2; normal, n = 6; IUGR, n = 5) and MCT10 (D2; normal, n = 3; IUGR, n = 3) were quantified by relative densitometry and shown as a ratio to β-actin protein expression (mean ± sem). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Discussion
In this study, we have demonstrated that whereas cytotrophoblasts isolated from normal placentae are unaffected by T3 treatment, cytotrophoblasts from IUGR placentae are responsive to T3 in terms of cell survival and apoptosis. This effect is associated with increased accumulation of intracellular T3 in IUGR cytotrophoblasts and increased protein expression of the TH transporter, MCT8. In this report, we show for the first time that the previously documented increase in apoptosis by IUGR placentae (11,12,13,14,15) may be partly mediated by T3.
In accordance with our previous study (26), we have confirmed that T3 does not affect the survival of normal cytotrophoblasts. In contrast, T3 adversely affects the survival of IUGR cytotrophoblasts. We also demonstrated that T3 induces apoptosis in IUGR, but not in normal cytotrophoblasts in vitro, indicating that the effect of T3 on the survival of IUGR cytotrophoblasts may be mediated via increased apoptosis. The regulation of apoptosis in the villous trophoblast is important for normal placental development. The syncytiotrophoblast layer of the placenta, which is the cell barrier that controls transplacental transport, is continuously renewed by cytotrophoblasts fusing into adjacent syncytiotrophoblast, whereas aged syncytiotrophoblast nuclei are extruded into the maternal circulation as “syncytial knots.” It is believed that this process is regulated by apoptotic mechanisms (33). IUGR has been associated with increased apoptosis in the human placenta, as demonstrated by more apoptotic nuclei seen histologically and increased caspase 3 activity in IUGR compared with normal placentae, particularly in syncytiotrophoblasts (11,12,13). It has been shown before that the IUGR placenta is more susceptible to apoptotic stimuli such as TNF-α and hypoxia (14,34). However, this is the first study to show that T3 can also induce apoptosis in IUGR cytotrophoblasts in vitro, whereas it has no effect on normal cytotrophoblasts.
Primary cultures of cytotrophoblasts isolated from term placentae syncytialize with time in culture to form multinucleate cells. This differentiation process is associated with increased secretion of hCG (28). Our finding of increased hCG secretion by IUGR compared with normal cytotrophoblasts is in agreement with other studies. Crocker et al. (14) have found that hCG secretion was higher from explants from IUGR placentae compared with normal controls. Newhouse et al. (15) reported that there was increased syncytialization of primary cytotrophoblast cultures from IUGR compared with normal placentae assessed by immunofluorescent staining for desmoplakin and by hCG secretion. A previous study by Nishii et al. (35) suggested that treatment with 10 nm T3 promotes hCG secretion in cytotrophoblasts. In our study, however, treatment with 10 nm T3 did not significantly affect hCG secretion by either normal or IUGR cytotrophoblasts.
TH transport may play a role in regulating the impact of T3 on cytotrophoblasts via regulating intracellular T3 availability. We observed in vitro that net T3 transport is increased in cytotrophoblasts from IUGR compared with normal placentae at 66 h after culture, whereas T3 efflux is unaltered, thus indicating that there is increased intracellular accumulation of T3 in IUGR cytotrophoblasts, which may contribute to their increased sensitivity to T3 treatment. The increased accumulation of T3 within IUGR cytotrophoblasts may be due to changes in TH transporter expression, changes in T3-binding capacity within cytotrophoblasts, as well as differences in individual cell volume due to the increased syncytialization of IUGR cytotrophoblasts.
We have previously reported that the protein expression of MCT8 is increased in whole placental biopsies from pregnancies complicated by severe IUGR requiring delivery in the early third trimester compared with gestationally matched controls, but not in placentae from IUGR pregnancies delivered after 37 wk (24,25). In contrast, the present results obtained using primary cultures of cytotrophoblasts (>95% pure) revealed that MCT8 protein expression was also increased in cytotrophoblasts from late third trimester IUGR placentae. This change was not accompanied by an increase in MCT8 mRNA expression indicating that posttranscriptional or posttranslational modulation occurs. We have previously reported such discrepancies between mRNA and protein expression for MCT8 (25), and similar findings have been reported for other plasma membrane transporters (36).
Increased protein expression of MCT8 is likely to contribute to the increased net T3 uptake observed in IUGR cytotrophoblasts. Although MCT8 can facilitate T3 efflux (31), our findings suggest that T3 uptake may be the more dominant role of MCT8 in cytotrophoblasts. Although we found no changes in the mRNA or protein expression of MCT10 or in the mRNA expression of LATs or OATPs in IUGR cytotrophoblasts, consistent with previous findings in whole placental biopsies (25), we cannot exclude the possibility that changes in the activity of these TH transporters may also contribute to the increased net T3 uptake by IUGR cytotrophoblasts. In addition, increased accumulation of T3 within IUGR cytotrophoblasts may occur as a result of the possible increased intracellular binding of T3 to TR isoforms, TRα1 and TRβ1, that have previously been found to be expressed at increased levels in IUGR placenta villi (22). If so, this could contribute to both increased intracellular accumulation of T3 and increased sensitivity of IUGR cytotrophoblasts to T3.
Unlike findings in central nervous system cell types (37), treatment of term cytotrophoblasts with T3 concentrations within the physiological range (10 nm or less) does not alter the expression of the deiodinase enzymes, D2 (activates T4 to T3) and D3 (inactivates T3) (23). Furthermore, no significant changes were observed in D2 and D3 mRNA expression and activity in biopsies from normal compared with IUGR placentae (23). This suggests that cytotrophoblasts cannot modulate intracellular T3 concentration through alterations in D2 and D3 activities, thus rendering IUGR cytotrophoblasts more vulnerable to the increased net T3 uptake demonstrated in this current study.
The increased T3 responsiveness of IUGR cytotrophoblasts could be a contributing factor to the underlying pathogenesis of IUGR or a consequence of this malplacentation syndrome. Maternal hyperthyroidism is one of the endocrine factors that have been associated with IUGR (16), suggesting that increased exposure to TH may be detrimental to fetoplacental development. Outside the context of maternal thyroid disorders, our findings that there is increased T3 accumulation within IUGR cytotrophoblasts and that T3 can adversely affect the survival and increase apoptosis of these cells raise the possibility that an abnormally high T3 concentration locally forms part of the dysregulated endocrine, paracrine, and autocrine environment, which occurs within the placenta in IUGR pregnancies. Further investigation into the possible etiologies and pathophysiology of altered T3 responsiveness within placental trophoblasts in IUGR is warranted.
Supplementary Material
Acknowledgments
We thank the United Kingdom Clinical Research Network-funded research midwifes at Birmingham Women’s Hospital for assistance with patient recruitment and Dr. Roger Holder (University of Birmingham) for statistical advice.
Footnotes
This work was supported the Medical Research Council (Grants G0501548, to S.-Y.C., M.D.K., J.A.F., and C.J.M.; and G0600285, to S.-Y.C. and M.D.K.), Action Medical Research (Grant SP4335, to M.D.K., S.-Y.C., L.S.L., and J.A.F.), and Wellbeing of Women (Grant RG/1082/09, to S.-Y.C., M.D.K., J.A.F., and L.S.L.). S.-Y.C. is supported by a Clinician Scientist Fellowship (6462/4335) awarded by the Health Foundation.
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 21, 2010
Abbreviations: D2, Deiodinase type 2; FCS, fetal calf serum; hCG, human chorionic gonadotropin; IUGR, intrauterine growth restriction; LAT, system L-amino acid transporter; MCT, monocarboxylate transporter; MTT, methyltetrazoleum; OATP, organic anion-transporting polypeptide; SDS, sodium dodecyl sulfate; SFM, serum free medium; TH, thyroid hormone; TR, TH receptor.
References
- Scifres CM, Nelson DM 2009 Intrauterine growth restriction, human placental development and trophoblast cell death. J Physiol 587:3453–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan TY, Yeo GS 2005 Intrauterine growth restriction. Curr Opin Obstet Gynecol 17:135–142 [DOI] [PubMed] [Google Scholar]
- Kok JH, den Ouden AL, Verloove-Vanhorick SP, Brand R 1998 Outcome of very preterm small for gestational age infants: the first nine years of life. Br J Obstet Gynaecol 105:162–168 [DOI] [PubMed] [Google Scholar]
- Sankaran S, Kyle PM 2009 Aetiology and pathogenesis of IUGR. Best Pract Res Clin Obstet Gynaecol 23:765–777 [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Ohadike C, Baker PN, Crocker IP, Mitchell C, Ong SS 2003 Stereological investigation of placental morphology in pregnancies complicated by pre-eclampsia with and without intrauterine growth restriction. Placenta 24:219–226 [DOI] [PubMed] [Google Scholar]
- Teasdale F 1984 Idiopathic intrauterine growth retardation: histomorphometry of the human placenta. Placenta 5:83–92 [DOI] [PubMed] [Google Scholar]
- Brosens I, Dixon HG, Robertson WB 1977 Fetal growth retardation and the arteries of the placental bed. Br J Obstet Gynaecol 84:656–663 [DOI] [PubMed] [Google Scholar]
- Brosens IA, Robertson WB, Dixon HG 1972 The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu 1:177–191 [PubMed] [Google Scholar]
- Khong TY, De Wolf F, Robertson WB, Brosens I 1986 Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol 93:1049–1059 [DOI] [PubMed] [Google Scholar]
- Sheppard BL, Bonnar J 1981 An ultrastructural study of utero-placental spiral arteries in hypertensive and normotensive pregnancy and fetal growth retardation. Br J Obstet Gynaecol 88:695–705 [DOI] [PubMed] [Google Scholar]
- Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T 2002 Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol 186:158–166 [DOI] [PubMed] [Google Scholar]
- Smith SC, Baker PN, Symonds EM 1997 Increased placental apoptosis in intrauterine growth restriction. Am J Obstet Gynecol 177:1395–1401 [DOI] [PubMed] [Google Scholar]
- Endo H, Okamoto A, Yamada K, Nikaido T, Tanaka T 2005 Frequent apoptosis in placental villi from pregnancies complicated with intrauterine growth restriction and without maternal symptoms. Int J Mol Med 16:79–84 [PubMed] [Google Scholar]
- Crocker IP, Tansinda DM, Baker PN 2004 Altered cell kinetics in cultured placental villous explants in pregnancies complicated by pre-eclampsia and intrauterine growth restriction. J Pathol 204:11–18 [DOI] [PubMed] [Google Scholar]
- Newhouse SM, Davidge ST, Winkler-Lowen B, Demianczuk N, Guilbert LJ 2007 In vitro differentiation of villous trophoblasts from pregnancies complicated by intrauterine growth restriction with and without pre-eclampsia. Placenta 28:999–1003 [DOI] [PubMed] [Google Scholar]
- Mestman JH 2004 Hyperthyroidism in pregnancy. Best Pract Res Clin Endocrinol Metab 18:267–288 [DOI] [PubMed] [Google Scholar]
- Abalovich M, Gutierrez S, Alcaraz G, Maccallini G, Garcia A, Levalle O 2002 Overt and subclinical hypothyroidism complicating pregnancy. Thyroid 12:63–68 [DOI] [PubMed] [Google Scholar]
- Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, Cunningham FG 2005 Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol 105:239–245 [DOI] [PubMed] [Google Scholar]
- Oki N, Matsuo H, Nakago S, Murakoshi H, Laoag-Fernandez JB, Maruo T 2004 Effects of 3,5,3′-triiodothyronine on the invasive potential and the expression of integrins and matrix metalloproteinases in cultured early placental extravillous trophoblasts. J Clin Endocrinol Metab 89:5213–5221 [DOI] [PubMed] [Google Scholar]
- Laoag-Fernandez JB, Matsuo H, Murakoshi H, Hamada AL, Tsang BK, Maruo T 2004 3,5,3′-Triiodothyronine down-regulates Fas and Fas ligand expression and suppresses caspase-3 and poly (adenosine 5′-diphosphate-ribose) polymerase cleavage and apoptosis in early placental extravillous trophoblasts in vitro. J Clin Endocrinol Metab 89:4069–4077 [DOI] [PubMed] [Google Scholar]
- Yen PM 2001 Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142 [DOI] [PubMed] [Google Scholar]
- Kilby MD, Verhaeg J, Gittoes N, Somerset DA, Clark PM, Franklyn JA 1998 Circulating thyroid hormone concentrations and placental thyroid hormone receptor expression in normal human pregnancy and pregnancy complicated by intrauterine growth restriction (IUGR). J Clin Endocrinol Metab 83:2964–2971 [DOI] [PubMed] [Google Scholar]
- Chan S, Kachilele S, Hobbs E, Bulmer JN, Boelaert K, McCabe CJ, Driver PM, Bradwell AR, Kester M, Visser TJ, Franklyn JA, Kilby MD 2003 Placental iodothyronine deiodinase expression in normal and growth-restricted human pregnancies. J Clin Endocrinol Metab 88:4488–4495 [DOI] [PubMed] [Google Scholar]
- Chan SY, Franklyn JA, Pemberton HN, Bulmer JN, Visser TJ, McCabe CJ, Kilby MD 2006 Monocarboxylate transporter 8 expression in the human placenta: the effects of severe intrauterine growth restriction. J Endocrinol 189:465–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loubière LS, Vasilopoulou E, Bulmer JN, Taylor PM, Stieger B, Verrey F, McCabe CJ, Franklyn JA, Kilby MD, Chan SY 2010 Expression of thyroid hormone transporters in the human placenta and changes associated with intrauterine growth restriction. Placenta 31:295–304 [DOI] [PubMed] [Google Scholar]
- Barber KJ, Franklyn JA, McCabe CJ, Khanim FL, Bulmer JN, Whitley GS, Kilby MD 2005 The in vitro effects of triiodothyronine on epidermal growth factor-induced trophoblast function. J Clin Endocrinol Metab 90:1655–1661 [DOI] [PubMed] [Google Scholar]
- Greenwood SL, Clarson LH, Sides MK, Sibley CP 1996 Membrane potential difference and intracellular cation concentrations in human placental trophoblast cells in culture. J Physiol 492:629–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss 3rd JF 1986 Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology 118:1567–1582 [DOI] [PubMed] [Google Scholar]
- James SR, Franklyn JA, Reaves BJ, Smith VE, Chan SY, Barrett TG, Kilby MD, McCabe CJ 2009 Monocarboxylate transporter 8 in neuronal cell growth. Endocrinology 150:1961–1969 [DOI] [PubMed] [Google Scholar]
- Kadyrov M, Kaufmann P, Huppertz B 2001 Expression of a cytokeratin 18 neo-epitope is a specific marker for trophoblast apoptosis in human placenta. Placenta 22:44–48 [DOI] [PubMed] [Google Scholar]
- Friesema EC, Jansen J, Jachtenberg JW, Visser WE, Kester MH, Visser TJ 2008 Effective cellular uptake and efflux of thyroid hormone by human monocarboxylate transporter 10. Mol Endocrinol 22:1357–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardosi J, Chang A, Kalyan B, Sahota D, Symonds EM 1992 Customised antenatal growth charts. Lancet 339:283–287 [DOI] [PubMed] [Google Scholar]
- Huppertz B, Kingdom JC 2004 Apoptosis in the trophoblast—role of apoptosis in placental morphogenesis. J Soc Gynecol Investig 11:353–362 [DOI] [PubMed] [Google Scholar]
- Crocker IP, Cooper S, Ong SC, Baker PN 2003 Differences in apoptotic susceptibility of cytotrophoblasts and syncytiotrophoblasts in normal pregnancy to those complicated with preeclampsia and intrauterine growth restriction. Am J Pathol 162:637–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishii H, Ashitaka Y, Maruo M, Mochizuki M 1991 Studies on the effect of thyroid hormone and epidermal growth factor on the cultured human cytotrophoblast. Endocrinol Jpn 38:279–286 [DOI] [PubMed] [Google Scholar]
- Zlender V, Breljak D, Ljubojeviæ M, Flajs D, Balen D, Brzica H, Domijan AM, Peraica M, Fuchs R, Anzai N, Saboliæ I 2009 Low doses of ochratoxin A upregulate the protein expression of organic anion transporters Oat1, Oat2, Oat3 and Oat5 in rat kidney cortex. Toxicol Appl Pharmacol 239:284–296 [DOI] [PubMed] [Google Scholar]
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR 2002 Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23:38–89 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.