Abstract
Context and Objective: The purpose of the present study was to investigate the cross-sectional and longitudinal associations of serum morning cortisol and aspects of insulin action in Latino children and adolescents (8–13 yr) at risk for type 2 diabetes.
Design and Participants: The present study includes a cross-sectional analysis in 211 participants and a longitudinal analysis in a subset of 143 participants.
Results: At baseline, cortisol was negatively associated with fasting glucose (r = 0.23; P < 0.01), β-cell function (disposition index, r = −0.24; P < 0.05), and acute insulin response to glucose (r = −0.27; P < 0.05). Baseline cortisol was also significantly related to the change in insulin sensitivity over 1 yr (r = −0.23; P < 0.05). These results did not differ by Tanner stage or sex.
Conclusions: Cortisol contributes to the reduction in insulin sensitivity at an early age in Latino children and adolescents. Specifically, cortisol is negatively associated with potential compensatory mechanisms for insulin resistance, such as increased β-cell function and increased insulin release to a glucose challenge, by exacerbating the progression toward insulin resistance in this population. The results underline the relevance of glucocorticoid reduction for the prevention of metabolic disease in Latino children and adolescents.
Cortisol concentrations are associated with a decrease in insulin sensitivity, suggesting a role for cortisol as a target for the prevention of metabolic disease in Latino youth.
Type 2 diabetes has emerged as a significant health issue in American youth, particularly among Latinos, with an estimated lifetime risk of approximately 50% (1). The ethnic disparity of diabetes risk has not been fully examined but likely includes a combination of insulin resistance and the inability of pancreatic β-cells to compensate through increased insulin secretion (2). Compensatory mechanisms to insulin resistance in children and early adolescence appear to be ethnic specific, and previous research from our laboratory has reported increased insulin release in response to an insulin-modified iv glucose challenge as a compensatory mechanism for insulin resistance in Latinos (3). Although this mechanism may be able to maintain the already low levels of insulin sensitivity (SI) in Latino youth in the short term, it may eventually lead to failure of the β-cell and the progression toward type 2 diabetes (3).
In addition to nutrition and physical activity, research has started to include the role of the social environment, particularly psychosocial stress on adiposity and insulin resistance (4). The physiological stress response leads to the release of cortisol, a glucocorticoid, from the adrenal glands. Designed to increase energy availability in the short term, cortisol acutely impairs insulin secretion and increases hepatic glucose output. An environment of prolonged glucocorticoid exposure, i.e. chronic stress, exerts diabetogenic effects by interfering with insulin action on several different levels (5,6,7), including a direct inhibition of insulin secretion from pancreatic β-cells (8), impaired insulin-mediated glucose uptake (9), and disruption of the insulin signaling cascade in skeletal muscle (10). Healthy adults appear able to compensate for glucocorticoid-induced insulin resistance with increased β-cell function or increased insulin release (11,12,13). This is evident even under rather chronic circumstances. In less insulin sensitive or obese individuals, however, those compensatory mechanisms fail to counteract glucocorticoid-induced insulin resistance, resulting in hyperglycemia (12,13).
Although research has demonstrated the negative association between cortisol and SI in adults (14,15), very little research has addressed the question of whether cortisol interferes with SI and potential compensatory mechanisms for insulin resistance in children and adolescents. To assess the effects of cortisol on SI in ethnic minorities is of particular interest, because they are exposed to greater chronic stress and life events (16). The hypercortisolism associated with chronic stress exposure may further compromise the already lower SI and exacerbate the progression to insulin resistance and type 2 diabetes in this population. In support of that idea, previous work from our laboratory has shown an association between higher morning cortisol and the metabolic syndrome in Latino youth (17). Hence, the purpose of the present paper was to investigate the relationship of cortisol with SI, β-cell function [disposition index (DI)], and acute insulin release (AIR), as well as their changes over a 1-yr period in a sample of Latino youth at risk for type 2 diabetes. We hypothesized that cortisol is negatively associated with those variables as well as the change in insulin parameters over a 1-yr period. The results of the present paper will provide additional insight regarding the relevance of stress reduction as a component of preventive measures against obesity and type 2 diabetes in minority youth.
Patients and Methods
Participants
The current analysis includes data from the first two annual visits of 211 participants (119 boys and 92 girls) of the longitudinal, observational SOLAR study (Study of Latino Adolescents at Risk of Diabetes) conducted at the University of Southern California (18). Inclusion criteria for participants were Latino ethnicity, a family history of type 2 diabetes, and a body mass index (BMI) at or above the 85th percentile for age and gender. BMI and BMI percentiles for age and gender were based on established Centers for Disease Control and Prevention normative curves (19).
Participants were between 8 and 13 yr old at study entry. Exclusionary criteria included major illness and medications or a condition known to affect body composition or SI. The study was approved by the Institutional Review Board of the University of Southern California, and informed consent as well as child assent was obtained before testing from parents and children, respectively. After consenting, children completed an oral glucose tolerance test (OGTT) (outpatient visit) to further determine eligibility for the study by excluding the presence of type 2 diabetes. Eligible participants completed an annual frequently sampled iv glucose tolerance test (FSIVGTT) (inpatient visit) establishing SI, β-cell function (DI), and AIR to a glucose challenge. A detailed description of both procedures are described below.
Oral glucose tolerance test
The annual OGTT was performed after an overnight fast in the General Clinical Research Center of the University of Southern California. After arrival at approximately 0800 h, a thorough medical history and physical exam were performed and Tanner stage was determined (20) by a licensed pediatric healthcare provider. Height was measured to the nearest 0.1 cm by a wall-mounted stadiometer, and weight was recorded to the nearest 0.1 kg by a balance-beam medical scale. Oral glucose tolerance was determined with two blood samples taken before (−5 min) as well as 2 h (120 min) after ingestion of 1.75 g oral glucose solution/kg body weight (up to a maximum of 75 g). Blood samples were analyzed for glucose and insulin concentrations. Impaired glucose tolerance was determined according to guidelines of the American Diabetes Association (21).
Intravenous glucose tolerance test
One to 2 wk after the OGTT, eligible participants were readmitted to the General Clinical Research Center in the afternoon. Total fat mass and total lean tissue mass was determined by whole-body dual-energy x-ray absorptiometry (QDR 4500W; Hologic, Bedford, MA) by a small group of trained research technicians blinded to patients treatment.
Waist circumference was measured to determine central adiposity, and waist-to-hip ratio was calculated. After body composition measurements were completed, participants were served dinner. After a 12-h fasting period, the FSIVGTT started at 0630 h the following morning. Intravenous catheters were placed in an anticubital vein of both arms. Two fasting blood samples (−15 and −5 min) were followed by a 0.3 g/kg body weight iv glucose administration at time point 0. Blood samples were taken at 2, 4, 8, and 19 min. At 20 min, iv insulin (0.02 U/kg body weight Humulin R; Eli Lilly, Indianapolis, IN) was administered. The remaining blood samples were taken at 22, 30, 40, 50, 70, 100, and 180 min. SI, AIR to a glucose challenge, and DI as an indicator for β-cell function was calculated with MINMOD Millenium 2003 (22).
The yr 1 fasting blood draw (−15 min) from the iv glucose tolerance test (IVGTT) also included aliquots for serum cortisol, lipids, IGF-I, IGF binding protein 1 (IGFBP-1), and IGF binding protein 3 (IGFBP-3).
Assays
Glucose samples from the OGTT were analyzed by the in vitro hexokinase method (Dade Behring, Deerfield, IL). Blood samples from the FSIVGTT were centrifuged for 10 min at 2500 rpm, and plasma aliquots were frozen at −80 C until additional analysis. Insulin concentrations were measured with a human insulin ELISA (Linco, St. Charles, MO) [intraassay coefficient of variation (CV), 4.7–7%; interassay CV, 9.1–1%]. Glucose concentrations were measured with the glucose oxidase technique (Yellow Springs Instrument analyzer; YIS Inc., Yellow Springs, OH). Morning serum cortisol concentrations were measured with RIA (Siemens, Deerfield, IL) (intraassay CV, 4.69%; interassay CV, 6.28% with minimal detection limit of 0.47 μg/dl). Fasting lipids were measured with Vitros Chemistry DT slides (Johnson & Johnson Clinical Diagnostics, Rochester, NY). IGF-I, IGFBP-1, and IGFBP-3 concentrations were determined using two site coated tube immunoradiometric assay kits (Active Diagnostic System Laboratories, Webster, TX). Samples were assayed in duplicate for IGF-I (intraassay CV, 8.2%; interassay CV, 11.3%), IGFBP-1 (intraassay CV, 6.8%; interassay CV, 11.4%), and IGFBP-3 (intraassay CV, 7.0%; interassay CV, 3.6%).
Statistics
The total sample size of 211 participants was used for descriptive analysis. Mean differences by Tanner stage were assessed with χ2 analysis. Differences by sex were determined with ANOVA. Effects of sex and Tanner stage on the relationship of cortisol and changes in SI were assessed with analysis of covariance, covarying for fat mass and fat-free mass. AIR was log transformed to achieve normality before analysis. Zero-order correlations were used to assess the relationship of cortisol with anthropometric measures, parameters of the OGTT [area under the curve (AUC), impaired fasting glucose, and impaired glucose tolerance], and IVGTT (SI, AIR, DI, and fasted insulin and glucose concentrations).
Change scores in parameters of the OGTT and FSIVGTT were calculated by subtracting yr 1 values from yr 2 values. A total of 143 participants (78 boys and 65 girls) had complete data from yr 1 and yr 2 and therefore were available for analyses, including change scores.
Zero-order correlations were then used to examine the relationship of yr 1 cortisol with the change in OGTT and IVGTT parameters from yr 1 to yr 2. All correlations and cross-sectional and change scores were then reexamined with adjusted partial correlations. Correlations were adjusted for age, sex, total fat mass, and total lean mass. Because of the considerable dropout between yr 1 and yr 2, analyses including change variables were performed, including all participants from yr 1 and yr 2, as well as with participants who had complete data for both years only.
The correlations of cortisol and change scores were additionally adjusted for baseline values. Data were analyzed using SPSS for Windows version 16.0 (SPSS Inc., Chicago, IL). Data in Table 1 are presented as means ± sds. A P value of 0.05 was a priori assigned significance.
Table 1.
Subject characteristics at baseline yr 1 and yr 2 (data presented as means ± sd)
| Boys
|
Girls
|
|||||
|---|---|---|---|---|---|---|
| Year 1 (n = 119) | Year 2 (n = 78) | Change | Year 1 (n = 92) | Year 2 (n = 65) | Change | |
| Tanner stage (1/2/3/4/5) | 85/62/18/30/17 | 27/26/9/10/6 | 33/42/15/32/23 | 6/15/6/21/17 | ||
| Age (yr) | 11.1 ± 1 | 12.1 ± 1 | 1.0 | 10.8 ± 1 | 11.8 ± 1 | 1.0 |
| Height (cm) | 149 ± 11.4 | 156 ± 11.3a | 6.6 ± 2.1 | 147.7 ± 11.9 | 152.9 ± 10.3a | 5.0 ± 2.7c |
| Weight (kg) | 63.2 ± 19.1 | 72.6 ± 20.4a | 9.7 ± 4.9 | 62.9 ± 20.7 | 70.6 ± 20.9a | 7.6 ± 4.2c |
| BMI (kg/m2) | 27.6 ± 5 | 29.3 ± 5a | 3.1 ± 10.1 | 28.0 ± 5.5 | 29.6 ± 5.7a | 5.6 ± 21.2 |
| Total fat mass (kg) | 23.35 ± 9.7 | 25.7 ± 9.4a | 2.5 ± 2.8 | 25.0 ± 10.6 | 28.1 ± 11.1a | 3.1 ± 3.0 |
| Fat mass (%) | 37.5 ± 7 | 36.0 ± 6.6 | −0.91 ± 2.8 | 39.8 ± 5.4 | 39.5 ± 5.1 | 0.32 ± 2.3c |
| Total lean mass (kg) | 36.9 ± 9.5 | 43.0 ± 11.2a | 6.26 ± 3.1 | 35.7 ± 10.3 | 39.8 ± 10.2a | 4.0 ± 2.3c |
| Cortisol (μg/dl) | 9.3 ± 3.2 | 9.7 ± 3.1 | 0.38 ± 3.9 | 9.2 ± 3.2 | 9.6 ± 3.3 | 0.44 ± 3.4 |
| IGF-I | 432.7 ± 263 | 590.1 ± 285 | 159 ± 198a | 533.6 ± 269 | 661 ± 256 | 82.3 ± 199 |
| IGFBP-1 | 18 ± 15.2 | 11.3 ± 11 | −6.7 ± 8.4 | 14.8 ± 15.8 | 8.9 ± 10.6 | −4.6 ± 6.3 |
| IGFBP-3 | 3871 ± 745 | 4509 ± 801 | 622 ± 715 | 4123 ± 848 | 4659 ± 716 | 509 ± 605 |
| Fasting insulin (μU/ml) | 17.2 ± 8.7b | 23.7 ± 12.9a | 6.7 ± 11.2 | 21.3 ± 13.1 | 27.3 ± 17.2a | 5.8 ± 9.4 |
| Fasting glucose (mg/dl) | 93.2 ± 5.9 | 93.8 ± 5.5 | 0.27 ± 6.4 | 92.4 ± 5.8 | 92.1 ± 6.0 | 1.7 ± 6.3 |
| Insulin AUC (μU/ml) | 261.2 ± 138.7b | 300.9 ± 191.2a,b | 50.0 ± 154 | 361.6 ± 246 | 371.7 ± 247 | 11.3 ± 216 |
| Glucose AUC (mg/dl) | 260.3 ± 29.4 | 257.9 ± 27.9 | −2.8 ± 26 | 267.5 ± 32 | 264.4 ± 37.2 | −2.2 ± 33.1 |
| SI [(×10−4/min−1)/(μU/ml)] | 2.3 ± 1.6 | 1.8 ± 1.2a | −0.48 ± 0.96 | 2.1 ± 1.4 | 1.6 ± 1.0a | −0.47 ± 0.81 |
| DI (×10−4/min−1) | 2795 ± 1235b | 2688 ± 1215b | −49.0 ± 1010 | 2231 ± 980 | 2023 ± 994a | −226 ± 864 |
| AIR (μU/ml) | 1663 ± 1233 | 1935 ± 1293a | 281 ± 840 | 1604 ± 1213 | 1681 ± 1236 | 72.4 ± 763 |
Difference between yr 1 and yr 2 for boys or girls significant at P < 0.05.
Difference between gender in the same year significant at P < 0.05.
Change scores are significantly different for gender at P < 0.05.
Results
The 211 participants included 119 male and 92 female participants. Table 1 displays subject participant characteristics separate for gender stratified by sex in yr 1 and yr 2 as well as the change between the two assessments. χ2 tests showed that neither cortisol, SI, DI, log AIR, nor the change from yr 1 to yr 2 were different for Tanner stages, and therefore results are reported for all Tanner stages combined. Sex differences were observed for fasting insulin in yr 1 but not in yr 2 with females having higher fasting insulin compared with males in yr 1. Fasting insulin concentrations increased for both sexes between the two assessments but the increase was not different. DI was higher in males compared with females in both years. However, the change between the 2 yr was not different by sex.
Changes between yr 1 and yr 2 were different by sex for height, weight, percentage fat mass, and total lean mass. None of the changes in insulin-related parameters was different by sex. Therefore, additional analysis was conducted for the whole group with sex as a covariate. Neither cortisol at yr 1 and yr 2 nor the change in cortisol was different by sex. Status of menarche had no effect on baseline SI, cortisol, or change in SI in females.
Cross-sectional analysis of baseline data
From the OGTT, cortisol was negatively associated with the 2-h AUC for glucose (r = 0.15; P < 0.05) but not with the 2-h insulin AUC. Cortisol was significantly higher in those participants with impaired fasting glucose compared with normal fasting glucose (10.9 ± 3.1 vs. 9.1 ± 3.1 μg/dl, respectively; P < 0.05; n = 26 vs. n = 185, respectively) and in those with impaired glucose tolerance (10.4 ± 3.7 vs. 9.0 ± 2.9 μg/dl, respectively, P < 0.01; n = 53 vs. n = 158, respectively).
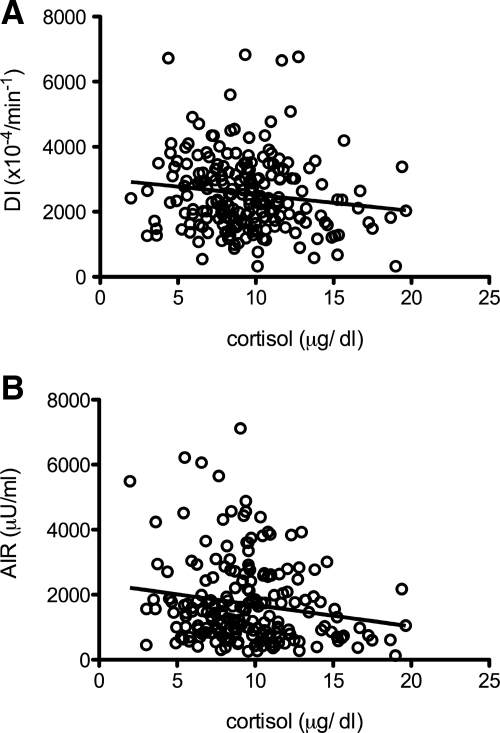
During the IVGTT, cortisol concentrations were associated with fasting glucose concentrations (r = 0.23; P < 0.01) but were not associated with fasting insulin concentrations, lipid concentrations, or SI. Cortisol was negatively associated with DI (r = −0.24; P < 0.05) (Fig. 1)and log AIR (r = −0.27; P < 0.05) (Fig. 1B) in yr 1. An analysis of covariance showed no main effect of sex or Tanner stage for these associations.
Figure 1.
A, B, Zero-order correlation of cortisol with DI (A) (r = −0.13; P < 0.05) and AIR (B) (r = −0.16; P < 0.05).
Longitudinal analysis
Change scores for OGTT parameters were not different by sex or Tanner stage. Insulin AUC significantly increased from yr 1 to yr 2 in males but not in females. Glucose AUC did not change from yr 1 to yr 2. Cortisol was not related to the change in insulin AUC.
Change scores for IVGTT parameters were not different by sex or by Tanner stage. Although fasting glucose did not increase significantly from yr 1 to yr 2, fasting insulin concentrations did increase significantly from yr 1 to yr 2. Cortisol, however, was not associated with the change in fasting insulin. SI decreased significantly from yr 1 to yr 2 for both sexes individually and for the whole group (2.2 ± 1.4 compared with 1.7 ± 1.1; respectively; P < 0.01). AIR increased significantly from yr 1 to yr 2 (1640 ± 1262 compared with 1817 ± 1277, respectively; P < 0.05) for males only. DI significantly decreased between the two annual assessments for females only. However, although the change in DI between the two assessments was not different by sex, DI was significantly higher in sex in yr 1 and yr 2.
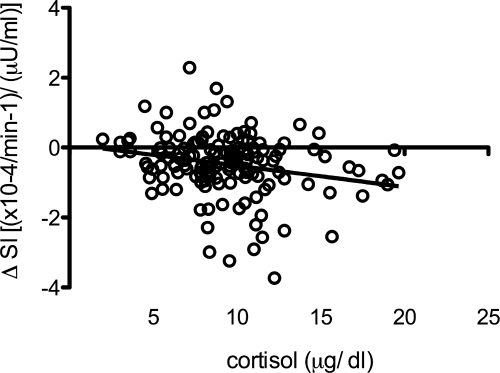
Baseline cortisol concentrations were negatively associated with change in SI from yr 1 to yr 2 (r = −0.23; P < 0.05) (Fig. 2). No relationship was observed between cortisol and the change in log AIR and DI. IGFs were not different for Tanner stage at baseline. Female participants had significantly higher concentrations of IGF-I and IGFBP-3 compared with male participants (432.1 ± 263 vs. 534.1 ± 270, P < 0.01 and 3871 ± 745 vs. 4120 ± 852, P < 0.05, respectively).
Figure 2.
Zero-order correlation of cortisol with change scores of SI (ΔSI) (r = −0.23; P < 0.01).
In adjusted correlations, cortisol was negatively associated with IGF-I (r = −0.15; P < 0.05) and IGFBP-3 (r = 0.18; P < 0.05) and positively with IGFBP-1 (r = −0.18; P < 0.05).
Zero-order and partial correlation coefficients for cortisol, the dependent variables from OGTT and FSIVGTT, as well as changes in the variables from yr 1 to yr 2 are summarized in Table 2. Analyses only including participants with complete data from both years (n = 143) were compared with analyses including all participants from yr 1 (n = 211). Results for both groups were the same. Most relevant, cortisol was still significantly associated with the decline in SI from yr 1 to yr 2, considering participants with measurements from both years only (r = −0.18; P < 0.05).
Table 2.
Zero order and partial correlations for cortisol, anthropometric, and metabolic parameters
| Cortisol (zero order) | Cortisol (partial) | |
|---|---|---|
| Baseline | ||
| Diastolic blood pressure | 0.19 | 0.24 |
| Systolic blood pressure | 0.19 | 0.35a |
| Weight (kg) | −0.12 | 0.08 |
| BMI (kg/m2) | −0.10 | 0.06 |
| Total fat mass (kg) | −0.12 | 0.0 |
| Total lean mass (kg) | −0.11 | −0.08 |
| Waist circumference | −0.11 | −0.03 |
| Hip circumference | −0.09 | 0.01 |
| Fasting insulin (μU/ml) | −0.03 | −0.05 |
| Fasting glucose (mg/dl) | 0.23a | 0.25a |
| IGF-I (ng/ml) | −0.19a | −0.15a |
| IGFBP-1 (ng/ml) | 0.15a | 0.18a |
| IGFBP-3 (ng/ml) | −0.18a | −0.18a |
| SI [(×10−4/min−1)/(μU/ml)] | 0.10 | 0.03 |
| DI (×10−4/min−1) | −0.13a | −0.21a |
| Log AIR (μU/ml) | −0.16a | −0.13a |
| Change (Δ yr 1–yr 2) | ||
| ΔSI [(×10−4/min−1)/(μU/ml)] | −0.23b | −0.17a |
| ΔDI (×10−4/min−1) | 0.08 | 0.06 |
| ΔAIR (μU/ml) | 0.13 | 0.12 |
Shown are zero-order and partial correlations for cortisol. Partial correlations were adjusted for age, gender, Tanner stage, fat mass, and fat-free mass. Correlations with change variables (Δ) were additionally adjusted for baseline values.
, P < 0.05;
, P < 0.01.
Discussion
The present paper demonstrates that serum cortisol is associated with increased fasting glucose concentrations and decreased AIR and β-cell function in overweight Latino youth. Moreover, we found that higher baseline cortisol was related to a greater decrease in SI over a 1-yr follow-up period. Although it has been demonstrated that cortisol affects SI in adults (7,23), the current study shows that cortisol may interfere with SI during adolescence as well.
The relationship between SI and insulin secretion has been described as hyperbolic (22,24). This means that individuals with lower SI compensate with higher insulin secretion to maintain normal glucose tolerance (13,24). Indeed, our laboratory has shown both increased AIR and DI as potential compensatory mechanisms for decreased SI in minority youths (3).
The current study suggests that cortisol may be associated with the decrease in SI in children and adolescents through decreasing β-cell function and the acute insulin response to a glucose stimulus, thus affecting the compensatory route for insulin resistance described above. This finding is further supported by results from research in adult individuals, showing a lack of adaption of islet function in response to a glucocorticoid-induced reduction in SI, specifically in individuals with low inherent SI, comparable with the Latino youths in our study (13). Both decreased AIR and decreased function of the β cell may then contribute to the decrease in SI in our study population, as evidenced in the negative association of cortisol and the change in SI after 1 yr. The present study is, to the best of our knowledge, the first report linking relative hypercortisolemia with indices of SI and a longitudinal change in SI in Latino youth.
Epidemiological and clinical studies in adults have shown that obesity, especially visceral obesity, and related metabolic comorbidities are associated with stress-related conditions (25,26,27). Our laboratory has found a significant relationship between the number of features of the metabolic syndrome and cortisol concentrations in Latino youth (17), suggesting that obesity and the related metabolic disturbances such as insulin resistance may in part result from a maladaptive physiological reaction to environmental stress factors in susceptible individuals (28). Despite the fact that differences in exposure to stress defined by ethnicity and socioeconomic status are systematically underestimated (16), very little research has addressed differences in the physiological stress reaction and their consequences for health in ethnic minorities.
Research in non-Latino white adults has linked obesity and insulin resistance with increased morning cortisol concentrations (29), whereas studies performed in Latino and African-American youth have shown lower morning cortisol and a flatter diurnal cortisol slope compared with their non-Latino white counterparts (4). Because of the one-time sampling of cortisol in the present study, we cannot draw conclusions regarding the overall stress levels in Latino children. It is possible that a maladaptive hypothalamus-pituitary-adrenal (HPA) axis physiology in cortisol slopes across the day may have not been observed in the present study as a result of one-time sampling in the morning. There is no uniformly accepted way of expressing HPA axis activity, and each way has inherent limitations. We previously found that, in obese Latino adolescents aged 14–17 yr, serum cortisol correlates with salivary cortisol awakening response (r = 0.42; P < 0.05) (Weigensberg M. J., E. Bomberg, C. J. Lane, S. Nguyen-Rodriguez, M. I. Goran, Z. Shoar, T. Wright, T. C. Adam, D. Spruijt-Metz, unpublished data), a frequently used measure of HPA activity in the psychoneuroendocrinology literature (for overview, see30). Findings in the literature suggest that even a single morning measurement of serum cortisol, although imperfect, reflects overall HPA activity (31) and justify our conclusions of this relationship, albeit with the stated limitations of a single cortisol measure.
Because cortisol has been generally associated with visceral fat accumulation (17,32), it is of interest that we did not find a relationship between cortisol and indices of visceral fat accumulation in our group of participants. This might be attributable to the age group or to the homogeneity of the group in terms of ethnicity and body weight. Visceral fat accumulation may be a consequence of prolonged glucocorticoid exposure, and the relationship may be present in somewhat older adolescents (33). Cortisol was negatively associated with IGF-I in the present study. IGF-I is an insulin sensitizer and has successfully been used as a surrogate to estimate GH secretion (26) based on the negative feedback relationship between the two. Epidemiological studies have shown that people with low IGF concentrations have a 2-fold increased risk of developing glucose intolerance or type 2 diabetes (34). Considering the physiological changes in the GH/IGF-I axis during puberty, the lack of difference in IGF-I between Tanner stages indicates a derangement of the GH/IGF-I axis in obesity. Our result is supportive of literature reporting an inhibitory effect of obesity on GH secretion, including peripheral factors such as IGF-I (35). IGF-I is mainly derived from the liver, which also is the sole site of splanchnic cortisol production, a fact that suggests a close interaction between cortisol and IGF-I. Although IGF has gained clinical significance predominantly as a mechanism linking obesity to cancer (36), in the present paper it is of particular interest because of its representation of GH concentrations. GH is greatly reduced in obesity and is particularly associated with visceral fat accumulation (32). The present paper would support work suggesting that low GH and high cortisol act in concert to reduce insulin resistance in adolescents (33).
Because of the correlational nature of the present study, we also cannot draw conclusions regarding the direction of the relationship between cortisol and our insulin parameters. However, we do have evidence that chronic stress and the related altered HPA activity are associated with features of the metabolic syndrome in adolescents (37). Elegant animal models have shown a direct inhibitory effect of glucocorticoids on insulin release (38). A causative effect of cortisol on several parameters relevant to SI is also substantiated by research investigating individuals with primary pathological hypercortisolism, e.g. Cushing’s disease (39).
In conclusion, the present study suggests that cross-sectionally, cortisol may affect SI through increased glucose concentrations, decreased β-cell function, decreased AIR, and IGF-I at an early age, contributing to a long-term decrease in SI. These results reinforce the fact that cortisol may play a permissive role for health disparities in metabolic diseases in ethnic minorities. Additional research is necessary to examine possible ethnic-specific stress exposure and more specifically the acute HPA responses to stress in African-American and Latino youth. Results from the present study underline the importance of prevention of chronic glucocorticoid exposure in minority children and adolescents as an additional preventive measure for metabolic disease.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant R01-DK-59211, the General Research Center, National Center for Research Resources/NIH Grant M01 RR 00043, and a 2009–2011 Fellowship in Health Disparities award from Pfizer’s Medical and Academic Partnership program.
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 21, 2010
Abbreviations: AIR, Acute insulin release; AUC, area under the curve; BMI, body mass index; CV, coefficient of variation; DI, disposition index; FSIVGTT, frequently sampled iv glucose tolerance test; HPA, hypothalamus-pituitary-adrenal; IGFBP, IGF binding protein; IVGTT, iv glucose tolerance test; OGTT, oral glucose tolerance test; SI, insulin sensitivity.
References
- Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF 2003 Lifetime risk for diabetes mellitus in the United States. JAMA 290:1884–1890 [DOI] [PubMed] [Google Scholar]
- Goran MI, Lane C, Toledo-Corral C, Weigensberg MJ 2008 Persistence of pre-diabetes in overweight and obese Hispanic children: association with progressive insulin resistance, poor β-cell function and increasing visceral fat. Diabetes 57:3007–3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goran MI, Bergman RN, Cruz ML, Watanabe R 2002 Insulin resistance and associated compensatory responses in African-American and Hispanic children. Diabetes Care 25:2184–2190 [DOI] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG 2007 Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. J Adolesc Health 41:3–13 [DOI] [PubMed] [Google Scholar]
- Adam TC, Epel ES 2007 Stress, eating and the reward system. Physiol Behav 91:449–458 [DOI] [PubMed] [Google Scholar]
- Dallman MF, Strack AM, Akana SF, Bradbury MJ, Hanson ES, Scribner KA, Smith M 1993 Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol 14:303–347 [DOI] [PubMed] [Google Scholar]
- Rosmond R 2003 Stress induced disturbances of the HPA axis: a pathway to Type 2 diabetes? Med Sci Monit 9:RA35–RA39 [PubMed] [Google Scholar]
- Lambillotte C, Gilon P, Henquin JC 1997 Direct glucocorticoid inhibition of insulin secretion. An in vitro study of dexamethasone effects in mouse islets. J Clin Invest 99:414–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderre L, Vallega GA, Pilch PF, Chipkin SR 1996 In vivo effects of dexamethasone and sucrose on glucose transport (GLUT-4) protein tissue distribution. Am J Physiol 271:E643–E648 [DOI] [PubMed] [Google Scholar]
- van Raalte DH, Ouwens DM, Diamant M 2009 Novel insights into glucocorticoid-mediated diabetogenic effects: towards expansion of therapeutic options? Eur J Clin Invest 39:81–93 [DOI] [PubMed] [Google Scholar]
- Beard JC, Halter JB, Best JD, Pfeifer MA, Porte Jr D 1984 Dexamethasone-induced insulin resistance enhances B cell responsiveness to glucose level in normal men. Am J Physiol 247:E592–E596 [DOI] [PubMed] [Google Scholar]
- Grill V, Pigon J, Hartling SG, Binder C, Efendic S 1990 Effects of dexamethasone on glucose-induced insulin and proinsulin release in low and high insulin responders. Metabolism 39:251–258 [DOI] [PubMed] [Google Scholar]
- Larsson H, Ahrén B 1999 Insulin resistant subjects lack islet adaptation to short-term dexamethasone-induced reduction in insulin sensitivity. Diabetologia 42:936–943 [DOI] [PubMed] [Google Scholar]
- Björntorp P 1997 Neuroendocrine factors in obesity. J Endocrinol 155:193–195 [DOI] [PubMed] [Google Scholar]
- Björntorp P, Rosmond R 2000 Obesity and cortisol. Nutrition 16:924–936 [DOI] [PubMed] [Google Scholar]
- Turner RJ, Avison WR 2003 Status variations in stress exposure: implications for the interpretation of research on race, socioeconomic status, and gender. J Health Soc Behav 44:488–505 [PubMed] [Google Scholar]
- Weigensberg MJ, Toledo-Corral CM, Goran MI 2008 Association between the metabolic syndrome and serum cortisol in overweight Latino youth. J Clin Endocrinol Metab 93:1372–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goran MI, Bergman RN, Avila Q, Watkins M, Ball GD, Shaibi GQ, Weigensberg MJ, Cruz ML 2004 Impaired glucose tolerance and reduced β-cell function in overweight Latino children with a positive family history for type 2 diabetes. J Clin Endocrinol Metab 89:207–212 [DOI] [PubMed] [Google Scholar]
- 1998 Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am J Clin Nutr 68:899–917 [DOI] [PubMed] [Google Scholar]
- Tanner JM 1981 Growth and maturation during adolescence. Nutr Rev 39:43–55 [DOI] [PubMed] [Google Scholar]
- 2000 Type 2 diabetes in children and adolescents. American Diabetes Association. Pediatrics 105:671–680 [DOI] [PubMed] [Google Scholar]
- Bergman RN, Phillips LS, Cobelli C 1981 Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and β-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 68:1456–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatruda JM, Livingston JN, Lockwood DH 1985 Cellular mechanisms in selected states of insulin resistance: human obesity, glucocorticoid excess, and chronic renal failure. Diabetes Metab Rev 1:293–317 [DOI] [PubMed] [Google Scholar]
- Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP, et al. 1993 Quantification of the relationship between insulin sensitivity and β-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 42:1663–1672 [DOI] [PubMed] [Google Scholar]
- Overgaard D, Gyntelberg F, Heitmann BL 2004 Psychological workload and body weight: is there an association? A review of the literature. Occup Med (Lond) 54:35–41 [DOI] [PubMed] [Google Scholar]
- Rosmond R, Dallman MF, Björntorp P 1998 Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab 83:1853–1859 [DOI] [PubMed] [Google Scholar]
- Rosmond R, Lapidus L, Björntorp P 1996 The influence of occupational and social factors on obesity and body fat distribution in middle-aged men. Int J Obes Relat Metab Disord 20:599–607 [PubMed] [Google Scholar]
- Björntorp P 1993 Visceral obesity: a “civilization syndrome.” Obes Res 1:206–222 [DOI] [PubMed] [Google Scholar]
- Vicennati V, Pasquali R 2000 Abnormalities of the hypothalamic-pituitary-adrenal axis in nondepressed women with abdominal obesity and relations with insulin resistance: evidence for a central and a peripheral alteration. J Clin Endocrinol Metab 85:4093–4098 [DOI] [PubMed] [Google Scholar]
- Pasquali R, Vicennati V, Cacciari M, Pagotto U 2006 The hypothalamic-pituitary-adrenal axis activity in obesity and the metabolic syndrome. Ann NY Acad Sci 1083:111–128 [DOI] [PubMed] [Google Scholar]
- Dorn LD, Lucke JF, Loucks TL, Berga SL 2007 Salivary cortisol reflects serum cortisol: analysis of circadian profiles. Ann Clin Biochem 44:281–284 [DOI] [PubMed] [Google Scholar]
- Scacchi M, Pincelli AI, Cavagnini F 1999 Growth hormone in obesity. Int J Obes Relat Metab Disord 23:260–271 [DOI] [PubMed] [Google Scholar]
- Misra M, Bredella MA, Tsai P, Mendes N, Miller KK, Klibanski A 2008 Lower growth hormone and higher cortisol are associated with greater visceral adiposity, intramyocellular lipids, and insulin resistance in overweight girls. Am J Physiol Endocrinol Metab 295:E385–E392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu MS, Heald AH, Gibson JM, Cruickshank JK, Dunger DB, Wareham NJ 2002 Circulating concentrations of insulin-like growth factor-I and development of glucose intolerance: a prospective observational study. Lancet 359:1740–1745 [DOI] [PubMed] [Google Scholar]
- Kreitschmann-Andermahr I, Suarez P, Jennings R, Evers N, Brabant G 2010 GH/IGF-I regulation in obesity—mechanisms and practical consequences in children and adults. Horm Res Paediatr 73:153–160 [DOI] [PubMed] [Google Scholar]
- Toledo-Corral CM, Roberts CK, Shaibi GQ, Lane CJ, Higgins PB, Davis JN, Weigensberg MJ, Goran MI 2008 Insulin-like growth factor-I is inversely related to adiposity in overweight Latino children. J Pediatr Endocrinol Metab 21:855–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravaja N, Keltikangas-Järvinen L, Viikari J 1996 Life changes, locus of control and metabolic syndrome precursors in adolescents and young adults: a three-year follow-up. Soc Sci Med 43:51–61 [DOI] [PubMed] [Google Scholar]
- Delaunay F, Khan A, Cintra A, Davani B, Ling ZC, Andersson A, Ostenson CG, Gustafsson J, Efendic S, Okret S 1997 Pancreatic β cells are important targets for the diabetogenic effects of glucocorticoids. J Clin Invest 100:2094–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivonello R, Faggiano A, Lombardi G, Colao A 2005 The metabolic syndrome and cardiovascular risk in Cushing’s syndrome. Endocrinol Metab Clin North Am 34:327–339, viii [DOI] [PubMed] [Google Scholar]