Abstract
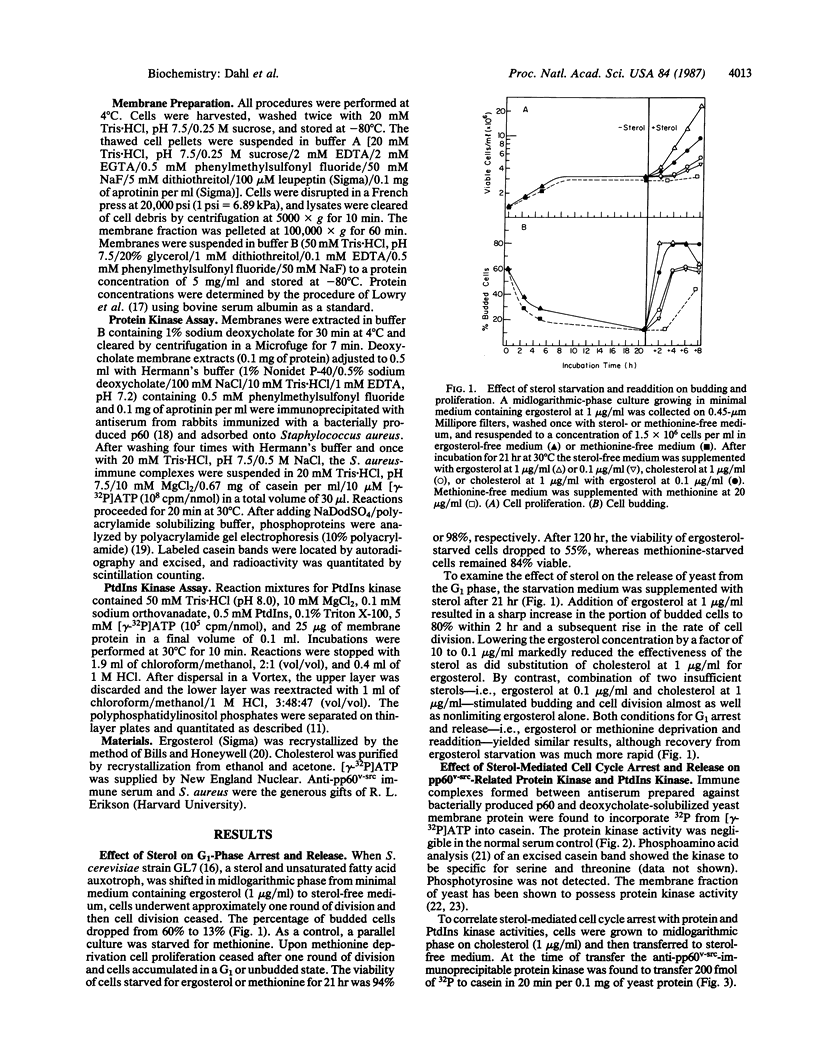
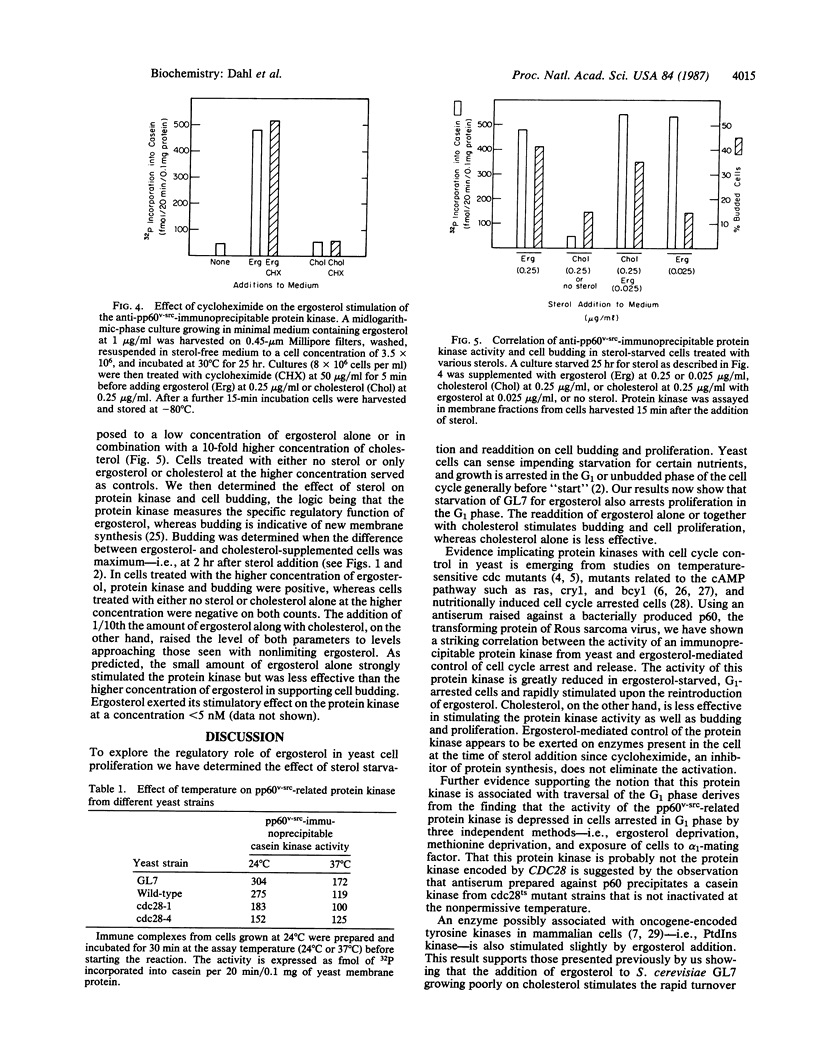
The effects of ergosterol, yeast's natural sterol, on cell cycling and a protein kinase antigenically related to pp60v-src were examined in a sterol auxotroph of Saccharomyces cerevisiae. Sterol-depleted cells accumulate in an unbudded, G1 state. Cell budding and proliferation are reinitiated upon addition of nonlimiting ergosterol or cholesterol with trace ergosterol, whereas cholesterol or trace ergosterol alone is less effective. Stimulation of a protein kinase associated with immune complexes of yeast protein and anti-pp60v-src shows a positive correlation with exit from the G1 phase following ergosterol addition. Ergosterol-stimulated cells also demonstrate an increase in phosphatidylinositol kinase activity. The data suggest that hormonal levels of ergosterol (effective concentration, approximately equal to 1 nM) participate in a signaling process associated with a protein kinase possibly involved in yeast cell cycle control.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos R. M., Mazón M. J. Identification of phosphotyrosine in yeast proteins and of a protein tyrosine kinase associated with the plasma membrane. J Biol Chem. 1985 Jul 15;260(14):8240–8242. [PubMed] [Google Scholar]
- Cerbón J. Relationship between phospholipid biosynthesis and the efficiency of the arsenate transport system in yeasts. J Bacteriol. 1970 Apr;102(1):97–105. doi: 10.1128/jb.102.1.97-105.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl J. S., Dahl C. E., Bloch K. Sterols in membranes: growth characteristics and membrane properties of Mycoplasma capricolum cultured on cholesterol and lanosterol. Biochemistry. 1980 Apr 1;19(7):1467–1472. doi: 10.1021/bi00548a032. [DOI] [PubMed] [Google Scholar]
- Dahl J. S., Dahl C. E. Coordinate regulation of unsaturated phospholipid, RNA, and protein synthesis in Mycoplasma capricolum by cholesterol. Proc Natl Acad Sci U S A. 1983 Feb;80(3):692–696. doi: 10.1073/pnas.80.3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl J. S., Dahl C. E. Stimulation of cell proliferation and polyphosphoinositide metabolism in Saccharomyces cerevisiae GL7 by ergosterol. Biochem Biophys Res Commun. 1985 Dec 31;133(3):844–850. doi: 10.1016/0006-291x(85)91211-2. [DOI] [PubMed] [Google Scholar]
- Feldman D., Do Y., Burshell A., Stathis P., Loose D. S. An estrogen-binding protein and endogenous ligand in Saccharomyces cerevisiae: possible hormone receptor system. Science. 1982 Oct 15;218(4569):297–298. doi: 10.1126/science.6289434. [DOI] [PubMed] [Google Scholar]
- Feldman D., Tökés L. G., Stathis P. A., Miller S. C., Kurz W., Harvey D. Identification of 17 beta-estradiol as the estrogenic substance in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4722–4726. doi: 10.1073/pnas.81.15.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmer T. M., Erikson R. L. Development of anti-pp60src serum with antigen produced in Escherichia coli. J Virol. 1983 Jan;45(1):462–465. doi: 10.1128/jvi.45.1.462-465.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollub E. G., Liu K. P., Dayan J., Adlersberg M., Sprinson D. B. Yeast mutants deficient in heme biosynthesis and a heme mutant additionally blocked in cyclization of 2,3-oxidosqualene. J Biol Chem. 1977 May 10;252(9):2846–2854. [PubMed] [Google Scholar]
- Hartwell L. H., McLaughlin C. S. Temperature-sensitive mutants of yeast exhibiting a rapid inhibition of protein synthesis. J Bacteriol. 1968 Nov;96(5):1664–1671. doi: 10.1128/jb.96.5.1664-1671.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Whitman M., Schaffhausen B., Raptis L., Garcea R. L., Pallas D., Roberts T. M., Cantley L. Phosphatidylinositol metabolism and polyoma-mediated transformation. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3624–3628. doi: 10.1073/pnas.83.11.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T., Powers S., Cameron S., Fasano O., Goldfarb M., Broach J., Wigler M. Functional homology of mammalian and yeast RAS genes. Cell. 1985 Jan;40(1):19–26. doi: 10.1016/0092-8674(85)90304-6. [DOI] [PubMed] [Google Scholar]
- Kawasaki S., Ramgopal M., Chin J., Bloch K. Sterol control of the phosphatidylethanolamine-phosphatidylcholine conversion in the yeast mutant GL7. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5715–5719. doi: 10.1073/pnas.82.17.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lörincz A. T., Reed S. I. Primary structure homology between the product of yeast cell division control gene CDC28 and vertebrate oncogenes. Nature. 1984 Jan 12;307(5947):183–185. doi: 10.1038/307183a0. [DOI] [PubMed] [Google Scholar]
- Macara I. G., Marinetti G. V., Balduzzi P. C. Transforming protein of avian sarcoma virus UR2 is associated with phosphatidylinositol kinase activity: possible role in tumorigenesis. Proc Natl Acad Sci U S A. 1984 May;81(9):2728–2732. doi: 10.1073/pnas.81.9.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Ishikawa T. Genetic analysis of the role of cAMP in yeast. Yeast. 1985 Sep;1(1):15–24. doi: 10.1002/yea.320010103. [DOI] [PubMed] [Google Scholar]
- McKenzie M. A., Carman G. M. Membrane-associated phosphatidylinositol kinase from Saccharomyces cerevisiae. J Bacteriol. 1983 Oct;156(1):421–423. doi: 10.1128/jb.156.1.421-423.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramgopal M., Bloch K. Sterol synergism in yeast. Proc Natl Acad Sci U S A. 1983 Feb;80(3):712–715. doi: 10.1073/pnas.80.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. I., Hadwiger J. A., Lörincz A. T. Protein kinase activity associated with the product of the yeast cell division cycle gene CDC28. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4055–4059. doi: 10.1073/pnas.82.12.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R. J., Taylor F. R., Parks L. W. A requirement for ergosterol to permit growth of yeast sterol auxotrophs on cholestanol. Biochem Biophys Res Commun. 1982 May 31;106(2):435–441. doi: 10.1016/0006-291x(82)91129-9. [DOI] [PubMed] [Google Scholar]
- Simanis V., Nurse P. The cell cycle control gene cdc2+ of fission yeast encodes a protein kinase potentially regulated by phosphorylation. Cell. 1986 Apr 25;45(2):261–268. doi: 10.1016/0092-8674(86)90390-9. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y., Erikson R. L. Phosphatidylinositol kinase activities in normal and Rous sarcoma virus-transformed cells. Mol Cell Biol. 1985 Nov;5(11):3194–3198. doi: 10.1128/mcb.5.11.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y., Whitman M., Cantley L. C., Erikson R. L. Evidence that the Rous sarcoma virus transforming gene product phosphorylates phosphatidylinositol and diacylglycerol. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2117–2121. doi: 10.1073/pnas.81.7.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talwalkar R. T., Lester R. L. The response of diphosphoinositide and triphosphoinostitide to perturbations of the adenylate energy charge in cells of Saccharomyces cerevisiae. Biochim Biophys Acta. 1973 Jun 21;306(3):412–421. doi: 10.1016/0005-2760(73)90180-x. [DOI] [PubMed] [Google Scholar]
- Tatchell K. RAS genes and growth control in Saccharomyces cerevisiae. J Bacteriol. 1986 May;166(2):364–367. doi: 10.1128/jb.166.2.364-367.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp M. L., Piñon R., Meisenhelder J., Hunter T. Identification of phosphoproteins correlated with proliferation and cell cycle arrest in Saccharomyces cerevisiae: positive and negative regulation by cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5973–5977. doi: 10.1073/pnas.83.16.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]




