Abstract
Context: Few studies have examined whether the inflammatory markers IL-6 and C-reactive protein (CRP) are associated with exceptional longevity.
Objective: Our objective was to determine the association of serum CRP and IL-6 with adult lifespan.
Design, Setting, and Participants: This was a prospective, population-based study of 610 men and 743 postmenopausal women, mean age 73 yr, who had serum IL-6 and CRP measurements at baseline (1984–1987) and who were followed for mortality for up to 23 yr (through 2008). Participants must have been old enough at baseline (57 yr) to have the potential to achieve age 80 during follow-up.
Main Outcome Measures: Relative survival time and age at death (lifespan) were assessed.
Results: During follow-up, overall mortality was 69%. After adjustment for cardiovascular disease risk factors, in men, each sd increase in IL-6 was associated with a 15% decrease in survival time and 0.94-yr shorter lifespan (P < 0.001); corresponding values for CRP were a 12% decrease in survival time and a 1.00-yr reduction in lifespan (P < 0.001). Among women not using estrogen therapy (n = 532), survival time decreased 7% per sd higher IL-6 and lifespan was 1.35 yr shorter (P < 0.001). IL-6 was not related to lifespan among women using estrogen. CRP levels were not significantly associated with survival time or lifespan in women regardless of estrogen status.
Conclusions: Higher levels of inflammatory markers predicted reduced survival time and shorter lifespan among older men, whereas only IL-6 was associated with longevity in postmenopausal women and only among those not using estrogen.
Higher levels of C-reactive protein and IL-6 predict reduced survival time and shorter lifespan among older men; only IL-6 is associated with longevity in postmenopausal women.
C-reactive protein (CRP) and IL-6 are well-studied proinflammatory cytokines (1,2,3,4,5). In the atherosclerotic process, IL-6 promotes endothelial dysfunction and smooth muscle cell proliferation and migration and recruits and activates inflammatory cells (5). Additionally, IL-6 stimulates lipolysis and oxidation of fat and is the primary regulator of CRP synthesis and secretion (1). CRP induces the secretion of cellular adhesion molecules and tissue factors (1) and promotes adhesion and chemotaxis of monocytes via monocyte chemotactic protein-1 (4,5). CRP also mediates low-density lipoprotein (LDL) uptake by macrophages (3). Many epidemiological studies have reported an association of higher circulating CRP and IL-6 levels with increased risk of cardiovascular disease (CVD) (6,7,8,9) and all-cause mortality (10,11,12).
There is also evidence for IL-6 and CRP associations with obesity and insulin resistance. About 25–30% of IL-6 is secreted by adipose tissue, thought to imply a low-grade inflammatory response in white adipose tissue, which might lead to insulin resistance and type 2 diabetes (1). CRP is correlated with insulin levels (2,13), and levels of both cytokines are elevated in individuals with type 2 diabetes and insulin resistance (14,15,16,17).
As the average age of the U.S. population increases, interest has grown in factors associated with exceptional longevity. Although there is good evidence that longevity is moderately heritable with approximately 15–30% attributed to genetic factors (18,19,20,21), there are many potentially modifiable factors that can reduce the quantity and quality of life. Although increased levels of proinflammatory cytokines such as CRP and IL-6 are associated with type 2 diabetes and CVD, and these diseases are generally associated with decreased survival, the associations between these inflammatory markers with lifespan and extreme longevity into the eighth and ninth decades of life are unknown. We report here the associations of CRP and IL-6 with both survival and lifespan among community-dwelling men and women.
Subjects and Methods
Study participants
The Rancho Bernardo Study is a prospective cohort study of community-dwelling adults, established between 1972 and 1974, when 82% of the adult residents of a San Diego, CA, suburb participated in a survey of heart disease risk factors. Eighty percent of the surviving cohort from the 1972–1974 survey participated in an examination between 1984 and 1987. Participants included in the present analysis are 610 men and 743 women who participated in the 1984–1987 follow-up examination when blood for CRP and IL-6 assays was obtained and who were old enough at this examination to have the possibility of reaching age 80 during the 23-yr follow-up period. The Rancho Bernardo Study was approved by the Institutional Review Board of the University of California, San Diego; participants gave written informed consent.
CRP and IL-6
Morning fasting blood samples were obtained by venipuncture from 1984–1987 and frozen at −70 C until 2000, when IL-6 and CRP were measured on these never previously thawed samples. IL-6 was measured using a high-sensitivity amplified commercial ELISA with an alkaline phosphatase signal amplification system (R&D Systems, Minneapolis, MN). The intra- and interassay coefficients of variation of IL-6 ranged from 7–12% and from 8–13%, respectively, and sensitivity was 0.094 pg/ml. Duplicate measurements of IL-6 were performed, and the mean of the measurements was used for analysis. High-sensitivity CRP was measured with an automated, high-sensitivity immunonephelometry method (Dade Behring, Inc., Deerfield, IL). This CRP assay has a sensitivity of 0.2 mg/liter. The intraassay coefficients of variation for standard concentrations at 15, 25, and 60 mg/liter are 4, 2.4, and 4.4% (n = 20), respectively. The interassay coefficients of variation for standards at various concentrations are 2.6–5.7%. Those with CRP higher than 15 mg/liter were excluded from analysis because such high levels usually indicate acute illness or trauma.
Longevity and survival time
Vital status was determined for 97% of the original cohort through December 31, 2008, using annual mailings and telephone contacts and was confirmed by death certificates in most cases. Underlying cause of death was coded by a certified nosologist using the ninth revision of the International Classification of Diseases, Adapted.
Covariates
Information on age, sex, smoking status, alcohol use, physical activity, medical history, and medication use was obtained via standard self-administered or interviewer-administered questionnaires. Physical activity was assessed by asking whether the participant reported current exercise three or more times per week. Weight and height were measured with participants wearing light clothing and no shoes. Body mass index was calculated using weight in kilograms divided by height in meters squared. Waist circumference was measured at the level of the iliac crest. Systolic and diastolic blood pressure was measured twice in seated subjects at rest for at least 5 min, and the average of two readings was used.
Serum creatinine was measured by SmithKline Beecham Clinical Laboratories (King of Prussia, PA), and estimated glomerular filtration rate (GFR) was calculated by the modification of diet in renal disease equation (22). Fasting plasma glucose and glucose 2 h after a 75-g oral glucose tolerance test were analyzed using a glucose oxidase method in a hospital diagnostic laboratory. Fasting plasma insulin levels were measured by double-antibody RIA in a subset of 974 participants. Homeostasis model for insulin resistance (HOMA-IR) was calculated for this subset as [fasting glucose (millimoles per liter) × fasting insulin (milliunits per liter)]/22.5. Fasting total cholesterol and triglycerides were measured by enzymatic methods using the ABA-200 biochromatic analyzer (Abbott Laboratories, Abbott Park, IL). High-density cholesterol (HDL) was determined by precipitation analysis using a protocol from the Lipid Research Clinic. LDL cholesterol was calculated with the Friedewald equation (23).
Comorbidities included diabetes, hypertension, CVD, and cancer. Diabetes was defined as self-report of physician diagnosis, antidiabetes medication use, fasting glucose of 126 mg/dl or higher, or 2-h postchallenge glucose of 200 mg/dl or higher. Hypertension was defined as self-report of physician diagnosis, antihypertensive medication use, systolic blood pressure 130 mm Hg or higher, or diastolic blood pressure 85 mm Hg or higher. Prevalent CVD included self-reported myocardial infarction, stroke, transient ischemic attack, or coronary artery revascularization. Cancer was based on self-report, excluding nonmelanoma skin cancer. Number of medications was summed for each participant and included antihypertensive, antidiabetic, lipid-lowering, thyroid hormone, anticoagulants, antiangina, aspirin, cortisone, antiarrhythmic, and digitalis medications.
Statistical methods
Because there is evidence that CRP and IL-6 levels differ between men and women (24), which may result in differential associations with longevity, all models were stratified by sex. Sex-specific characteristics were compared between those who survived and those who died using t test, χ2, or Wilcoxon tests as appropriate.
Associations of IL-6 and CRP with survival were evaluated using accelerated failure time (AFT) models with a Weibull distribution. AFT models are parametric survival models that can be used as an alternative to Cox models and provide parameter estimates in terms of percent relative survival time (25). Associations of IL-6 and CRP with age at death (i.e. adult lifespan) among those who died were assessed using linear regression models. Univariate associations of CRP and IL-6 with both longevity and age at death were evaluated first, followed by staged models used to examine potential confounders. CRP and IL-6 were also entered together into a single model. For women, interactions of estrogen use with IL-6 and CRP in fully adjusted survival time and lifespan models were also examined.
To assess the appropriate functional form of IL-6 and CRP with age at death (lifespan), generalized additive models with a cubic B-spline function were used to construct splines. Generalized additive models extend the generalized linear model by allowing fit of nonparametric functions to estimate the associations of predictors and outcomes. To examine the functional form of IL-6 and CRP with survival/longevity, penalized cubic B-splines were constructed, which add a smoothness penalty to the Cox likelihood.
Splines were constructed in SPlus version 8.1 (Tibco Software, Inc., Seattle, WA); all other analyses were conducted in SAS version 9.1.3 (SAS Institute, Cary, NC). P values < 0.05 were considered statistically significant.
Results
Baseline characteristics
At baseline, the mean ± sd age of men and women was similar (73.0 ± 7.5 and 73.0 ± 7.4 yr, respectively). During follow-up, 935 of 1353 (69%) participants died, 465 of 610 (76%) men and 470 of 743 (63%) women. The median follow-up time was 13.3 yr, with a maximum follow-up of 23 yr. Average age at death was 84.7 yr for men and 86.5 yr for women. Mean ± sd CRP and IL-6 levels for women on estrogen were 3.4 ± 3.0 mg/liter and 2.9 ± 2.8 pg/ml, respectively; for women not on estrogen, mean ± sd CRP and IL-6 levels were 2.7 ± 2.7 mg/liter and 3.2 ± 2.5 pg/ml, respectively.
Splines indicated approximately linear associations of both CRP and IL-6 with age at death and longevity for both sexes. Among men, those who survived differed significantly from those who died by age, physical activity, diabetes, CVD, hypertension, systolic blood pressure, estimated GFR, number of medications, and number of comorbidities (Table 1). Among women, age, alcohol use, physical activity, CVD, hypertension, systolic blood pressure, HDL cholesterol, number of medications, estrogen use, and number of comorbidities differed between those who died and those who did not (Table 1). IL-6 levels differed by vital status for both men and women; CRP levels differed for men but not women (Table 1).
Table 1.
Characteristics by mortality and sex of Rancho Bernardo Study participants
| Men
|
Women
|
|||||
|---|---|---|---|---|---|---|
| Survived n = 145 | Died n = 465 | P valuea | Survived n = 273 | Died n = 470 | P valuea | |
| Age (yr) | 67.8 ± 6.0 | 74.7 ± 7.1 | <0.001 | 69.2 ± 6.1 | 75.2 ± 7.2 | <0.001 |
| Current smoking [n (%)] | 15 (10.3) | 48 (10.3) | 0.99 | 36 (13.2) | 68 (14.5) | 0.63 |
| Daily alcohol use [n (%)] | 81 (55.9) | 245 (52.7) | 0.50 | 125 (45.8) | 167 (35.5) | 0.006 |
| Exercise ≥ 3×/wk [n (%)] | 133 (91.7) | 393 (84.5) | 0.03 | 231 (84.6) | 360 (76.6) | 0.009 |
| Body mass index (kg/m2) | 26.0 ± 2.8 | 25.5 ± 3.2 | 0.17 | 24.5 ± 3.8 | 24.1 ± 3.8 | 0.11 |
| Waist circumference (cm) | 93.1 ± 8.2 | 93.5 ± 8.8 | 0.66 | 78.6 ± 9.4 | 79.2 ± 9.8 | 0.42 |
| Diabetes [n (%)] | 13 (9.0) | 94 (20.2) | 0.002 | 38 (13.9) | 61 (13.0) | 0.72 |
| CVD [n (%)] | 22 (15.2) | 158 (34.0) | <0.001 | 38 (13.9) | 129 (27.5) | <0.001 |
| Hypertension [n (%)] | 86 (59.3) | 358 (77.0) | <0.001 | 179 (65.6) | 354 (75.3) | 0.004 |
| Systolic blood pressure (mm Hg) | 134.0 ± 17.6 | 143.3 ± 20.8 | <0.001 | 136.1 ± 18.3 | 145.8 ± 23.1 | <0.001 |
| Diastolic blood pressure (mm Hg) | 78.3 ± 8.6 | 62.8 ± 14.2 | 0.22 | 74.6 ± 9.4 | 74.8 ± 9.5 | 0.74 |
| Estimated GFR (ml/min · 1.73 m2) | 65.2 ± 11.0 | 62.8 ± 14.2 | 0.02 | 59.2 ± 11.5 | 58.6 ± 14.2 | 0.11 |
| HDL cholesterol (mg/dl) | 53.7 ± 14.3 | 53.3 ± 14.4 | 0.73 | 70.2 ± 18.7 | 66.5 ± 18.6 | 0.009 |
| LDL cholesterol (mg/dl) | 134.4 ± 33.2 | 132.5 ± 34.1 | 0.59 | 139.2 ± 38.6 | 140.0 ± 38.9 | 0.80 |
| Total cholesterol (mg/dl) | 211.7 ± 35.8 | 209.3 ± 37.9 | 0.52 | 233.1 ± 39.9 | 229.9 ± 40.2 | 0.29 |
| Triglycerides (mg/dl) | 122.2 ± 84.1 | 123.1 ± 84.0 | 0.67 | 122.1 ± 75.5 | 122.7 ± 73.8 | 0.72 |
| Fasting glucose (mg/dl) | 100.7 ± 12.6 | 105.3 ± 22.8 | 0.21 | 99.4 ± 22.2 | 98.5 ± 21.7 | 0.32 |
| Fasting insulin (mU/liter)b | 12.0 (8.0, 15.0) | 12.0 (8.0, 16.0) | 0.63 | 11.0 (8.0, 14.0) | 11.0 (8.0, 16.0) | 0.47 |
| HOMA-IRb | 2.8 (1.9, 3.8) | 2.9 (1.9, 4.2) | 0.47 | 2.7 (1.8, 3.4) | 2.6 (1.9, 3.9) | 0.69 |
| No. of medications | 0.8 ± 1.1 | 1.1 ± 1.2 | <0.001 | 0.8 ± 0.9 | 1.2 ± 1.2 | <0.001 |
| Estrogen use [n (%)] | 105 (38.5) | 106 (22.6) | <0.001 | |||
| No. of comorbidities | 1.6 ± 1.2 | 2.4 ± 1.5 | <0.001 | 1.8 ± 1.2 | 2.1 ± 1.2 | <0.001 |
| IL-6 (pg/ml) | 2.6 ± 1.6 | 3.6 ± 2.8 | <0.001 | 2.6 ± 2.4 | 3.2 ± 2.5 | <0.001 |
| CRP (mg/liter) | 2.0 ± 2.1 | 2.8 ± 2.7 | <0.001 | 2.8 ± 2.7 | 3.1 ± 3.1 | 0.22 |
P value obtained by t test, Wilcoxon test, or χ2 test as appropriate.
Median (quartile 1, quartile 3).
Associations of IL-6 and CRP with survival (Table 2)
Table 2.
Association of IL-6 and CRP with survival among Rancho Bernardo Study participants
| Women
|
||||||
|---|---|---|---|---|---|---|
| Men
|
Estrogen use
|
No estrogen use
|
||||
| Time ratio (95% CI) | P value | Time ratio (95% CI) | P value | Time ratio (95% CI) | P value | |
| IL-6 per sda | ||||||
| Unadjusted | 0.82 (0.79, 0.86) | <0.001 | 0.93 (0.86, 1.01) | 0.07 | 0.85 (0.80, 0.89) | <0.001 |
| Age adjusted | 0.85 (0.81, 0.88) | <0.001 | 0.95 (0.87, 1.03) | 0.21 | 0.90 (0.86, 0.94) | <0.001 |
| +Body size/LS/comorbiditiesb | 0.85 (0.81, 0.89) | <0.001 | 0.97 (0.89, 1.06) | 0.49 | 0.90 (0.86, 0.95) | <0.001 |
| +Lipids/blood pressurec | 0.85 (0.81, 0.89) | <0.001 | 0.98 (0.90, 1.06) | 0.56 | 0.92 (0.87, 0.96) | <0.001 |
| +CRP | 0.87 (0.83, 0.92) | <0.001 | 0.97 (0.89, 1.06) | 0.54 | 0.92 (0.87, 0.97) | 0.003 |
| CRP per sda | ||||||
| Unadjusted | 0.85 (0.8, 0.89) | <0.001 | 0.95 (0.86, 1.06) | 0.37 | 0.97 (0.92, 1.03) | 0.29 |
| Age adjusted | 0.86 (0.82, 0.91) | <0.001 | 0.97 (0.88, 1.06) | 0.46 | 0.96 (0.91, 1.01) | 0.14 |
| +Body size/LS/comorbiditiesb | 0.89 (0.84, 0.93) | <0.001 | 0.98 (0.89, 1.09) | 0.76 | 0.96 (0.92, 1.02) | 0.17 |
| +Lipids/blood pressurec | 0.88 (0.84, 0.93) | <0.001 | 0.99 (0.89, 1.11) | 0.91 | 0.91 (0.93, 1.03) | 0.47 |
| +IL-6 | 0.91 (0.86, 0.97) | 0.003 | 1.01 (0.89, 1.14) | 0.90 | 1.01 (0.96, 1.07) | 0.63 |
CI, Confidence interval; LS, lifestyle.
The sd values are 2.6 pg/ml for IL-6 and 2.6 mg/liter for CRP in men and 2.5 pg/ml for IL-6 and 2.9 mg/liter for CRP in women.
Models include age, body mass index, waist circumference, daily alcohol use, current smoking, diabetes, CVD, hypertension, cancer, estimated GFR, and total number of medications.
Models include previous model variables plus systolic blood pressure, diastolic blood pressure, HDL cholesterol, LDL cholesterol, and triglycerides.
In unadjusted AFT models in men, each sd increase in IL-6 was associated with an 18% decrease in survival time. This association was not materially changed after adjustment for lifestyle, comorbidities, and traditional CVD risk factors; in the adjusted model, each sd increase in IL-6 was associated with a 15% decrease in survival time (P < 0.001). For CRP, each sd increase was associated with a 15% decrease in survival time for men (P < 0.001); the association remained significant although somewhat attenuated after adjustment for potential confounders, with a 12% decrease (P < 0.001).
In women, there were no significant interactions of CRP or IL-6 with estrogen use for survival time (P = 0.47 and P = 0.12, respectively). However, to maintain consistency with other analyses and because of well-known differences in CRP levels in women using oral estrogen compared with those who do not use estrogen, survival time results in women were stratified by estrogen use. In an unadjusted model, each sd increase in IL-6 was associated with a 15% decrease in survival time in women not on estrogen (P < 0.001) and not significantly related to survival time in women on estrogen (P = 0.07). The association in women not on estrogen was attenuated to an 8% decrease with adjustment for lifestyle, comorbidites, and traditional CVD risk factors (P = 0.003). Regardless of estrogen use status, CRP was not significantly associated with survival time in women in unadjusted or fully adjusted models.
Additional adjustment of CRP models for IL-6 (and vice versa) did not substantially alter results for either sex (Table 2). Associations were also unaltered in sensitivity analyses stratifying by age 75 at baseline and in separate models adjusting for use of antihypertensive medications, use of cholesterol-lowering medications, or HOMA-IR (data not shown). The dependence of CRP-survival associations on short-term mortality was examined in separate fully adjusted models by eliminating deaths that occurred during the first 3 and 5 yr of follow-up. The association of CRP with reduced survival time in men was essentially unchanged in these analyses [time ratios = 0.91 (95% confidence interval = 0.88–0.95) and 0.93 (95% confidence interval = 0.90–0.97), for eliminating the first 3 and 5 yr, respectively], and the absence of a significant CRP association for women was not altered regardless of estrogen status (data not shown).
Associations of IL-6 and CRP with adult lifespan (Table 3)
Table 3.
Association of IL-6 and CRP with lifespan among those who died during follow-up in the Rancho Bernardo Study
| Women
|
||||||
|---|---|---|---|---|---|---|
| Men
|
Estrogen use
|
No estrogen use
|
||||
| βa (95% CI) | P value | βa (95% CI) | P value | βa (95% CI) | P value | |
| IL-6 per sdb | ||||||
| Unadjusted | −0.66 (−1.21, −0.11) | 0.02 | 0.41 (−0.91, 1.72) | 0.54 | −1.01 (−1.70, −0.32) | 0.004 |
| Age adjusted | −1.06 (−1.46, −0.65) | <0.001 | −0.10 (−0.99, 0.80) | 0.83 | −1.56 (−2.02, 1.09) | <0.001 |
| +Body size/LS/comorbiditiesc | −0.94 (−1.34, −0.54) | <0.001 | 0.13 (−0.90, 1.16) | 0.81 | −1.41 (−1.89, −0.94) | <0.001 |
| +Lipids/blood pressured | −0.94 (−1.35, −0.53) | <0.001 | 0.12 (−0.93,1.17) | 0.82 | −1.35 (−1.84, −0.86) | <0.001 |
| +CRP | −0.69 (−1.13, −0.26) | 0.002 | −0.02 (−1.10, 1.06) | 0.97 | −1.45 (−2.06, −0.85) | <0.001 |
| CRP per sdb | ||||||
| Unadjusted | −1.38 (−1.94, −0.83) | <0.001 | 0.55 (−0.40, 1.94) | 0.44 | −0.65 (−1.37, 0.06) | 0.07 |
| Age adjusted | −1.25 (−1.66, −0.83) | <0.001 | 0.42 (−0.52, 1.36) | 0.38 | −0.41 (−0.90,0.09) | 0.11 |
| +Body size/LS/comorbiditiesc | −0.92 (−1.33, −0.51) | <0.001 | 0.87 (−0.14, 1.89) | 0.09 | −0.20 (−0.71, 0.32) | 0.45 |
| +Lipids/blood pressured | −0.89 (−1.31, −0.47) | <0.001 | 0.79 (−0.33, 1.91) | 0.16 | −0.17 (−0.68, 0.34) | 0.52 |
| +IL-6 | −0.61 (−1.06, −0.16) | 0.008 | 0.79 (−0.36, 1.95) | 0.17 | 0.46 (−0.11, 1.02) | 0.11 |
CI, Confidence interval; LS, lifestyle.
β-Coefficient corresponds to years of life lost or gained.
sd values are 2.6 pg/ml for IL-6 and 2.6 mg/liter for CRP in men, 2.5 pg/ml for IL-6 and 2.9 mg/liter for CRP in women who did not use estrogen, and 2.6 pg/ml for IL-6 and 3.0 mg/liter for CRP for women who were on estrogen.
Models include age, body mass index, waist circumference, daily alcohol use, current smoking, diabetes, CVD, hypertension, cancer, estimated GFR, and total number of medications.
Models include previous model variables plus systolic blood pressure, diastolic blood pressure, HDL cholesterol, LDL cholesterol, and triglycerides.
In linear regression analyses among the 465 men who died during follow-up, each sd increase in IL-6 corresponded to approximately 0.7 yr shorter adult lifespan in unadjusted models (P = 0.02). After adjustment for covariates, this association was strengthened to an approximately 1-yr reduction in lifespan (P < 0.001). Each sd increase in CRP levels in men was associated with a 1.4-yr shorter lifespan in unadjusted models (P < 0.001) and with approximately 1 yr of reduced lifespan after multivariate adjustment (P < 0.001). CRP and IL-6 levels were moderately correlated in men (r = 0.37; P < 0.001). To examine the independence of CRP and IL-6 associations, we included both in the same multiply adjusted model. Adjusting for the other cytokine reduced associations to 0.7 (P < 0.001) and 0.6 (P < 0.003) years of life lost, respectively, for CRP and IL-6.
Among the 470 women who died during follow-up, the association of CRP and IL-6 with adult lifespan varied by estrogen use status (P for interaction = 0.001 for IL-6, and 0.04 for CRP). Lifespan analyses stratified by estrogen use were significantly different. For women not using estrogen, each sd increase in IL-6 was associated with an approximately 1-yr shorter lifespan (P = 0.004), which persisted after adjustment for covariates. In women using estrogen, levels of IL-6 were not significantly related to lifespan. CRP was not significantly related to adult lifespan in women regardless of estrogen status. Inclusion of CRP and IL-6 in the same adjusted models increased the strength of the IL-6-lifespan association in women not on estrogen but did not alter the null association for CRP or the absence of any cytokine-lifespan association in women using estrogen.
Additional adjustment for HOMA-IR, antihypertensive medications, and cholesterol-lowering medications separately did not change results for IL-6 or CRP (data not shown). Sensitivity analyses stratifying by age 75 at baseline did not alter the significance of the associations between either CRP or IL-6 and lifespan for either men or women (data not shown).
Associations of IL-6 and CRP with cardiovascular and noncardiovascular mortality
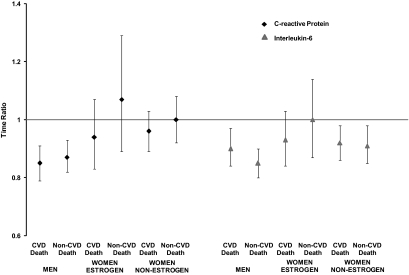
Overall, 465 of 935 deaths (49%) in this cohort were attributed to CVD, 227 of which were in men and 229 in women. We examined whether sex differences in IL-6 and CRP mortality associations were specific to cardiovascular deaths. Figure 1 displays IL-6 and CRP associations with CVD vs. non-CVD mortality in men and women based on AFT models. In men, IL-6 and CRP were significantly and positively associated with both CVD and non-CVD death. For women on estrogen, IL-6 and CRP were not significantly associated with either CVD death or non-CVD death. For women not using estrogen, IL-6 but not CRP, was significantly associated with both CVD death and non-CVD death.
Figure 1.
Results of association of CRP and IL-6 with CVD and non-CVD mortality by sex and estrogen status (women). The x-axis shows the non-CVD and CVD mortality by sex/estrogen status, and the y-axis displays the corresponding time ratios with 95% confidence intervals from the AFT models. Models are adjusted for age, body mass index, waist circumference, daily alcohol use, current smoking, diabetes, CVD, hypertension, cancer, estimated GFR, total number of medications, systolic and diastolic blood pressure, LDL and HDL cholesterol, and triglycerides.
Discussion
CRP and IL-6 have been associated with CVD and all-cause mortality in a variety of populations; however, whether they are associated with exceptional longevity has not been well studied. Overall, this study found that higher levels of inflammatory markers were associated with reduced survival and shorter adult lifespan in men and women who were 57 yr or older at the time of the cytokine measurements, but with important differences by sex and by use of estrogen therapy in women. In men, higher levels of IL-6 and CRP were associated with both a decrease in survival time and adult lifespan independent of lifestyle, comorbidities, and other traditional cardiovascular risk factors including insulin resistance. For women in this study, higher IL-6 levels were significantly associated with decreased survival time and shorter lifespan, but only among women who were not using estrogen therapy at the time of the cytokine measurements. CRP was not significantly associated with either survival time or lifespan in women with or without estrogen therapy. Almost half of all deaths were attributed to CVD. These results did not vary by CVD vs. non-CVD mortality for either sex.
There is some evidence that the association of CRP with mortality may wane over time, i.e. the further the mortality event is from the time of CRP measurement, and that CRP may in fact be a better near-term mortality predictor (26,27,28). This has been indicated for both general population studies (27) as well as in those with existing vascular disease (26,28). In the present study, the association of higher CRP with reduced longevity in men persisted when early deaths (those occurring during the first 3 and 5 yr of follow-up) were eliminated from the analyses, implying that CRP may possibly predict longer-term survival in men who have already lived beyond middle age.
Few studies have evaluated the association of CRP or IL-6 with exceptional longevity. Arai et al. (29) did not find either CRP or IL-6 associated with all-cause mortality in men and women centenarians in models adjusted for traditional cardiovascular risk factors, physical function, and total number of comorbidities. In a group of men and women nonagenarians, Jylhä et al. (30) also found no association of CRP or IL-6 with all-cause mortality in models adjusted for traditional risk factors. In another study of nonagenarians, Kravitz et al. (31) found that higher CRP was associated with increased risk of all-cause mortality but not dementia. However, these studies had small sample sizes (208–285 participants) and much shorter follow-up periods (∼4–6 yr) and concentrated on cohorts that had already achieved 90+ or 100+ yr old, so inflammatory markers were measured much more proximal to time of death. In contrast, the present study has a much larger sample size and longer follow-up of participants who were younger at baseline (median age 73 yr). Additionally, we employed statistical methods that gave us percent decrease in survival time or years of decrease in lifespan as opposed to hazard ratios, which indicate only relative risk for CRP and IL-6 with all-cause mortality. These results indicate that inflammation measured years earlier may play an important role in survival to age 80 and beyond, particularly for men.
The present study found no significant associations of CRP with survival and lifespan in women regardless of estrogen use status. Recent studies suggest that it is unlikely that CRP is causally related to CVD (32,33) and may more appropriately be defined as an indicator of overall inflammatory state or immune response. Although it is acknowledged that chronic inflammation is a threat to survival (34), inflammatory processes are an essential component of the protective immune response (35). Low levels of inflammatory cytokines in an elderly woman could indicate immune deficiency, and in turn, higher levels could possibly indicate an intact and/or healthy immune system. Our discrepant results for CRP in men and women could also indicate the presence of an unknown or unadjusted confounder for women that counteracts detrimental effects of higher CRP on longevity. Because adiposity is associated with levels of inflammatory markers (36), and adipose tissue distributions vary by sex (37), we adjusted for waist circumference and body mass index, but more precise measures of regional adiposity such as visceral fat or sc fat were not available.
Among women who were estrogen users, there was a significant association of higher IL-6 with shorter lifespan, which was not seen in women on estrogen therapy. Previous reports of an association between IL-6 with exogenous estrogen have been inconsistent with some studies finding estrogen use associated with higher levels of IL-6, whereas other studies have found no association or an inverse association (38,39). And although the association of CRP with lifespan in women was not significant, there was a suggestion of association of higher CRP with longer lifespan among estrogen users and shorter lifespan among non-estrogen users. This association could be an artifact of estrogen use affecting CRP levels and not due to any underlying inflammatory state per se. It is well established that women who use oral hormone replacement therapy have higher levels of CRP (39,40,41,42) and that women who use estrogen tend to live longer, possibly due to healthy-user bias.
Our study has many strengths but also some limitations. The Rancho Bernardo Study is a large prospective cohort who is well characterized for covariates and has nearly complete follow-up for vital status. Even though plasma samples were collected at a 1984–1987 examination, the measurement of CRP and IL-6 occurred in 2000, allowing the use of high-sensitivity methods. The Rancho Bernardo cohort comprises participants of primarily Caucasian and middle to upper-middle class socioeconomic status, which limits generalizability but concurrently greatly reduces unmeasured confounding due to these factors. As in all observational studies, residual confounding is possible. We did not incorporate information on estrogen dose or perform drug-specific analyses; however, more than 80% of women taking estrogen at this time were taking unopposed oral conjugated equine estrogen at a daily dose of 0.625 mg.
Our results indicate that higher levels of IL-6 and CRP predict reduced survival and lifespan even among older men who live an exceptionally long life, with a significant IL-6 association only in women not taking estrogen. Inflammation is often associated with chronic diseases such as diabetes and CVD, which are in turn associated with decreased survival. Replication of these findings is needed as well as further investigation of the association of CRP with survival and lifespan in women. Studying factors associated with exceptional longevity is of utmost importance as the size of the elderly population increases.
Footnotes
This work was supported by a Sandra A. Daugherty Foundation Cardiovascular Epidemiology Award and National Heart, Lung and Blood Institute Grant HL089622. The Rancho Bernardo Study was funded by research Grants AG028507 and AG018339 from the National Institute on Aging and Grant DK31801 from the National Institute of Diabetes and Digestive and Kidney Diseases. G.A.L. is funded by American Heart Association Award 0930073N.
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 21, 2010
Abbreviations: AFT, Accelerated failure time; CRP, C-reactive protein; CVD, cardiovascular disease; GFR, glomerular filtration rate; HDL, high-density lipoprotein; HOMA-IR, homeostasis model for insulin resistance; LDL, low-density lipoprotein.
References
- Abeywardena MY, Leifert WR, Warnes KE, Varghese JN, Head RJ 2009 Cardiovascular biology of interleukin-6. Curr Pharm Des 15:1809–1821 [DOI] [PubMed] [Google Scholar]
- Garg R, Tripathy D, Dandona P 2003 Insulin resistance as a proinflammatory state: mechanisms, mediators, and therapeutic interventions. Curr Drug Targets 4:487–492 [DOI] [PubMed] [Google Scholar]
- Packard RR, Libby P 2008 Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem 54:24–38 [DOI] [PubMed] [Google Scholar]
- Pasceri V, Willerson JT, Yeh ET 2000 Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation 102:2165–2168 [DOI] [PubMed] [Google Scholar]
- Schuett H, Luchtefeld M, Grothusen C, Grote K, Schieffer B 2009 How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thromb Haemost 102:215–222 [DOI] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, Pahor M 2003 Inflammatory markers and onset of cardiovascular events: results from the health ABC study. Circulation 108:2317–2322 [DOI] [PubMed] [Google Scholar]
- Cushman M, Arnold AM, Psaty BM, Manolio TA, Kuller LH, Burke GL, Polak JF, Tracy RP 2005 C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation 112:25–31 [DOI] [PubMed] [Google Scholar]
- Folsom AR, Aleksic N, Catellier D, Juneja HS, Wu KK 2002 C-reactive protein and incident coronary heart disease in the atherosclerosis risk in communities (ARIC) study. Am Heart J 144:233–238 [DOI] [PubMed] [Google Scholar]
- Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH 1998 Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation 98:731–733 [DOI] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger Jr WH, Heimovitz H, Cohen HJ, Wallace R 1999 Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med 106:506–512 [DOI] [PubMed] [Google Scholar]
- Strandberg TE, Tilvis RS 2000 C-reactive protein, cardiovascular risk factors, and mortality in a prospective study in the elderly. Arterioscler Thromb Vasc Biol 20:1057–1060 [DOI] [PubMed] [Google Scholar]
- Tuomisto K, Jousilahti P, Sundvall J, Pajunen P, Salomaa V 2006 C-reactive protein, interleukin-6 and tumor necrosis factor α as predictors of incident coronary and cardiovascular events and total mortality. A population-based, prospective study. Thromb Haemost 95:511–518 [DOI] [PubMed] [Google Scholar]
- Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E, Ahmad S 2001 Insulin inhibits intranuclear nuclear factor κB and stimulates IκB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab 86:3257–3265 [DOI] [PubMed] [Google Scholar]
- Barzilay JI, Abraham L, Heckbert SR, Cushman M, Kuller LH, Resnick HE, Tracy RP 2001 The relation of markers of inflammation to the development of glucose disorders in the elderly: the cardiovascular health study. Diabetes 50:2384–2389 [DOI] [PubMed] [Google Scholar]
- Lacquemant C, Vasseur F, Lepretre F, Froguel P 2005 [Adipocytokins, obesity and development of type 2 diabetes]. Med Sci (Paris) 21 Spec No:10–18 (French) [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM 2001 C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286:327–334 [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Cook NR, Buring JE, Manson JE, Ridker PM 2003 C-reactive protein is independently associated with fasting insulin in nondiabetic women. Arterioscler Thromb Vasc Biol 23:650–655 [DOI] [PubMed] [Google Scholar]
- Capri M, Salvioli S, Sevini F, Valensin S, Celani L, Monti D, Pawelec G, De Benedictis G, Gonos ES, Franceschi C 2006 The genetics of human longevity. Ann NY Acad Sci 1067:252–263 [DOI] [PubMed] [Google Scholar]
- Herskind AM, McGue M, Holm NV, Sørensen TI, Harvald B, Vaupel JW 1996 The heritability of human longevity: A population-based study of 2872 danish twin pairs born 1870–1900. Hum Genet 97:319–323 [DOI] [PubMed] [Google Scholar]
- McGue M, Vaupel JW, Holm N, Harvald B 1993 Longevity is moderately heritable in a sample of Danish twins born 1870–1880. J Gerontol 48:B237–B244 [DOI] [PubMed] [Google Scholar]
- Mitchell BD, Hsueh WC, King TM, Pollin TI, Sorkin J, Agarwala R, Schäffer AA, Shuldiner AR 2001 Heritability of life span in the Old Order Amish. Am J Med Genet 102:346–352 [DOI] [PubMed] [Google Scholar]
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D 1999 A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med 130:461–470 [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS 1972 Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502 [PubMed] [Google Scholar]
- Cartier A, Côté M, Lemieux I, Pérusse L, Tremblay A, Bouchard C, Després JP 2009 Sex differences in inflammatory markers: what is the contribution of visceral adiposity? Am J Clin Nutr 89:1307–1314 [DOI] [PubMed] [Google Scholar]
- Swindell WR 2009 Accelerated failure time models provide a useful statistical framework for aging research. Exp Gerontol 44:190–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criqui MH, Ho LA, Denenberg JO, Ridker PM, Wassel CL, McDermott MM 2010 Biomarkers in peripheral arterial disease patients and near and longer term mortality. J Vasc Surg 52:85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny NS, Yanez ND, Psaty BM, Kuller LH, Hirsch CH, Tracy RP 2007 Inflammation biomarkers and near-term death in older men. Am J Epidemiol 165:684–695 [DOI] [PubMed] [Google Scholar]
- Vidula H, Tian L, Liu K, Criqui MH, Ferrucci L, Pearce WH, Greenland P, Green D, Tan J, Garside DB, Guralnik J, Ridker PM, Rifai N, McDermott MM 2008 Biomarkers of inflammation and thrombosis as predictors of near-term mortality in patients with peripheral arterial disease: a cohort study. Ann Intern Med 148:85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai Y, Takayama M, Gondo Y, Inagaki H, Yamamura K, Nakazawa S, Kojima T, Ebihara Y, Shimizu K, Masui Y, Kitagawa K, Takebayashi T, Hirose N 2008 Adipose endocrine function, insulin-like growth factor-1 axis, and exceptional survival beyond 100 years of age. J Gerontol A Biol Sci Med Sci 63:1209–1218 [DOI] [PubMed] [Google Scholar]
- Jylhä M, Paavilainen P, Lehtimäki T, Goebeler S, Karhunen PJ, Hervonen A, Hurme M 2007 Interleukin-1 receptor antagonist, interleukin-6, and C-reactive protein as predictors of mortality in nonagenarians: the vitality 90+ study. J Gerontol A Biol Sci Med Sci 62:1016–1021 [DOI] [PubMed] [Google Scholar]
- Kravitz BA, Corrada MM, Kawas CH 2009 High levels of serum C-reactive protein are associated with greater risk of all-cause mortality, but not dementia, in the oldest-old: results from the 90+ study. J Am Geriatr Soc 57:641–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J, Pepys MB 2009 C-reactive protein and coronary disease: is there a causal link? Circulation 120:2036–2039 [DOI] [PubMed] [Google Scholar]
- Elliott P, Chambers JC, Zhang W, Clarke R, Hopewell JC, Peden JF, Erdmann J, Braund P, Engert JC, Bennett D, Coin L, Ashby D, Tzoulaki I, Brown IJ, Mt-Isa S, McCarthy MI, Peltonen L, Freimer NB, Farrall M, Ruokonen A, Hamsten A, Lim N, Froguel P, Waterworth DM, Vollenweider P, Waeber G, Jarvelin MR, Mooser V, Scott J, Hall AS, Schunkert H, Anand SS, Collins R, Samani NJ, Watkins H, Kooner JS 2009 Genetic loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA 302:37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM 2007 Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: implications for longevity. Nutr Rev 65(12 Pt 2):S253–S259 [DOI] [PubMed] [Google Scholar]
- Gauldie J 2007 Inflammation and the aging process: devil or angel. Nutr Rev 65(12 Pt 2):S167–S169 [DOI] [PubMed] [Google Scholar]
- Kanaya AM, Harris T, Goodpaster BH, Tylavsky F, Cummings SR; Health, Aging, and Body Composition (ABC) Study 2004 Adipocytokines attenuate the association between visceral adiposity and diabetes in older adults. Diabetes Care 27:1375–1380 [DOI] [PubMed] [Google Scholar]
- Beasley LE, Koster A, Newman AB, Javaid MK, Ferrucci L, Kritchevsky SB, Kuller LH, Pahor M, Schaap LA, Visser M, Rubin SM, Goodpaster BH, Harris TB; Health ABC study 2009 Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity (Silver Spring) 17:1062–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachoñ D 2005 Role of tumor necrosis factor (TNF) and interleukin-6 (IL-6) in the pathogenesis of late complications of menopause. effects of hormone replacement therapy on TNF and IL-6 expression. Pol Merkur Lekarski 18:724–727 [PubMed] [Google Scholar]
- Davison S, Davis SR 2003 New markers for cardiovascular disease risk in women: Impact of endogenous estrogen status and exogenous postmenopausal hormone therapy. J Clin Endocrinol Metab 88:2470–2478 [DOI] [PubMed] [Google Scholar]
- Albert MA, Ridker PM 2006 C-reactive protein as a risk predictor: do race/ethnicity and gender make a difference? Circulation 114:e67–e74 [DOI] [PubMed] [Google Scholar]
- Cushman M, Meilahn EN, Psaty BM, Kuller LH, Dobs AS, Tracy RP 1999 Hormone replacement therapy, inflammation, and hemostasis in elderly women. Arterioscler Thromb Vasc Biol 19:893–899 [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Rifai N, Buring JE, Manson JE 1999 Hormone replacement therapy and increased plasma concentration of C-reactive protein. Circulation 100:713–716 [DOI] [PubMed] [Google Scholar]