Abstract
Context: Mandibuloacral dysplasia (MAD) is an autosomal recessive progeroid disorder associated with type A (partial) or B (generalized) lipodystrophy and is due to mutations in lamin A/C (LMNA) or zinc metalloproteinase (ZMPSTE24) genes.
Objective: The objective of the study was to report a novel syndrome with some overlapping features with MAD.
Results: We report seven patients with mandibular hypoplasia, deafness, progeroid features (MDP), and associated lipodystrophy. These patients have similar features to MAD patients such as hypoplastic mandible, beaked nose, stiff joints, and sclerodermatous skin. However, the patients did not harbor any disease causing variants in LMNA or ZMPSTE24 and showed distinct characteristics such as sensorineural hearing loss and absence of clavicular hypoplasia and acroosteolysis. All males with MDP had undescended testes and were hypogonadal. One adult female showed lack of breast development. Skinfold thickness, dual-energy X-ray absorptiometry and whole-body magnetic resonance imaging for body fat distribution revealed a lack of lipodystrophy in a prepubertal female but a progressive loss of sc fat presenting with partial lipodystrophy in young adults and generalized lipodystrophy in older patients.
Conclusions: Patients with MDP syndrome have a few overlapping but some distinct clinical features as compared with MAD, suggesting that it is a novel syndrome. The molecular basis of MDP syndrome remains to be elucidated.
We report 5 males and 2 females with a novel autosomal recessive syndrome of mandibular hypoplasia, deafness, and progeroid features with lipodystrophy, undescended testes, and male hypogonadism.
Mandibuloacral dysplasia (MAD; Online Mendelian Inheritance in Man 248370, 608612) is a heterogeneous autosomal recessive disorder characterized by mandibular hypoplasia, dysplastic clavicles, acroosteolysis, prominent eyes, stiff joints, beaked nose, sclerodermatous skin, alopecia, and mottled hyperpigmentation (1,2,3). We described two distinct patterns of lipodystrophy in patients with MAD: type A with partial loss of sc fat from the extremities and excess fat deposition in the neck and trunk and type B with more generalized loss of sc fat (1,2). Type A MAD phenotype was then linked to mutations in the lamin A/C (LMNA) gene (4). Genotyping of our initial patients with MAD revealed lack of disease causing LMNA mutations in some with type B phenotype (5). Based on a candidate gene approach, we discovered the second MAD locus, zinc metalloproteinase (ZMPSTE24) (6). Limited data show that children with MAD due to ZMPSTE24 mutations may have mild lipodystrophy of the trunk and extremities, whereas adults may have more severe loss of sc fat (6,7,8).
In our original paper, a 25-yr-old male with MAD type B had no acroosteolysis or clavicular hypoplasia (1) and had no mutations in LMNA or ZMPSTE24 genes (5,6). We now report six additional patients, all of whom share many phenotypic characteristics, which are distinct from those reported in classical MAD patients with LMNA or ZMPSTE24 mutations.
Patients and Methods
All patients or their parents gave written informed consent. The study protocol was approved by the Institutional Review Boards of the University of Texas Southwestern and the National Institutes of Health. The salient clinical features of the patients are presented in Table 1, Fig. 1, and below. All of them had mandibular hypoplasia, a beaked nose with bird-like facies, prominent eyes, small mouth, growth retardation, limb muscle atrophy, skin atrophy, and hearing loss in childhood but none of them had acroosteolysis or clavicular hypoplasia. All five males had undescended testes and hypogonadism requiring testosterone replacement. All of them were sporadic, isolated cases with no consanguinity among parents (Supplemental Fig. 1, published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org.).
Table 1.
Clinical characteristics, body composition, and metabolic variables of the MDP patients
| 100.3 | 200.5 | 300.4 | 400.3 | 500.4 | 600.4 | 700.3 | |
|---|---|---|---|---|---|---|---|
| Age/sex | 63/M | 36/M | 19/F | 10/F | 25/M | 21/M | 18/M |
| Age at onset (yr) | 4 | 7 | 4 | 2 | 2.5 | 4 | 3 |
| Ethnicity | American | American | Italian | Canadian | Indian | Hispanic | German |
| Birth weight (kg) | 3.4 | 3.2 | 3.8 | 3.6 | 3 | 4.1 | 3.9 |
| Height (m) | NA | 1.75 | 1.60 | 1.19 | 1.68 | 1.61 | 1.79 |
| Weight (kg) | NA | 45.4 | 45 | 17.2 | 41.3 | 55.1 | 47 |
| BMI (kg/m2) | NA | 14.9 | 17.6 | 12.2 | 14.6 | 21.3 | 14.7 |
| Lipodystrophy | + | + | + | − | + | + | + |
| Joint contractures | + | + | − | + | − | + | + |
| Telangiectasia | NA | + | − | + | + | − | + |
| Deafness (age of onset, yr) | + (18) | + (15) | + (7) | + (8) | + (16) | + (14) | + (6) |
| Lack of breast development | NA | NA | + | NA | NA | NA | NA |
| Corneal keratosis | NA | + | − | − | − | + | − |
| Hepatic steatosis | NA | − | + | − | NA | + | + |
| Hepatomegaly | NA | − | + | + | NA | − | + |
| Hypertriglyceridemia (age of onset, yr) | + (26) | + (7) | + (16) | − | + (16) | + (21) | + (15) |
| Diabetes mellitus (age of onset, yr) | + (52) | − | − | − | + (23) | − | − |
| HDL-C (mg/dl) | NA | 27 (>40) | 16 (>50) | 34 (>50) | NA | 25 (>40) | 42 (>40) |
| Leptin (ng/ml) | NA | 1.77 (6.0 ± 0.42) | 8.22 (12.9 ± 8.9) | 4.03 | 4.36 (4.5 ± 0.48) | 7.08 (5.3 ± 0.28) | 1.48 |
| Fasting insulin (μU/ml) | NA | 8.7 (7.87 ± 0.17) | 33.8 (7.87 ± 0.17) | 34.1 | 24.4 (7.87 ± 0.17) | 105.4 (9.6 ± 0.24) | 17.4 |
| Two-hour PP insulin μU/ml) | NA | 400.3 | 290.6 | 202.9 | NA | 558.4 | NA |
| Whole-body fat (% body mass) | NA | 15.3 (26.6 ± 6.0) | 20.2 (35.1 ± 7.2) | 29.9 | 22.6 (24.6 ± 6.5) | 21.1 (24.4 ± 6.1) | 14.3 |
| Truncal fat (% regional mass) | NA | 19.3 | 23.7 | 28.3 | 30 | 25.8 | 19.2 |
| Arm fat (% regional mass) | NA | 9.3 | 20.4 | 43.9 | 6.9 | 21.3 | 4.8 |
| Leg fat (% regional mass) | NA | 5.3 | 13.9 | 34.3 | 7.5 | 11.8 | 4.7 |
| Truncal fat (%)/leg fat (%) | NA | 3.64 (1.02 ± 0.16) | 1.71 (0.76 ± 0.16) | 0.83 | 4.27 (0.93 ± 0.15) | 2.19 (0.89 ± 0.13) | 4.08 |
Normal values for some variables are shown in parentheses as mean ± sd and for HDL-C as >40 for males and >50 for females. Normal values of leptin are from Ruhl and Everhart (12); insulin from Meng et al. (18); and total and percent truncal fat/percent leg fat from Kelly et al. (11). Normal values for leptin in children younger than age 20 yr are not available. Normal values for regional body fat (truncal, arm, and leg) are not available. M, Male; F, female; NA, not available; PP, postprandial; HDL-C, high-density lipoprotein cholesterol.
Figure 1.
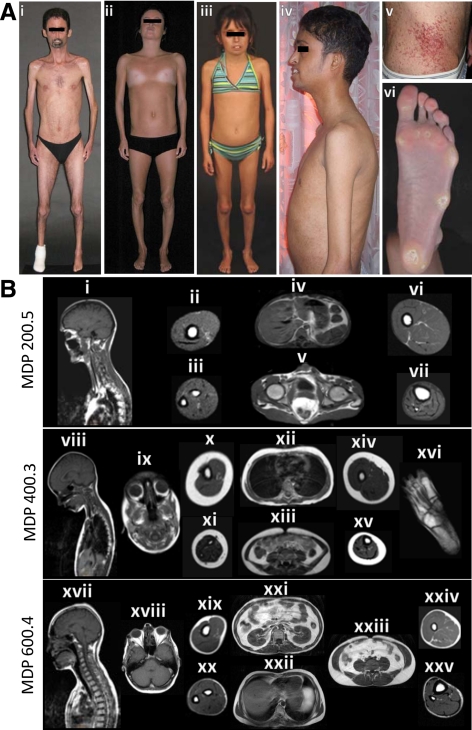
MDP patients and their body fat distribution pattern on MRI. A, i, MDP 200.5, anterior view of a 36-yr-old male showing generalized lipodystrophy, reduced muscle mass, and prominent maxillary incisors. He had pinched nose, small mouth, and contractures of the elbows. There was no loss of scalp hair and he had graying of hair on the chin. ii, MDP 300.4, anterior view of a 19-yr-old Italian female showing generalized lipodystrophy and lack of breast development. She had pinched nose and small mouth. iii, MDP 400.3, anterior view of a 10-yr-old French Canadian girl with small mouth, pinched nose, and bilateral coxa valga. Her body fat distribution was normal. iv, MDP 500.4, lateral view of a 25-yr-old Indian male showing generalized lipodystrophy, muscle wasting, and prominent maxillary incisors. v, Telangiectasias on the abdomen of MDP 200.5. vi, Marked loss of sc fat from the sole and calluses in the patient MDP 200.5. B, Sagittal T-1-weighted MRIs of the head, neck, and thorax through midline or orbit and axial MRIs of the chest, abdomen, arm, forearm, thigh, calf, and foot from various MDP patients. MRIs of MDP 200.5 show near total loss of sc fat from the head, neck, and thorax (i), arm (ii), forearm (iii), abdomen (iv and v), thigh (vi), and calf (vii). Retroorbital, ip, and bone marrow fat depots were well preserved. MRIs of MDP 400.3 show normal amounts of sc fat in the head and neck (viii and ix), and thorax (xii), normal retroorbital (ix), ip (xiii), retroperitoneal, and bone marrow fat as well as in the arm (x), forearm (xi), thigh (xiv), and calf (xv). Plantar fat (xvi) was reduced. MRIs of MDP 600.4 show moderate loss of sc fat in all the areas including the head and neck (xvii), thorax (xxii), arm (xix), forearm (xx), thigh (xxiv), and calf (xxv). Retroorbital (xviii), intraabdominal, and bone marrow fat depots were well preserved. A significant amount of intraabdominal (xxi and xxiii) fat was noted.
Mandibular hypoplasia, deafness, progeroid features (MDP) patient 100.3
This 63-yr-old male has been published previously (9,10). Briefly, he noted thinning of extremities and crowded teeth at age 4 yr. His atrophic, undescended testes were surgically removed at age 12 yr. He had a high-pitched voice. He underwent a three-vessel coronary artery bypass graft at age 55 yr, developed diabetic neuropathy, and had an amputation above the left knee at age 58 yr.
MDP patient 200.5
Since the last report as MAD 400.5 (5), this 36-yr-old male developed bilateral corneal opacities in the lower quadrant and had surgery for exposure keratitis. His eyelashes were long. He had a very small uvula and mouth. The skin was taut distally over the feet and multiple telangiectasia were noted on the trunk and thighs. He had limited mobility of the ankle and knee joints. Radiographs of the right foot revealed a hypoplastic fourth metatarsal, a surgically removed fifth metatarsal and eroded distal phalanx of the great toe. The left foot appeared normal.
MDP patient 300.4
This 19-yr-old Italian female had reduced growth and thin limbs since age 4 yr. She had menarche at age 12 yr. At age 16 yr, hepatic steatosis, insulin resistance, impaired glucose tolerance, and hypertriglyceridemia were noted; and rosiglitazone, gemfibrozil, and fish oil were started. Liver biopsy at age 18 yr revealed nonalcoholic steatohepatitis with moderate fibrosis. She underwent bilateral mammoplasty recently for lack of breast development but had regular menstrual periods. She had generalized loss of sc fat and skin on the distal extremities was extremely tight.
MDP patient 400.3
This 10-yr-old Canadian girl started losing fat from the extremities and subsequently from the face, neck, and chest at age 2 yr. She had bilateral coxa valga and could not make a fist. She had long eyelashes and the skin around the eyes was tight. She had a prominent forehead, low-set ears, and prominent upper teeth. She had no breast development. She had some telangiectasia on the ankles. She had notable fat loss from the face, palms, and soles, but her extremities and abdomen were spared. She had mild contractures of the wrists, elbows, ankles, and knees.
MDP patient 500.4
This 25-yr-old Indian male noted thinning of the limbs with associated weakness at age 2 yr. Subsequently he developed scleroderma-like skin changes with ulceration, telangiectasia, and tiny hemangiomas. Hypertriglyceridemia developed at age 16 yr followed by diabetes and osteoporosis. He was on thiazolidinedione and testosterone therapy. Radiographs at age 18 yr revealed congenital fusion of the trapezoid, capitates, triquetral and pisiform carpal bones, scalloping of articular margins of lumbar 1 and 2 vertebrae, and slipped femoral epiphyses bilaterally.
MDP patient 600.4
This 21-yr-old Hispanic male developed generalized loss of sc fat at age 4 yr. He had surgical removal of undescended testes. He had received GH from age 8 to 13 yr and hydrocortisone for adrenal insufficiency. He also had nephrolithiasis and recurrent nose bleeds. He had generalized loss of sc fat and tight skin over the feet and arms. His upper eyelashes were long, and he had bilateral exophthalmos and corneal opacities in the right eye. He had a small uvula. He had contractures of both the ankles and knees. He had plantar calluses and little body hair. Radiographs revealed short fifth metatarsal bilaterally and lack of closure of the distal radial epiphyses (Supplemental Fig. 2).
MDP patient 700.3
This 18-yr-old German male had prominent eyes and a pinched nose. Malformation of the ears was noted at age 6 yr. He underwent bilateral orchiopexy at the age of 3 yr but has been on testosterone replacement since age 15 yr. Generalized lipodystrophy, hypertriglyceridemia, and hepatic steatosis were diagnosed at age 15 yr. He had telangiectasia on the chest and neck and contractures of the fingers, hands, elbows, knees, and ankles.
Methods
Anthropometric measurements, whole-body magnetic resonance imaging (MRI), and dual-energy x-ray absorptiometry for regional and total body fat were performed as described previously (1). Fasting serum insulin and leptin levels were determined by immunoassays using commercial kits (Millipore, Billerica, MA). Fasting serum glucose and lipoproteins were analyzed as part of a systematic multichannel analysis (Synchron CX9 ALX clinical system; Beckman, Fullerton, CA). A standard oral glucose tolerance test for 180 min was performed (1). The exons and exon-intron boundaries of the LMNA and ZMPSTE24 genes were sequenced in genomic DNA obtained from patients’ blood according to Simha et al. (5) and Agarwal et al. (6). Three highly polymorphic short tandem repeats from ABI linkage mapping sets version 2.5 (Applied Biosystems, Foster City, CA) around LMNA and ZMPSTE24 were genotyped in five pedigrees according to the manufacturer’s protocol.
Results
Measurement of skinfold thickness revealed severe generalized loss of sc fat in MDP 200.5. MDP 600.4 showed moderate fat loss from the extremities but preserved truncal fat (Supplemental Fig. 3). The prepubertal girl, MDP 400.3, did not have evidence of fat loss.
MDP 200.5, 300.4, 600.4, and 700.3 had moderate to severe reduction in body fat compared with age- and gender-matched normal data (11), whereas MDP 400.3 and 500.4 did not show reduction in body fat (Table 1). The ratio of truncal fat to leg fat, however, was markedly increased in all adult patients (1.7 to 4.1 compared with normal mean values of 0.8 to 1.0; Table 1). Whole-body MRI studies further confirmed the generalized lack of body fat in patients MDP 200.5 and 600.4 with preservation of retroorbital, intraabdominal, and bone marrow fat depots (Fig. 1B). MRIs of MDP 400.3 showed only loss of plantar fat (Fig. 1B). MRI of MDP 700.3 demonstrated a drastic reduction of sc fat.
Serum leptin levels ranged from low to normal (Table 1) (12). Despite a normal glucose tolerance test, MDP 400.3 and MDP 600.4 had markedly elevated fasting and postprandial insulin levels (Table 1). MDP 200.5 and MDP 300.4 had impaired glucose tolerance and hyperinsulinemia (Table 1). MDP 500.4 had diabetes and fasting hyperinsulinemia.
Screening of the exons and the adjacent introns and splice sites revealed no disease causing variants in LMNA or ZMPSTE24 genes in any of the patients. Haplotype analysis of short tandem repeats ruled out linkage to LMNA in MDP 200, 300, and 500 pedigrees and to ZMPSTE24 in MDP 200 pedigree (Supplemental Fig. 1).
Discussion
Our current reports and the review of the literature suggest that these patients represent a novel variety of progeroid syndrome with lipodystrophy. Freidenberg et al. (2) also reported two males (16 and 17 yr old) who had some clinical features similar to MDP patients such as bilateral sensorineural hearing loss, normal clavicles, hypogonadism, and partial lipodystrophy. Unfortunately, no pictures or other details of the patients were published.
None of our patients had any disease causing mutations in LMNA or ZMPSTE24 genes, and linkage to these loci was excluded in some pedigrees. Review of the pedigrees reveals lack of consanguinity or other affected siblings. Thus, MDP syndrome may be due to either autosomal recessive inheritance or de novo heterozygous mutations. So far, there is no evidence of transmission because all of the affected males have been hypogonadal. However, females may not be infertile as evidenced by one of our post pubertal patients who had regular menstrual cycles.
MDP patients share only some clinical features with MAD patients reported to harbor LMNA or ZMPSTE24 mutations (13) but reveal many distinct features (Supplemental Table 1). For example, all three varieties have mandibular hypoplasia, short stature, some joint contractures, and sclerodermatous skin with mottled pigmentation. Interestingly, the onset of sc fat loss reportedly started at or before age 4 yr, which is close to that noted in those with LMNA and ZMPSTE24 mutations. However, patients with MDP have generalized loss of sc fat, especially evident in the older patients compared with those with LMNA and ZMPSTE24 mutations. There is no radiological evidence of acroosteolysis and clavicular hypoplasia in MDP patients, whereas all MAD patients develop acroosteolysis by 2–4 yr of age. All males with MDP had hypogonadism and undescended testes, which were surgically removed in three of the patients and treated by orchidopexy in one patient.
Most interestingly, all MDP patients developed sensorineural hearing loss during childhood, which is not a feature of MAD patients with LMNA and ZMPSTE24 mutations. Interestingly, mild sensorineural hearing deficit was reported on audiological examination in patients with Hutchinson-Gilford progeria syndrome (14) who harbor heterozygous c.1824C>T LMNA mutation. We have reported sensorineural hearing loss in a pedigree with atypical progeroid syndrome due to heterozygous p.P4R LMNA mutation (15), and Rankin et al. (16) reported it in a 20-yr-old female with type 2 diabetes and heterozygous p.R644C LMNA mutation, but her mother who carried the same mutation did not have it. However, hearing loss is not an accompanying feature of other laminopathies (17).
Interestingly, none of the patients with MDP had alopecia and/or sparse, thin hair seen in the other varieties of MAD (Supplemental Table 1). Other interesting features include the presence of corneal opacities and dry eyes in MDP patients, presumably due to exophthalmos and exposure keratitis requiring surgery in two patients. We also observed extremely long eyelashes in three patients, but it is unclear at this time whether these findings are characteristic feature of MDP syndrome.
MDP patients seem to have better long-term survival than patients with MAD due to LMNA and ZMPSTE24 mutations. All MDP patients are alive despite morbidities such as diabetes mellitus, hypertriglyceridemia, low high-density lipoprotein cholesterol, nonalcoholic steatohepatitis, and coronary artery disease. The oldest patient is 63 yr old. On the other hand, two patients with MAD due to LMNA mutations died at the young age of 10 and 16 yr and three with ZMPSTE24 mutations have died at the ages 2, 27, and 37 yr.
In conclusion, patients with MDP-associated lipodystrophy constitute a novel syndrome whose genetic basis remains to be elucidated.
Supplementary Material
Acknowledgments
We thank Dr. Phillip Gorden and Elaine Cochran, R.N. (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health) for allowing us to include their patient. We also thank Dr. Pedro A. Sanchez for referring patient MDP 600.4 to us and Monica D’Adamo for helping us in clinical evaluation of MDP 300.4. We thank Sarah Masood and Crystal Kittisopikul for help with illustrations and mutational screening, Ross Wilson for genotyping short tandem repeats in the pedigrees, and Claudia Quittner for assistance in patient care during evaluations.
Footnotes
This work was supported by National Institutes of Health Grants R01-DK54387, Clinical and Translational Science Awards Grant UL1 RR024982, the Southwest Medical Foundation, and Italian Agenzia Italiana del Farmaco Research Program Grant FARM6FMSH5.
Disclosure Summary: The authors have nothing to declare.
First Published Online July 14, 2010
Abbreviations: MAD, Mandibuloacral dysplasia; MDP, mandibular hypoplasia, deafness, progeroid features.
References
- Simha V, Garg A 2002 Body fat distribution and metabolic derangements in patients with familial partial lipodystrophy associated with mandibuloacral dysplasia. J Clin Endocrinol Metab 87:776–785 [DOI] [PubMed] [Google Scholar]
- Freidenberg GR, Cutler DL, Jones MC, Hall B, Mier RJ, Culler F, Jones KL, Lozzio C, Kaufmann S 1992 Severe insulin resistance and diabetes mellitus in mandibuloacral dysplasia. Am J Dis Child 146:93–99 [DOI] [PubMed] [Google Scholar]
- Young LW, Radebaugh JF, Rubin P, Sensenbrenner JA, Fiorelli G, McKusick VA 1971 New syndrome manifested by mandibular hypoplasia, acroosteolysis, stiff joints and cutaneous atrophy (mandibuloacral dysplasia) in two unrelated boys. Birth Defects Orig Artic Ser 7:291–297 [PubMed] [Google Scholar]
- Novelli G, Muchir A, Sangiuolo F, Helbling-Leclerc A, D'Apice MR, Massart C, Capon F, Sbraccia P, Federici M, Lauro R, Tudisco C, Pallotta R, Scarano G, Dallapiccola B, Merlini L, Bonne G 2002 Mandibuloacral dysplasia is caused by a mutation in LMNA-encoding lamin A/C. Am J Hum Genet 71:426–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simha V, Agarwal AK, Oral EA, Fryns JP, Garg A 2003 Genetic and phenotypic heterogeneity in patients with mandibuloacral dysplasia-associated lipodystrophy. J Clin Endocrinol Metab 88:2821–2824 [DOI] [PubMed] [Google Scholar]
- Agarwal AK, Fryns JP, Auchus RJ, Garg A 2003 Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum Mol Genet 12:1995–2001 [DOI] [PubMed] [Google Scholar]
- Agarwal AK, Zhou XJ, Hall RK, Nicholls K, Bankier A, Van Esch H, Fryns JP, Garg A 2006 Focal segmental glomerulosclerosis in patients with mandibuloacral dysplasia owing to ZMPSTE24 deficiency. J Investig Med 54:208–213 [DOI] [PubMed] [Google Scholar]
- Miyoshi Y, Akagi M, Agarwal AK, Namba N, Kato-Nishimura K, Mohri I, Yamagata M, Nakajima S, Mushiake S, Shima M, Auchus RJ, Taniike M, Garg A, Ozono K 2008 Severe mandibuloacral dysplasia caused by novel compound heterozygous ZMPSTE24 mutations in two Japanese siblings. Clin Genet 73:535–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Thurmon T, Salvaggio J 1973 Werner’s Syndrome. Cutis 12:76–81 [Google Scholar]
- Ng D, Stratakis CA 2000 Premature adrenal cortical dysfunction in mandibuloacral dysplasia: a progeroid-like syndrome. Am J Med Genet 95:293–295 [DOI] [PubMed] [Google Scholar]
- Kelly TL, Wilson KE, Heymsfield SB 2009 Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS One 4:e7038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl CE, Everhart JE 2001 Leptin concentrations in the United States: relations with demographic and anthropometric measures. Am J Clin Nutr 74:295–301 [DOI] [PubMed] [Google Scholar]
- Ahmad Z, Zackai E, Medne L, Garg A, Early onset mandibuloacral dysplasia due to compound heterozygous mutations in ZMPSTE24. Am J Med Genet, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merideth MA, Gordon LB, Clauss S, Sachdev V, Smith AC, Perry MB, Brewer CC, Zalewski C, Kim HJ, Solomon B, Brooks BP, Gerber LH, Turner ML, Domingo DL, Hart TC, Graf J, Reynolds JC, Gropman A, Yanovski JA, Gerhard-Herman M, Collins FS, Nabel EG, Cannon 3rd RO, Gahl WA, Introne WJ 2008 Phenotype and course of Hutchinson-Gilford progeria syndrome. N Engl J Med 358:592–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg A, Subramanyam L, Agarwal AK, Simha V, Levine B, D'Apice MR, Novelli G, Crow Y 2009 Atypical progeroid syndrome due to heterozygous missense LMNA mutations. J Clin Endocrinol Metab 94:4971–4983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin J, Auer-Grumbach M, Bagg W, Colclough K, Nguyen TD, Fenton-May J, Hattersley A, Hudson J, Jardine P, Josifova D, Longman C, McWilliam R, Owen K, Walker M, Wehnert M, Ellard S 2008 Extreme phenotypic diversity and nonpenetrance in families with the LMNA gene mutation R644C. Am J Med Genet A 146A:1530–1542 [DOI] [PubMed] [Google Scholar]
- Jacob KN, Garg A 2006 Laminopathies: multisystem dystrophy syndromes. Mol Genet Metab 87:289–302 [DOI] [PubMed] [Google Scholar]
- Meng YX, Ford ES, Li C, Quarshie A, Al-Mahmoud AM, Giles W, Gibbons GH, Strayhorn G 2007 Association of C-reactive protein with surrogate measures of insulin resistance among nondiabetic U.S. adults from National Health and Nutrition Examination Survey, 1999–2002. Clin Chem 53:2152–2159 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.