Summary
Background
Enhanced platelet activation in HIV-1 infected patients has been reported and shown to strongly correlate with plasma viral load. Activated platelets are known to express and to release a variety of proteins that can modulate the immune system. Specifically, platelet derived CD154 has shown to be directly involved in the development of autoimmune thrombocytopenia (ITP). The mechanism by which HIV-1 infection leads to platelet activation and the effect of this activation on the development of HIV-1 ITP, however, is not fully understood.
Objective
We have investigated the effect of HIV-1 Tat on platelet activation.
Results
We report that HIV-1 Tat directly interacts with platelets and induces platelet activation resulting in platelet micro-particle release. This activation by Tat requires the chemokine receptor CCR3 and β3-integrin expression on platelets, as well as calcium flux. Tat-induced activation of platelets releases platelet CD154, an immune modulator. Enhanced B cell activity is found in mouse spleen B cells co-cultured with platelets treated with Tat in vitro. An early antibody response against adenovirus is found in Tat injected mouse immunized with adenovirus suggesting an enhanced immune response in vivo.
Conclusions
We have described a role of Tat-induced platelet activation in the modulation of the immune system, with implications for the development of HIV-1 associated thrombocytopenia.
Introduction
HIV-related thrombocytopenia (HIV-1-ITP) occurs in 15–60% of patients with AIDS and is seen in approximately 10% of patients during HIV infection.[1] The pathogenesis of HIV-1-ITP is not completely understood, although it appears that the etiology of HIV-1-ITP in the early and the late stages of infection are different. HIV-1-ITP occurring during the early stage of infection is largely due to immune destruction of platelets by autoantibody.[2] In its late stage, HIV-1-ITP is likely due to defective thrombopoiesis resulting from HIV-induced impaired bone marrow production.[1, 2] We have previously identified peptides derived from HIV-1 proteins (Nef, GP120) which cross react with an autoantibody generated against platelet integrin GPIIIa49-66, and thus demonstrated a role for molecular mimicry in the induction of the dominant autoantibody found in HIV-1-ITP.[3] We have also shown that anti-GPIIIa49-66 antibody-induced platelet fragmentation requires a specific conformational change of the GPIIIa integrin.[4] While we have a better understanding of the generation of anti-GPIIIa49-66 antibody and how it induces platelet fragmentation, the mechanism by which HIV-1 infection induces a hyperactive immune response and leads to the generation of autoantibody against platelet is still ambiguous.[5, 6] Platelet activation has been reported in HIV-1 patients [7, 8]. Since activated platelets release CD154 and elevated CD154 has been correlated with the development of autoimmune thrombocytopenia, we investigated the role of platelet activation in the development of HIV-1 ITP.[9, 10]
Upon activation, platelets not only play a critical role in thrombotic events but are also involved in inflammatory reaction, angiogenesis, and immune responses.[11, 12] They also play an important role in the immune response by releasing considerable quantities of chemokines and cytokines as well as expressing a multitude of immune receptors on their membrane.[11]
Among these platelet-derived molecules, CD154 has been intensively investigated due to its important role in regulating the B cell response. CD154 is a trimeric transmembrane protein of the tumor necrosis factor family present on many different cell types.[13] Studies on the cellular distribution of CD154 indicate that 95% of the circulating CD154 is derived from platelets.[14, 15] CD154 is cryptic in resting platelets but is rapidly presented on the platelet surface after platelet activation.[11, 16–18] The surface-expressed CD154 is subsequently cleaved over a period of minutes to hours, generating a soluble fragment termed sCD154 that remains trimeric.[15] B-cell activation relies on the interaction between CD154 (CD40 ligand) and CD40. Interaction of CD154 with its receptor CD40 on B cells is essential for B-cell proliferation, differentiation, isotype switching, memory B-cell generation, and germinal center formation.[13, 19] As little as a twofold change of CD154 in serum has been shown to have a profound effect on B cell activity.[20] It has been suggested that the presence of high level of CD154 is associated with the development of autoimmune diseases.[9, 10, 21, 22] Blockade of the CD40/CD154 interaction can block the production of autoantibodies and induce generation of autoantigen-specific anergic CD4+ T cells.[22–25]
It is therefore conceivable that over-expression or release of platelet associated CD154 may lead to immune dysregulation. In fact, platelet associated CD154 has been reported to be involved in the development of non-HIV associated autoimmune thrombocytopenia (ITP).[9, 10] However, the role of platelet associated CD154 in HIV-1 associated autoimmune diseases and the effect of HIV infection on platelet activity have not been examined.
HIV-1 Tat (Trans Activating Factor) encodes a viral regulatory protein with multifunctional activities.[26, 27] Tat interacts with a variety of receptors both on the surface and inside the cells including the integrin αVβ3 and the chemokine receptors CCR1, CCR3, CCR4, and CXCR4. The interaction of Tat with the integrin occurs through the RGD (Arg-Gly-Asp) motif in Tat’s C-terminal domain while its interactions with chemokine receptors are mediated through CCF/Y, SYXR motifs located between Tat’s cysteine-rich region domain and basic domain.[28–31] It has been reported that Tat interacts with mast cells through CCR3 and may contribute to high IgE level in HIV-1-infected patients.[32] CCR3 is expressed on platelets as well as mast cells[33, 34], but it is not known whether Tat is able to activate platelets through CCR3. In this study, we thus investigated the interaction between Tat and platelets and whether this interaction is able to activate platelets and modulate B cell immune response.
Materials and Methods
Materials
All reagents were obtained from Sigma (St. Louis, MO) unless otherwise designated. HPLC purified HIV-1 Tat protein is from Immunodiagnostics, Inc (Scottsdale, AZ). CD154−/−mice, β3−/−mice with C57BL/6J background, C57BL/6J and BALB/c wild type mice were obtained from Jackson Laboratory. The genotype of knockout mice was confirmed by PCR. The animal protocol was approved by the New York University Medical Center Institutional Review Board. The following reagents were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: pcDNA3.1+/tat101-flag from Dr. Eric Verdin and pGEX2T-Tat from Dr. Andrew Rice. Control plasmids pGEX4T-2 was from GE Healthcare (Piscataway, NJ 08855) and pET28b-Adamts18-66 was previously constructed in the lab[35]. CCR3 inhibitor SB328437 is from Calbiochem (San Diego, CA). CCL5 (Recombinant Mouse Rantes) is from Shenandoah Biotechnology, Inc (Warwick, PA). The adenovirus vector was kindly provided by Dr.Chuanju Liu from Hospital for Joint Diseases of New York University Medical Center. Retrovirus vector MSCV-puro is from Clontech (Mountain View, CA).
Tat cloning, expression and purification
Tat cloning
Two clone vectors have been used to express TAT in both E. coli and eukaryotic cells respectively. The Tat gene (86aa) contained in a pGEX2T-Tat plasmid was subcloned into the expression vector pET28b and the Tat gene (101aa) from pcDNA3.1+/tat101-flag plasmid was subcloned into retroviral vector MSCV-puro.
Tat expression and purification
Rosetta BL21 (DE3) competent cells were transformed with pGEX2T-Tat and pET28b-Tat. Bacterial clones were cultured in 2YT medium until A600 reached 0.3–0.6. The expression of GST-Tat was induced by IPTG (Isopropyl-β-D-thio-galactoside). GST-Tat was purified using GST purification modules (GE Healthcare). Trace contamination of endotoxin was removed by Endotoxin Removing Gel (Thermo Scientific, Rockford, IL). Control proteins GST from pGEX4T-2 and His-Ad18-66 from pET28b-Adamts18-66 were prepared parallel with Tat protein to ensure no artifact due to contamination.
Flow Cytometry Assay of platelet activation
Gel-filtered human or mouse platelets were prepared from platelet-rich plasma obtained from blood collected in 0.38% sodium citrate via heart puncture. 1 × 107 gel-filtered platelets were incubated under different stimulation conditions at 37°C for 30 minutes. For in vitro platelet activation, Tat 20ng/ml, CCL5 10µg/ml, or control Ad18-66 20ng/ml were used. To test the role of chemokine receptor CCR3, calcium flux and cAMP in Tat-induced platelet activation, CCR3 inhibitor SB328437 3nM, calcium flux inhibitor EGTA 100µM and prostaglandin E1 (PGE1, a cAMP elevator) 5µM were used separately to treat platelets at 37°C for 30 minutes before incubation with Tat, CCL5 or control as above. To test the role of β3 integrin, platelets from β3 integrin knock out mice were used to incubate with Tat, CCL5, thrombin, and control. Treated platelets were then stained with anti-CD154-FITC and anti-CD62P-PE antibody on ice for one hour. Fluorescent-labeled platelets were measured by flow cytometry (FACScan, Becton-Dickinson, CA).
In vivo Tat expression stimulate platelet activation
Transfection of 4T1 cells with MSCV-Tat and control MSCV-TatR
MSCV-Tat and MSCV-TatR (reversed insertion Tat as control) pseudo-retrovirus were generated and concentrated by ultracentrifugation as described.[36, 37] 4T1 cells (a carcinoma cell line syngeneic with BALB/c mouse) were infected with MSCV-Tat or MSCV-TatR pseudo-retrovirus in 35mm dishes. Cells were selected with puromycin (3µg/ml, InvivoGen). Positive 4T1 cells were collected to confirm the expression of Tat by both RT-PCR and Western Blot.
Injection 4T1 cells and pseudo-retrovirus to BALB/c mcie
5 × 106 positive 4T1 cells (transfected with MSCV-Tat or MSCV-TatR) were injected into BALB/c mice by intraperitoneal injection. 24 hours after the injection, and platelets were collected in Tyrode’s buffer for flow cytometry assay as above.
Concentrated MSCV-Tat or MSCV(control) pseudo-retrovirus particles were injected into mice via tail vein to mimic HIV infection. Platelets were collected for flow cytometry assay two weeks after three times injection. Serum level of Tat was examined by Western Blot.
Sera were collected from both 4T1 cell line and pseudo-retrovirus injected mice. The titer of soluble IgG1 and IgG2b were detected with anti-mouse IgG1-HRP and anti-mouse IgG2b-HRP mAb (Santa Cruz Biotech, Inc, Santa Cruz, CA) by ELISA.
Tat interaction with platelets
S35 labeling of Tat
S35-methionine labeled Tat and control protein were translated from pET28b/Tat and luciferase T7 control DNA plasmids using an in vitro translation kit (TNT® Coupled Reticulocyte Lysate Systems, Promega, Madison, WI 53711) following the manufacturer’s protocol. The S35-Tat or S35-luciferase proteins were incubated with 1 × 107 platelets for 30 minutes at room temperature. Platelets were then washed with PBS three times to remove unbound protein. The total protein of the platelets was extracted and resolved on SDS-PAGE gel. The film was exposed to the dried gel at 4°C for two weeks to detect binding of Tat to platelets.
GST-Tat pulling down of β3 integrin
Tat interaction with the β3 integrin was determined by incubating purified GST-Tat protein or GST protein (5µg) with the protein lysates from 1 × 108 platelets 2 hours at 4°C. Glutathione-conjugated beads were used to pull down the GST-Tat or GST. The total proteins were resolved on the SDS-PAGE gel and transferred to PVDF membranes for western blotting to detect β3 integrin protein.
Measure CD154 release in Tat-treated platelets
To assess Tat-induced platelet CD154 release, fresh isolated mouse platelets were incubated with Tat or control Ad18-66. Supernatant were collected and concentrated for Western blot to detect CD154.
Electron Microscopy
1 × 107 gel-filtered mouse platelets platelets were incubated with Tat 20ng/ml or Ad18-66 20ng/ml at 37°C for 30 minutes, followed by fixation in an equal volume of 0.1% glutaraldehyde in White saline.[38] After fixation and dehydration, the platelet sample was embedded in an Epon/Aarldite resin mixture. Sample sections were cut using an ultramicrotome and mounted on copper grids for EM images in the Imagine Core Facility of Skirball Institute of Biomolecular Medicine at NYU.
In vitro induction of B cell activity
Splenic B cells were isolated from RBC-depleted splenocytes of CD154−/− mice and cultured at 1 × 106 cell/ml in 10% FCS-RPMI 1640 with 0.05mM 2-ME, plus IL-4 (100U/ml) and IL-10 (100ng/ml). 1 × 108 platelets treated with Tat 20ng/ml or control Ad18-66 20ng/ml was then added into the splenic B cell culture. Cells were collected on day 5 and double stained with anti-mouse IgG1-FITC mAb or anti-mouse IgG2b-FITC mAb and anti-mouse CD45R-PE mAb (BD Biosciences, San Jose, CA) for analysis of surface Ig expression on a FACSCalibur flow cytometry (BD Biosciences, San Jose, CA).[39]
Antibody generation and B cell activity in vivo
Immunization of Balb/c mice with Adenovirus
5 × 106 293-HEK cells were infected with adenoviruses (AdEasy adenoviral vector system, Stratagene, Santa Clara, CA). Adenovirus infected cells were frozen and thawed three times to collect supernatant for virus. Viruses were inactivated by heating to 100°C for 10 minutes before mixing with an equal volume of mineral oil for immunization. BALB/c mice were injected with Tat (50ng) or control Ad18-66 (50ng) in 100 PBS through the tail before immunization with 100ul of antigen mixture (about 1 × 108 inactive viruses) by intraperitoneal injection. Tat and control Ad18-66 were injected every two days and the immunization was boost every four days until day 12. Serum from each mouse was collected from mouse tail every two days to monitor the anti-adenovirus antibody titer by ELISA.
Flow cytometry of B cell from BALB/c mcie
To examine B cell activity in vivo, BALB/c mice were immunized and treated with Tat and control Ad18-66 as above. B cells were collected from the spleen at day 6. Purified B cells were double stained with anti-mouse IgG1-FITC or anti-mouse IgG2b-FITC mAb and anti-mouse CD45R-PE mAb and analyzed with a FACSCalibur flow cytometry.
Results
Tat activates platelets
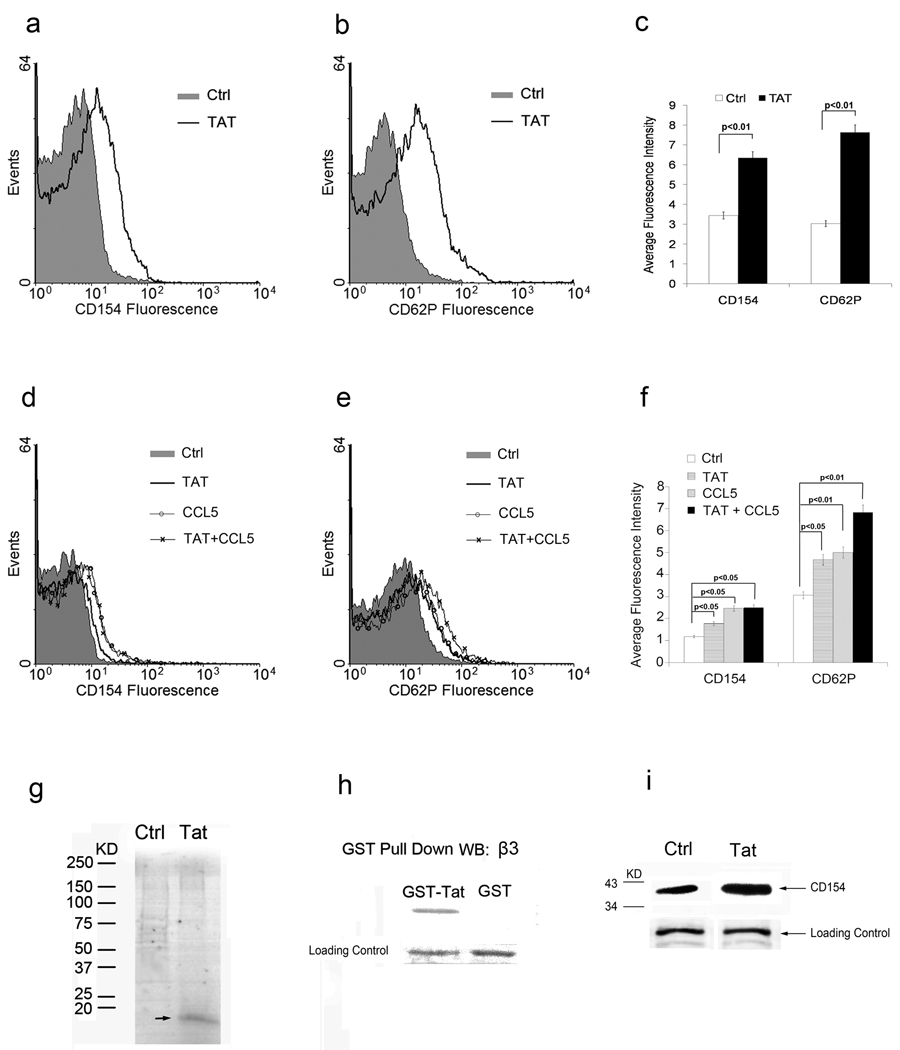
Tat has been reported to mimic the ligand of chemokine receptor CCR3 and to activate monocyte. Since platelets also express CCR3, we tested whether Tat is able to interact with CCR3 on the platelet surface and activate platelets. The concentration of Tat found in the serum of many HIV-1 positive patients is 2 ng to 40 ng/ml.[40] We therefore treated platelets with 20ng/ml and measured platelet activation by assessing the expression of the well-described platelet activation markers CD62P and CD154 by flow cytometry. As shown in Figure 1(a,b,c), Tat is able to induce platelet activation as compared to the control protein Ad18-66. To confirm that CCR3 is involved in Tat-induced platelet activation we then compared Tat- induced platelet activation with platelet activation induced by the CCR3 ligand chemokine CCL5. Tat and CCL5 induce similar platelet activation while additional CD62P expression was found in the Tat plus CCL5 group (Figure 1 d,e,f).
Figure 1. Tat induces human platelet activation.
Histogram of flow cytometry analysis of the platelets treated with Tat or Ad18-66 (Ctrl, filled histogram) with CD154 (a) or CD62P (b). Histogram is an example of one of three experiments. Bar graph summary of Tat induced platelet activation and CD154 expression (c). Both Tat and CCL5 induce mouse platelet activation. Histogram of flow cytometry analysis of the platelets treated with Tat, CCL5 or Ad18-66 (Ctrl, filled histogram). Histogram is an example of one of three experiments. Summary of Tat and CCL5 induced mouse platelet activation (f). Tat interacts with platelets through integrin β3. Platelets incubated with S35- Tat or S35-luciferase in PBS for 30 minutes at 37°C, washed and lysed for total protein were resolved on a SDS-PAGE gel (g). Western blot analysis for β3 with GST-Tat or GST pulls down protein from platelet lysates (h). Western blot analysis of CD154 release in supernatant of platelets incubated with Tat or with PBS buffer as control (i).
Tat interacts with platelet integrin β3 to release CD154
Tat has previously been reported to interact with integrin αVβ3 by Tat’s RGD motif.[28, 29] To test whether Tat is able to interact with platelet integrin β3, S35-methionine labeled Tat or Luciferase proteins were incubated with human platelets. Unbound proteins were removed by washing with PBS. Platelets were then lysed to obtain total proteins and resolved by SDS-PAGE for autoradiography. As shown in Figure 1g, a 14 KDa band Tat band was resolved in the gel, whereas luciferase was not, suggesting that Tat specifically interacts with platelets. To further confirm that Tat is able to interact with integrin β3, GST-Tat was expressed in E coli. and purified with glutathione-conjugated beads. Total protein was extracted from platelets and was incubated with GST-Tat or GST protein follwed by protein immunoprecipitation with glutathione-conjuagted beads. The protein samples were subjected for Western Blot to detect integrin β3 (Figure 1h). A strong integrin β3 band was found in the GST-Tat pull down proteins but not in the GST protein pull down sample, suggesting that Tat can interact with integrin β3 on the platelets. In addition, elevated CD154 levels were found in the supernatant of platelets incubated with Tat, suggesting that Tat not only induces the expression of CD154 on the surface of platelets but also promotes the release of soluble CD154 (Figure 1i).
Tat- induced platelet activation requires integrin β3 and CCR3
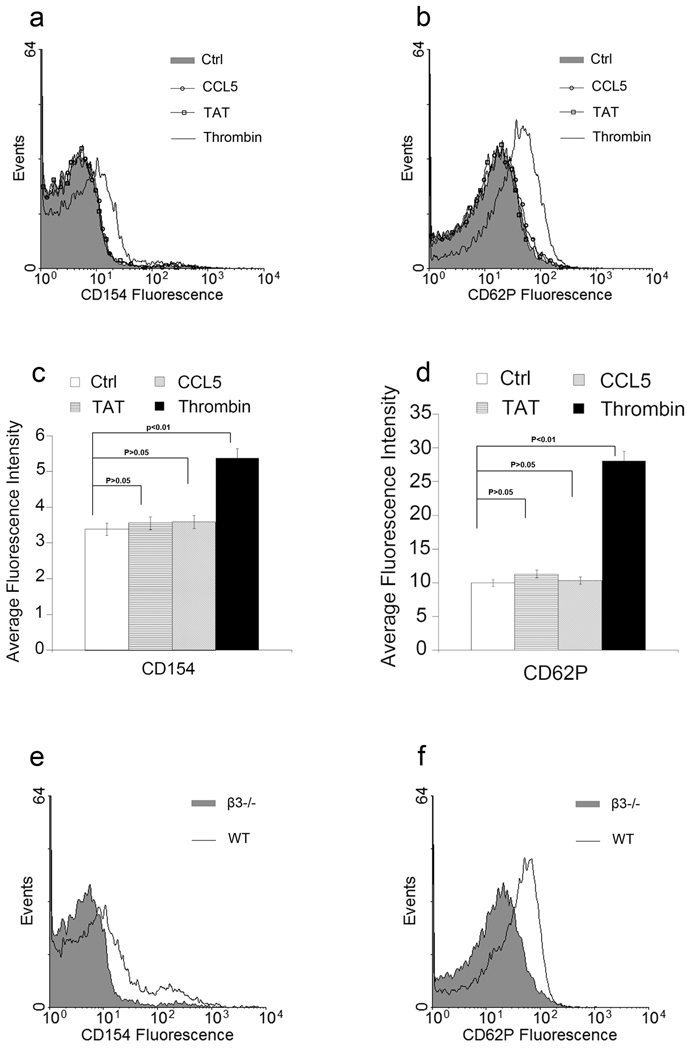
Since Tat is able to interact with both β3 integrin and CCR3 on platelets, we next examined the role of integrin β3 and CCR3 in Tat-induced platelet activation. Gel-filtered platelets from integrin β3 knockout mice (β3−/−) were treated with Tat, CCL5 and thrombin. Flow cytometry analysis revealed lack of both CCL5 and Tat-induced platelet activation in integrin β3 deficient mouse platelets. In contrast, thrombin still induces platelet activation suggesting that Tat and CCL5 induced platelet activation requires integrin β3 but thrombin does not (Figure 2, Supplemental Figure 1).
Figure 2. Tat-induced platelet activation is integrin β3 dependent.
Histogram of flow cytometry analysis of platelets treated with Tat, CCL5, Thrombin and Ad18-66 (Ctrl, filled histogram) with CD154 (a) or CD62P (b). Histogram is an example of one of three experiments. Platelets from integrin β3 knockout mice were employed. Summary of flow cytometry result of Tat with integrin β3 knock out platelets (c, d). Histogram of flow cytometry analysis of platelets for CD154 (e) or CD62P (f) from integrin β3 knockout mice compared with platelets from wild type mice, both were treated with Tat.
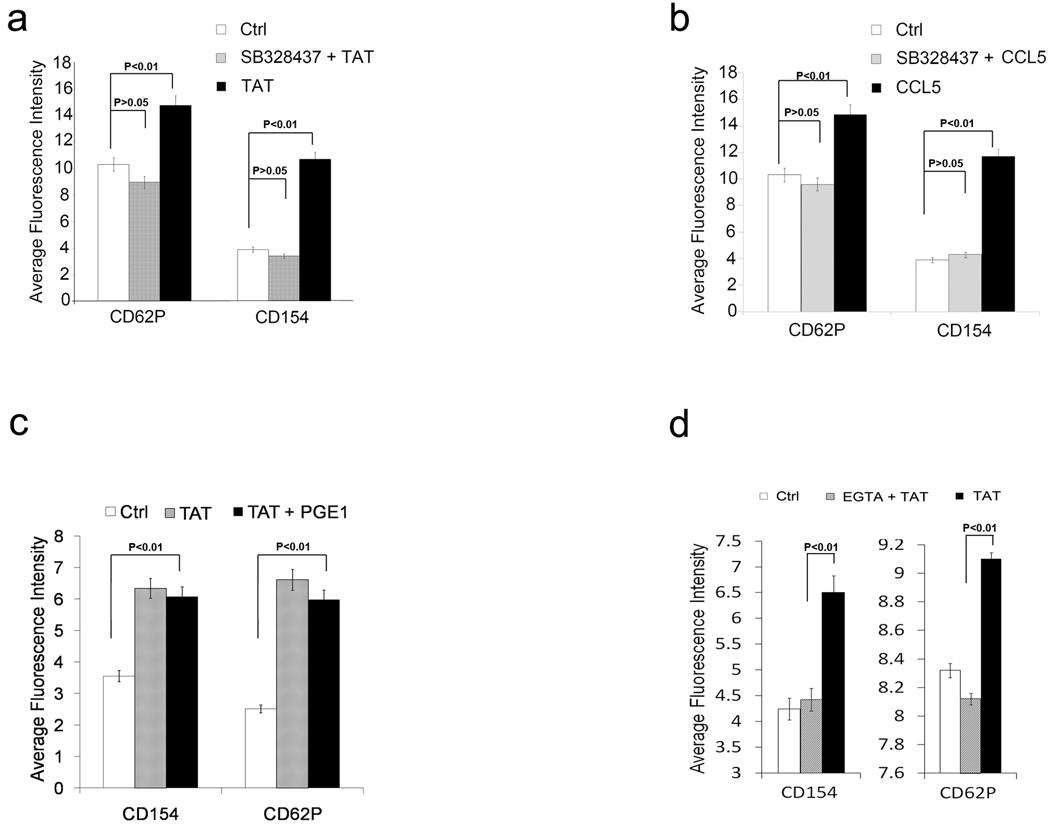
To examine the role of CCR3 in Tat-induced platelet activation, platelets were incubated with the CCR3 inhibitor SB328437 prior to exposure to Tat. SB328437 completely inhibited both Tat and CCL5 induced platelet activation but did not inhibit thrombin-induced platelet activation suggesting the CCR3 is required for Tat-induced platelet activation but not required for thrombin-induced platelet activation (Figure 3 a,b, Supplemental Figure1).
Figure 3. CCR3 is required for Tat induced platelet activation.
Bar graphs summary of flow cytometry analysis of platelets treated with Tat with or without CCR3 inhibitor SB328437 compared with Ad18-66 (Ctrl) (a, n=6), with CCL5 with or without CCR3 inhibitor SB328437 (b, n=6), with Tat with or without PGE1 compared (c, n=6), with Tat with or without Calcium flux inhibitor EGTA (d, n=3).
Tat induces platelet activation is calcium flux dependent but cAMP independent
Since both calcium flux and cAMP are implicated in the regulation of platelet activation by a variety of agonists, we next examined their roles in Tat-induced platelet activation. Tat-treated platelets were incubated with and without prostaglandin E1 (PGE1) 5µM, a cAMP elevator, and analyzed by flow cytometry (Figure 3c). No significant inhibition was found with PGE1 suggesting that the effect of cAMP on Tat-induced platelet activation is not significant while PGE1 partially inhibited CCL5-induced platelet activation (Supplemental Figure 2). However, the calcium chelator EGTA completely inhibited both Tat and CCL5 induced platelet activation suggesting the role of calcium flux in Tat-induced platelet activation and a minor difference between Tat-induced platelet activation and CCL5-induced platelet activation (Figure 3d, Supplemental Figure 2).
Tat expression cell line and MSCV-Tat virus induce platelet activation in vivo
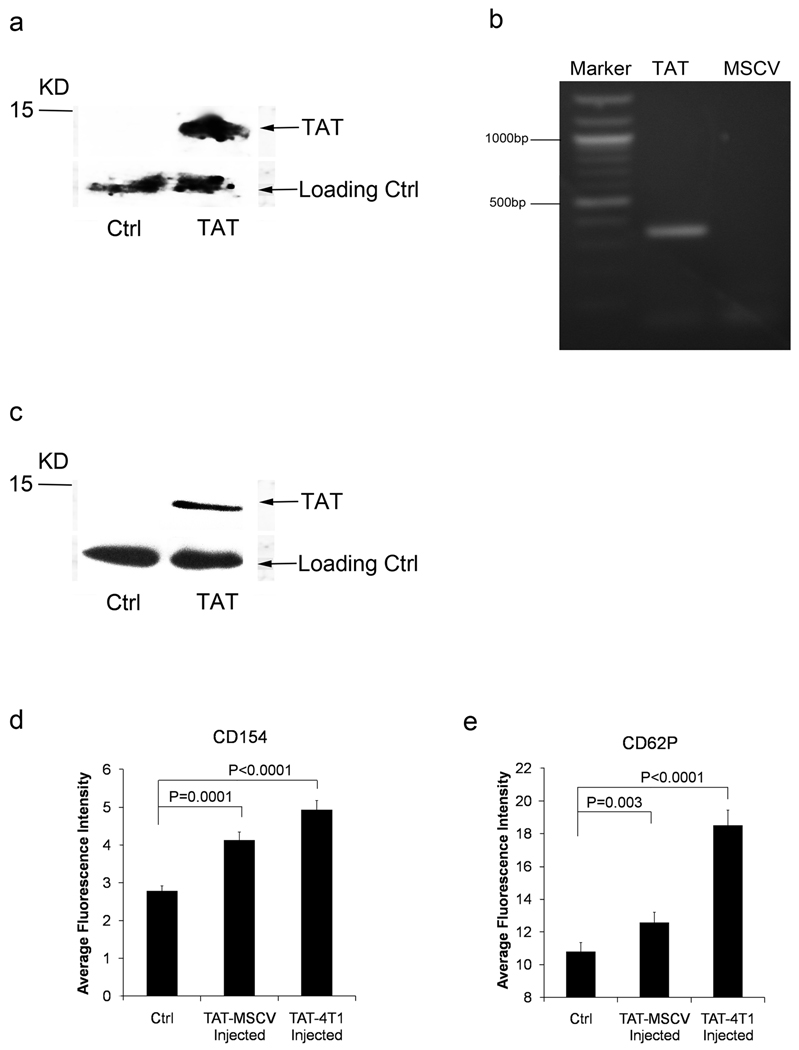
Although we have shown that the Tat protein is able to induce platelet activation and release CD154, it is not clear whether platelet activation as seen in HIV-1 infected patients is indeed due to the Tat expression. To determine whether Tat is able to induce platelet activation in vivo, we investigated the effect of Tat expressing cells and a Tat retrovirus on platelet activation in BALB/c mice. 4T1 cells (a carcinoma cell line syngeneic with BALB/c mouse) were transfected with full length Tat expression retrovirus (MSCV-Tat) or reverse insert Tat (MSCV-TatR) as control. Tat expressing 4T1 cells or control 4T1 cells were injected intraperitoneally into BALB/c mice. Gel-filtered platelets were prepared at 24 hours, and platelet activation was assessed by flow cytometry. To mimic HIV-1 infection, MSCV-Tat retrovirus or MSCV retrovirus as control were also directly injected into BALB/c mice via tail vein every three days. Platelet activation was assessed by flow cytometry two weeks after the first injection of virus. We confirmed the expression of Tat in 4T1 cells by Western Blot and RT-PCR (Figure 4 a,b). Tat protein was elevated in the serum of MSCV-Tat infected BALB/c mice (Figure 4 c). Both 4T1-Tat cells and MSCV-Tat retrovirus induced platelet activation in vivo as demonstrated by (Fig 4 d,e). suggesting that Tat released by HIV-1 infected cells is able to induce platelet activation in vivo.
Figure 4. Tat expression cells induce platelet activation in vivo.
Western Blot to detect the Tat expression in TAT-4T1 cells (a). RT-PCR of Tat with RNA from TAT-4T1 cells. Specific Tat band 359bp was found with the MSCV-TAT cells (left lane), MSCV cells (right lane) (b). Western Blot to detect the Tat expression in serum of mouse injected with TAT-MSCV (c). Summary of flow cytometry analysis of CD154 expression (d) and platelet activation (CD62P) (e) induced by TAT-MSCV retrovirus or by TAT-4T1 cell line injection (n=3).
Tat-activated platelets are able to induce B activation through platelet associated CD154 both in vitro and in vivo
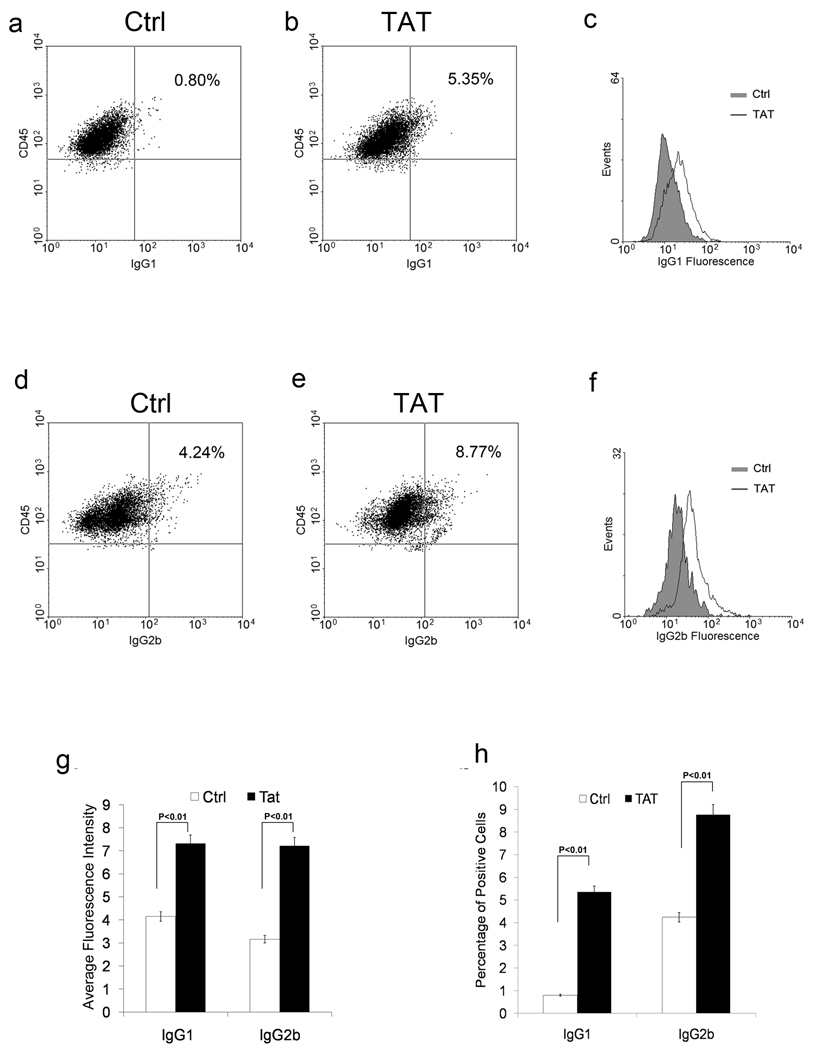
Since elevated CD154 correlates with the development of ITP [9, 10] and activated platelets are known to release CD154, we next examined whether Tat-induced platelet-associated CD154 contributes to the development of autoantibody by promoting immunoglobulin production in B cells. To avoid endogenous CD154 from T cells, CD154−/− mouse spleen cells were co-cultured with wild type platelets (CD154+/+) pretreated with Tat. Parallel co-cultures were setup with an Ad18-66 control protein. The surface IgG1 and IgG2b positive B cell population were then examined by flow cytometry (Figure 5). A significant increase of subpopulation of IgG1 (Figure 5 a,b,c,g) and IgG2b (Figure 5 d,e,f,h) positive B cells was found in spleen cells co-cultured with platelets treated with Tat compared to those treated with Ad18-66.
Figure 5. Tat activated platelets enhance the B cell activity in vitro.
An example of flow cytometry analysis of splenic B cells co-cultured with platelets with Tat or Ad18-66 (Ctrl). The population with high expression of CD45 was gated and the percentages with positive IgG1 (a,b,c) and IgG2b (d,e,f) are shown. Bar-graph summary of flow data for average fluorescent intensity (g) and percentage of positive cells (h) (n=6, p<0.05 for both).
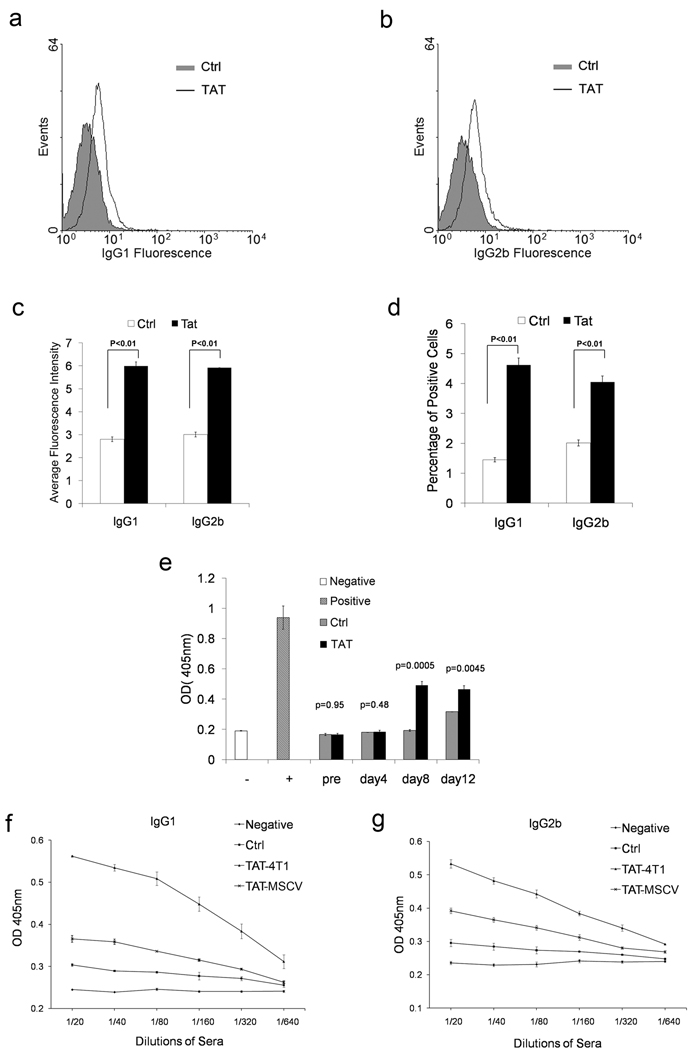
We next examined whether Tat-induced platelet activation and up-regulation of CD154 can effect immunoglobulin expression in vivo. Approximately a two-fold increase in IgG1 and IgG2b positive cells were found in 4T1-Tat cells injected mice (Figure 6 a, b). Serum levels of both IgG1 and IgG2b were increased approximately two-fold in both MSCV-Tat virus and 4T1-Tat cells injected mice (Figure 6 c, d). Thus, our data suggests that Tat-induced platelet CD154 enhances B cell activity in vitro and in vivo.
Figure 6. Tat activated platelets enhance the B cell activity in vivo.
Bar-graph summary of flow cytometry result of positive IgG1 and IgG2b cells in peripheral blood from TAT expression 4T1 cells injected BALB/c mice for average fluorescent intensity (a) and percentage of positive cells (b) (n=6, p<0.05 for both). ELISA results of IgG1 (c) and IgG2b (d) in serum from TAT-MSCV virus and TAT-4T1 cells injected BALB/c mice (n=3). Histogram of flow cytometry analysis of splenic B cells from BALB/c mice injected with Tat or Ad18-66 (Ctrl) for IgG1 (e) and IgG2b (f). Enhanced immune response was found in mice treated with Tat. BALB/c mice were injected with Tat or Ad18-66 (Ctrl) protein through intravenous injection before immunization with Adenovirus. The generation of antibody against Adenovirus was monitored by ELISA (n=3) (g).
Enhanced B cell antibody response was found in Tat-treated mouse immunized with adenovirus
To assess the role of Tat-induced platelet activation in the B cell immune response in vivo, BALB/c mice were immunized with adenovirus and intravenously injected with Tat. Serum was collected at various time points. Positive IgG1 and IgG2b cells were monitored by flow cytometry and the titer of anti-adenovirus antibody was tested by ELISA. An increase of positive IgG1 and IgG2 cell population was found in mice injected with Tat (Figure 6 e, f). An early antibody response to adenovirus was seen in the group of mice injected with Tat at day 8 compared with the control mice at day 12 (Figure 6g). Thus, our data suggest that Tat-induced platelet activation and release of CD154 could be involved in the early antibody response and potentially contribute the HIV-1 autoimmune disease, such as autoimmune thrombocytopenia.
Electron microscopy (EM) of microparticles released by TAT- activated platelets
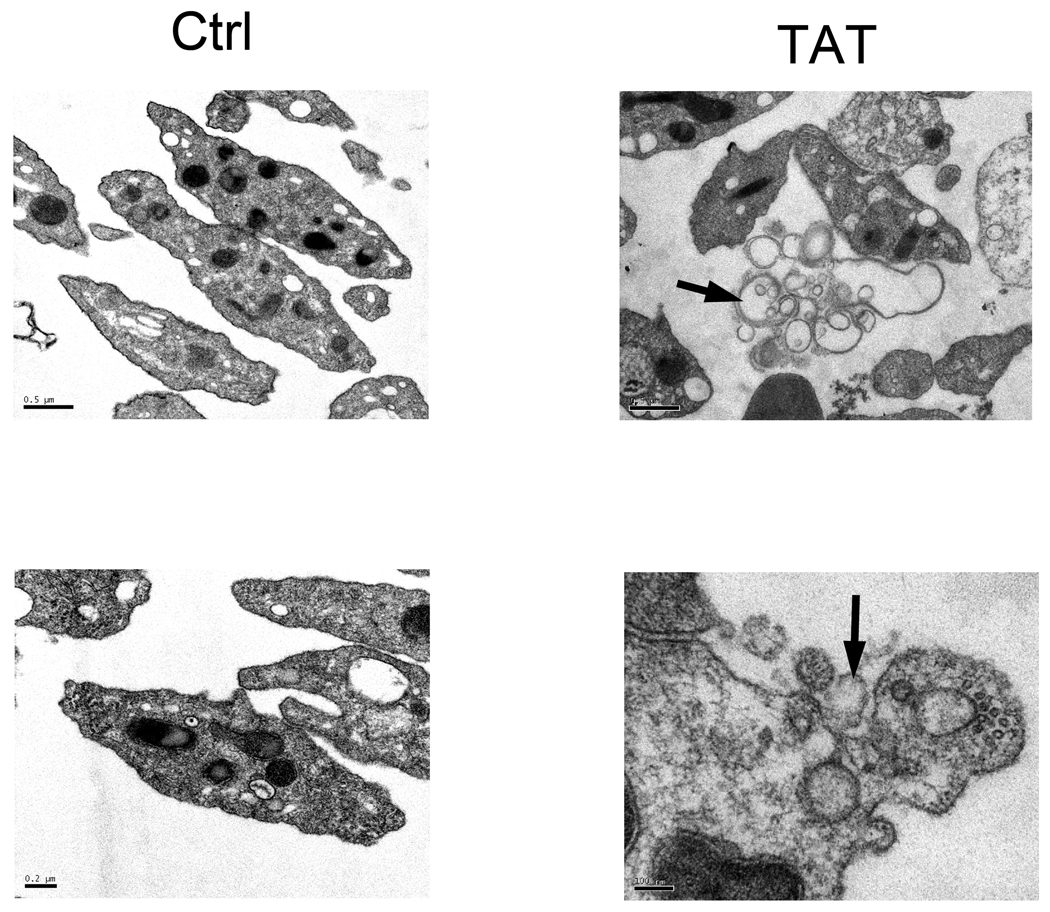
The release of platelet-derived microparticles (PMPs) is a sign of platelet activation. Chronic platelet activation leads to high level of PMPs in peripheral blood and is associated with cardiovascular diseases.[41] It has been shown recently that CD154 in PMPs is sufficient to facilitate the augmentation of adenovirus-specific antibodies.[42] To test whether Tat is able to induce the release of PMPs in platelets, platelets were treated with Tat or Ad18-66 before fixation for electron microscopy. A significant number of PMP buds were seen at the plasma membrane of Tat-activated platelets (Figure 7). Thus, Tat is able to induce platelet activation and to release PMPs.
Figure 7. Electron Microscopy of platelets treated with Tat or control.
Left upper panel: Control, low magnification; Right upper panel: Tat, low magnification. Left bottom panel: Control, high magnification. Right bottom panel: Tat, high magnification. The release of platelet-derived micro-particles (PMPs) (upper right) and the PMP buds at the plasma membrane of Tat activated platelets (bottom right) are indicated by arrow.
Discussion
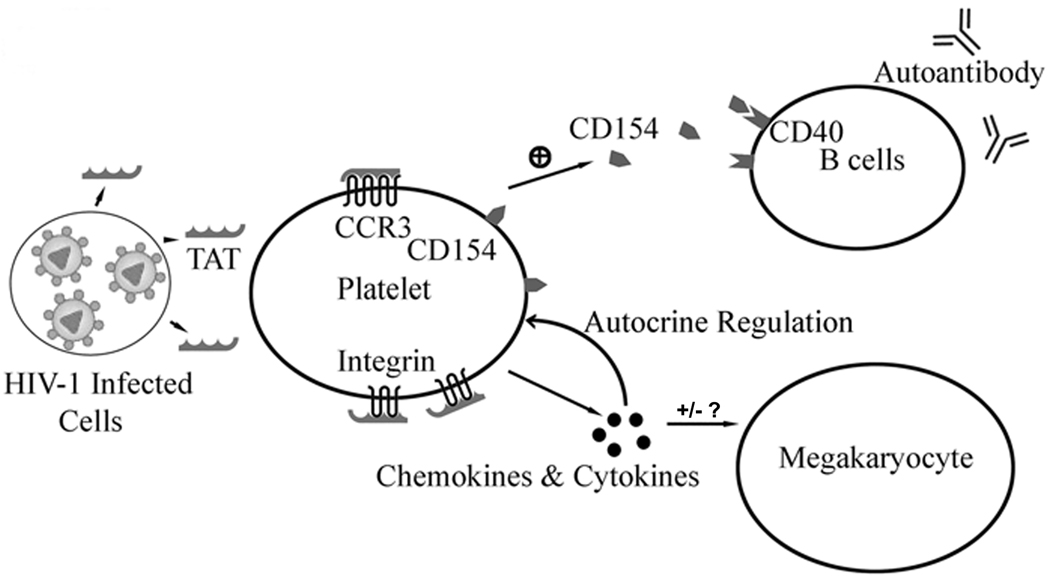
Two mechanisms may be involved in the HIV-1 induced platelet activation. One is via deregulated chemokines and cytokines. An alternative mechanism involves HIV derived protein(s) directly interacting with platelets (Figure 8). HIV infected cells release cellular or HIV viral proteins (such as Tat or Nef) that effect bystander cells [26, 43]. The extra-cellular functions of Tat and Nef have been studied intensively in several cell types. Tat is not only able to mimic the CCR3 ligand but also the integrin ligand with its RGD motif. Tat binds to integrin αVβ3 with an affinity similar to fibrinogen and vitronectin with dissociation constants (Kd) of 32nM (Tat), 27nM (fibrinogen) and 64nM (vitronectin) respectively.[29] Due to the unique character of Tat protein, we examined the interaction between Tat and platelet and its potential role in HIV-1 ITP. Our data demonstrate that Tat induced platelet activation requires both chemokine receptor CCR3 and integrin β3 (Figures 2 and 3). In addition, while calcium flux is required for Tat-induced platelet activation (Figure 3j,k,l), PGE1 does not inhibit Tat-induced platelet activation suggesting a limited role for cAMP in this process (Figure 3g,h,i). Thus, we have described a new role of Tat on platelet activation. It is likely that Tat released by HIV infected cells (such as CD4+ T cells in the spleen) is able to first activate the platelets through CCR3 and then further activate platelets through integrin β3. Tat-activated platelets release platelet-derived micro-particle (PMPs) containing CD154, and PMPs may increase the activity of B cells and contribute to autoimmune response.
Figure 8. Model of the role of HIV-1 induced platelet activation in HIV-1 ITP.
Tat is expressed by HIV-1 infected cells and activates platelets through chemokine receptor CCR3 and integrin β3. The activated platelets release CD154 and other chemokines and cytokines. CD154 enhance B cell activity and contribute to autoimmune response to platelet. Platelet derived chemokines and cytokines may also affect the megakaryopoiesis.
CD154 can play opposing roles in HIV infection.[24] On one hand, it promotes the immune response to HIV. On the other hand, it can activate CD4+ T cells through the activation of dendritic cells and macrophages to help HIV replication. In fact, it has been shown that macrophages stimulated by CD154 release sCD23 and sICAM to render T cells permissive to HIV-1 infection.[24, 44, 45] Thus, it is possible platelet released CD154 is required for effective viral replication. Since anti-Tat antibodies frequently develop in HIV-infected patients, it needs to be determined whether anti-Tat antibody has any effect on Tat-induced platelet activation in HIV-infected patients.[46, 47]
Antiretroviral therapy (ART) improves the thromboyctopenina while frequently inducing platelet activation in HIV-infected patients. Although the mechanism of ART-induced platelet activation is different from Tat-induced platelet activation, ART is frequently accompanied by increased cardiovascular disease and myocardial infarction in HIV-infected patients.[48] Thus, ART-induced platelet activation represents another aspect of HIV associated platelet activation and needs to be studied further.[49]
Platelet agonists can be broadly grouped into two groups depending on their effectiveness in inducing platelet activation: strong agonists capable of inducing stable aggregation and weak agonists causing brief, unstable aggregation requiring a second agonist to induce complete aggregation. Thrombin and ADP are classic strong agonists, whereas epinephrine and serotonin are examples of weak agonists. Chemokines induce weak platelet activation. We found that Tat- as well as CCL5-induced platelet activation require both CCR3 and integrin β3, suggesting a synergy of two receptor signals may be required for these two weak platelet receptors. Since a variety of platelet agonists only induces weak platelet activation, it is not clear what the biological significance of such activation is. We demonstrate here that one consequence is the presentation of CD154, which can have important immunomodulator effects. Whether the property of moderate platelet activation to serve as a source of additional reactive molecules (chemokines, cytokines etc.) represents another important aspect of platelet function is yet to be determined.
In conclusion, we have demonstrated that: 1. Tat is able to induce the release of platelet CD154 and microparticles. 2. Tat-induced platelet activation requires chemokine receptor CCR3 and β3 integrin. 3. Tat-induced platelet activation is cAMP independent and calcium flux dependent. 4. Tat expression cell line 4T1 and TAT-MSCV retrovirus induce platelet activation in vivo. 5. Platelet derived CD154 stimulates B cell activity by enhancing Ig production. Therefore, our data suggest that Tat-induced platelet CD154 may play a role in and contribute to the development of HIV-1 ITP. Additional studies will help us understand better the implications of HIV-1 Tat protein and platelet activation in the development of HIV-1 ITP.
Supplementary Material
Histogram of flow cytometry analysis thrombin induced platelet activation in β3−/− platelets pre-treated CCR3 inhibitor SB328437 and control (nil). (n=3) (a). Summary of flow cytometry result of thrombin induced platelet activation in β3−/− platelets pre-treated CCR3 inhibitor SB328437 and control (nil) (b).
Flow cytometry analysis of platelets treated with CCL5 with or without PGE1 or EGTA. Control (nil) n=3. Histogram of flow cytometry analysis CD154 (a) and CD62P (b).
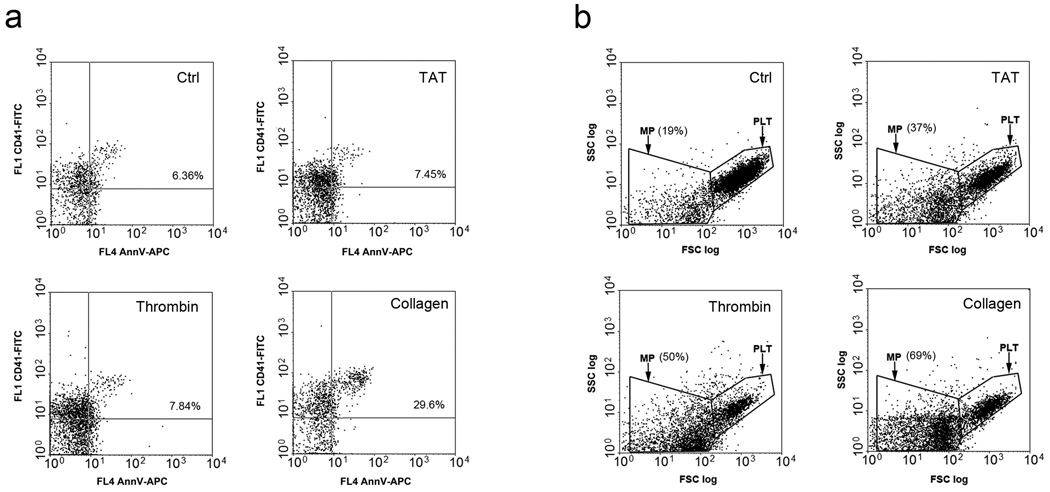
Reference Figure 1. Tat induces microparticles release from platelets.
Dot plot of flow cytometry analysis of platelets incubated with control (buffer), Tat 0.5µg/ml, thrombin (1U/ml), collagen (1mg/ml) 37°C one hour. a. Dot plot of gated microparticles staining with CD41 and Annexin V (AnnV), percentages of double positive mircoparticles are shown. b. Dot plot of platelets and microparticles gated with CD41. Percentages of microparticles are shown.
Acknowledgments
This study is supported by grants DA020816 and DA004315 of the National Institute of Health. We thank Dr. Simon Karpatkin for reading the manuscript and his suggestions, and Dr. Lawrence Gardner for help with the retrovirus package. Electromicroscopy data were obtained with the assistance of Dr. Fengxia Liang in Imagine Core Facility of Skirball Institute of Biomolecular Medicine.
Footnotes
Authorship
J.W. performed the in vitro platelet co-culture and in vivo platelet activation experiments; M.N. helped for the human platelet activation assay; W.Z. helped to set the in vitro platelet co-culture experiment; Z.L. conceived the idea and performed cDNA cloning, protein purification and testing of Tat.
Conflict of Interest Disclosure
The authors declare no competing financial interests.
References
- 1.Bierling P, Bettaieb A, Oksenhendler E. Human immunodeficiency virus-related immune thrombocytopenia. Semin Thromb Hemost. 1995;21:68–75. doi: 10.1055/s-2007-1000380. [DOI] [PubMed] [Google Scholar]
- 2.Najean Y, Rain JD. The mechanism of thrombocytopenia in patients with HIV infection. J Lab Clin Med. 1994;123:415–420. [PubMed] [Google Scholar]
- 3.Li Z, Nardi MA, Karpatkin S. Role of molecular mimicry to HIV-1 peptides in HIV-1-related immunologic thrombocytopenia. Blood. 2005;106:572–576. doi: 10.1182/blood-2005-01-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z, Nardi MA, Wu J, Pan R, Zhang W, Karpatkin S. Platelet fragmentation requires a specific structural conformation of human monoclonal antibody against beta3 integrin. J Biol Chem. 2008;283:3224–3230. doi: 10.1074/jbc.M705902200. [DOI] [PubMed] [Google Scholar]
- 5.Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev. 2002;1:329–337. doi: 10.1016/s1568-9972(02)00086-1. [DOI] [PubMed] [Google Scholar]
- 6.Levy JA. HIV pathogenesis: knowledge gained after two decades of research. Adv Dent Res. 2006;19:10–16. doi: 10.1177/154407370601900104. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad R, Iannello A, Samarani S, Morisset R, Toma E, Grosley M, Ahmad A. Contribution of platelet activation to plasma IL-18 concentrations in HIV-infected AIDS patients. AIDS. 2006;20:1907–1909. doi: 10.1097/01.aids.0000244217.46445.45. [DOI] [PubMed] [Google Scholar]
- 8.Holme PA, Muller F, Solum NO, Brosstad F, Froland SS, Aukrust P. Enhanced activation of platelets with abnormal release of RANTES in human immunodeficiency virus type 1 infection. FASEB J. 1998;12:79–89. doi: 10.1096/fasebj.12.1.79. [DOI] [PubMed] [Google Scholar]
- 9.Meabed MH, Taha GM, Mohamed SO, El-Hadidy KS. Autoimmune thrombocytopenia: flow cytometric determination of platelet-associated CD154/CD40L and CD40 on peripheral blood T and B lymphocytes. Hematology. 2007;12:301–307. doi: 10.1080/10245330701383957. [DOI] [PubMed] [Google Scholar]
- 10.Solanilla A, Pasquet JM, Viallard JF, Contin C, Grosset C, Dechanet-Merville J, Dupouy M, Landry M, Belloc F, Nurden P, Blanco P, Moreau JF, Pellegrin JL, Nurden AT, Ripoche J. Platelet-associated CD154 in immune thrombocytopenic purpura. Blood. 2005;105:215–218. doi: 10.1182/blood-2003-07-2367. [DOI] [PubMed] [Google Scholar]
- 11.Elzey BD, Sprague DL, Ratliff TL. The emerging role of platelets in adaptive immunity. Cell Immunol. 2005;238:1–9. doi: 10.1016/j.cellimm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Elzey BD, Tian J, Jensen RJ, Swanson AK, Lees JR, Lentz SR, Stein CS, Nieswandt B, Wang Y, Davidson BL, Ratliff TL. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity. 2003;19:9–19. doi: 10.1016/s1074-7613(03)00177-8. [DOI] [PubMed] [Google Scholar]
- 13.Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2001;58:4–43. doi: 10.1007/PL00000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andre P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation. 2002;106:896–899. doi: 10.1161/01.cir.0000028962.04520.01. [DOI] [PubMed] [Google Scholar]
- 15.Henn V, Steinbach S, Buchner K, Presek P, Kroczek RA. The inflammatory action of CD40 ligand (CD154) expressed on activated human platelets is temporally limited by coexpressed CD40. Blood. 2001;98:1047–1054. doi: 10.1182/blood.v98.4.1047. [DOI] [PubMed] [Google Scholar]
- 16.Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 17.Sprague DL, Sowa JM, Elzey BD, Ratliff TL. The role of platelet CD154 in the modulation in adaptive immunity. Immunol Res. 2007;39:185–193. doi: 10.1007/s12026-007-0074-3. [DOI] [PubMed] [Google Scholar]
- 18.von Hundelshausen P, Weber C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ Res. 2007;100:27–40. doi: 10.1161/01.RES.0000252802.25497.b7. [DOI] [PubMed] [Google Scholar]
- 19.Elzey BD, Grant JF, Sinn HW, Nieswandt B, Waldschmidt TJ, Ratliff TL. Cooperation between platelet-derived CD154 and CD4+ T cells for enhanced germinal center formation. J Leukoc Biol. 2005;78:80–84. doi: 10.1189/jlb.1104669. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Melgosa M, Hollenbaugh D, Wilson CB. Cutting edge: CD40 ligand is a limiting factor in the humoral response to T cell-dependent antigens. J Immunol. 1999;163:1123–1127. [PubMed] [Google Scholar]
- 21.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 22.Toubi E, Shoenfeld Y. The role of CD40-CD154 interactions in autoimmunity and the benefit of disrupting this pathway. Autoimmunity. 2004;37:457–464. doi: 10.1080/08916930400002386. [DOI] [PubMed] [Google Scholar]
- 23.Kuwana M, Nomura S, Fujimura K, Nagasawa T, Muto Y, Kurata Y, Tanaka S, Ikeda Y. Effect of a single injection of humanized anti-CD154 monoclonal antibody on the platelet-specific autoimmune response in patients with immune thrombocytopenic purpura. Blood. 2004;103:1229–1236. doi: 10.1182/blood-2003-06-2167. [DOI] [PubMed] [Google Scholar]
- 24.Kornbluth RS. The emerging role of CD40 ligand in HIV infection. J Leukoc Biol. 2000;68:373–382. [PubMed] [Google Scholar]
- 25.Kuwana M, Kawakami Y, Ikeda Y. Suppression of autoreactive T-cell response to glycoprotein IIb/IIIa by blockade of CD40/CD154 interaction: implications for treatment of immune thrombocytopenic purpura. Blood. 2003;101:621–623. doi: 10.1182/blood-2002-07-2157. [DOI] [PubMed] [Google Scholar]
- 26.Pugliese A, Vidotto V, Beltramo T, Petrini S, Torre D. A review of HIV-1 Tat protein biological effects. Cell Biochem Funct. 2005;23:223–227. doi: 10.1002/cbf.1147. [DOI] [PubMed] [Google Scholar]
- 27.Noonan D, Albini A. From the outside in: extracellular activities of HIV Tat. Adv Pharmacol. 2000;48:229–250. doi: 10.1016/s1054-3589(00)48008-7. [DOI] [PubMed] [Google Scholar]
- 28.Urbinati C, Bugatti A, Giacca M, Schlaepfer D, Presta M, Rusnati M. alpha(v)beta3-integrin-dependent activation of focal adhesion kinase mediates NF-kappaB activation and motogenic activity by HIV-1 Tat in endothelial cells. J Cell Sci. 2005;118:3949–3958. doi: 10.1242/jcs.02518. [DOI] [PubMed] [Google Scholar]
- 29.Urbinati C, Mitola S, Tanghetti E, Kumar C, Waltenberger J, Ribatti D, Presta M, Rusnati M. Integrin alphavbeta3 as a target for blocking HIV-1 Tat-induced endothelial cell activation in vitro and angiogenesis in vivo. Arterioscler Thromb Vasc Biol. 2005;25:2315–2320. doi: 10.1161/01.ATV.0000186182.14908.7b. [DOI] [PubMed] [Google Scholar]
- 30.de Paulis A, De Palma R, Di Gioia L, Carfora M, Prevete N, Tosi G, Accolla RS, Marone G. Tat protein is an HIV-1-encoded beta-chemokine homolog that promotes migration and up-regulates CCR3 expression on human Fc epsilon RI+ cells. J Immunol. 2000;165:7171–7179. doi: 10.4049/jimmunol.165.12.7171. [DOI] [PubMed] [Google Scholar]
- 31.Albini A, Ferrini S, Benelli R, Sforzini S, Giunciuglio D, Aluigi MG, Proudfoot AEI, Alouani S, Wells TNC, Mariani G, Rabin RL, Farber JM, Noonan DM. HIV-1 Tat protein mimicry of chemokines. Proc Natl Acad Sci U S A. 1998;95:13153–13158. doi: 10.1073/pnas.95.22.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marone G, Florio G, Petraroli A, Triggiani M, de Paulis A. Human mast cells and basophils in HIV-1 infection. Trends Immunol. 2001;22:229–232. doi: 10.1016/s1471-4906(01)01903-2. [DOI] [PubMed] [Google Scholar]
- 33.Gear AR, Camerini D. Platelet chemokines and chemokine receptors: linking hemostasis, inflammation, and host defense. Microcirculation. 2003;10:335–350. doi: 10.1038/sj.mn.7800198. [DOI] [PubMed] [Google Scholar]
- 34.Clemetson KJ, Clemetson JM, Proudfoot AE, Power CA, Baggiolini M, Wells TN. Functional expression of CCR1, CCR3, CCR4, and CXCR4 chemokine receptors on human platelets. Blood. 2000;96:4046–4054. [PubMed] [Google Scholar]
- 35.Li Z, Nardi MA, Li YS, Zhang W, Pan R, Dang S, Yee H, Quartermain D, Jonas S, Karpatkin S. C-terminal ADAMTS-18 fragment induces oxidative platelet fragmentation, dissolves platelet aggregates and protects against carotid artery occlusion and cerebral stroke. Blood. 2009 doi: 10.1182/blood-2008-07-170571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawley RG, Lieu FH, Fong AZ, Hawley TS. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1994;1:136–138. [PubMed] [Google Scholar]
- 37.Soneoka Y, Cannon PM, Ramsdale EE, Griffiths JC, Romano G, Kingsman SM, Kingsman AJ. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 1995;23:628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White JG. Electron microscopy methods for studying platelet structure and function. Methods Mol Biol. 2004;272:47–63. doi: 10.1385/1-59259-782-3:047. [DOI] [PubMed] [Google Scholar]
- 39.Cerutti A, Zan H, Schaffer A, Bergsagel L, Harindranath N, Max EE, Casali P. CD40 ligand and appropriate cytokines induce switching to IgG, IgA, and IgE and coordinated germinal center and plasmacytoid phenotypic differentiation in a human monoclonal IgM+IgD+ B cell line. J Immunol. 1998;160:2145–2157. [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao H, Neuveut C, Tiffany HL, Benkirane M, Rich EA, Murphy PM, Jeang KT. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc Natl Acad Sci U S A. 2000;97:11466–11471. doi: 10.1073/pnas.97.21.11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan KT, Tayebjee MH, Lynd C, Blann AD, Lip GY. Platelet microparticles and soluble P selectin in peripheral artery disease: relationship to extent of disease and platelet activation markers. Ann Med. 2005;37:61–66. doi: 10.1080/07853890410018943. [DOI] [PubMed] [Google Scholar]
- 42.Sprague DL, Elzey BD, Crist SA, Waldschmidt TJ, Jensen RJ, Ratliff TL. Platelet-mediated modulation of adaptive immunity: unique delivery of CD154 signal by platelet-derived membrane vesicles. Blood. 2008;111:5028–5036. doi: 10.1182/blood-2007-06-097410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fackler OT, Baur AS. Live and let die: Nef functions beyond HIV replication. Immunity. 2002;16:493–497. doi: 10.1016/s1074-7613(02)00307-2. [DOI] [PubMed] [Google Scholar]
- 44.Swingler S, Brichacek B, Jacque JM, Ulich C, Zhou J, Stevenson M. HIV-1 Nef intersects the macrophage CD40L signalling pathway to promote resting-cell infection. Nature. 2003;424:213–219. doi: 10.1038/nature01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin G, Roy J, Barat C, Ouellet M, Gilbert C, Tremblay MJ. Human immunodeficiency virus type 1-associated CD40 ligand transactivates B lymphocytes and promotes infection of CD4+ T cells. J Virol. 2007;81:5872–5881. doi: 10.1128/JVI.02542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Re MC, Furlini G, Vignoli M, Ramazzotti E, Zauli G, La Placa M. Antibody against human immunodeficiency virus type 1 (HIV-1) Tat protein may have influenced the progression of AIDS in HIV-1-infected hemophiliac patients. Clin Diagn Lab Immunol. 1996;3:230–232. doi: 10.1128/cdli.3.2.230-232.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Re MC, Vignoli M, Furlini G, Gibellini D, Colangeli V, Vitone F, La Placa M. Antibodies against full-length Tat protein and some low-molecular-weight Tat-peptides correlate with low or undetectable viral load in HIV-1 seropositive patients. J Clin Virol. 2001;21:81–89. doi: 10.1016/s1386-6532(00)00189-x. [DOI] [PubMed] [Google Scholar]
- 48.Phillips AN, Neaton J, Lundgren JD. The Role of HIV in Serious Diseases Other than AIDS. AIDS. 2008;22:2409–2418. doi: 10.1097/QAD.0b013e3283174636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Satchell CS, Cotter AG, O'Connor EF, Peace AJ, Tedesco AF, Clare A, Clare A, Sheehan GJ, Kenny D, Mallon PW. Platelet function and HIV: a case-control study. AIDS. 24:649–557. doi: 10.1097/QAD.0b013e328336098c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Histogram of flow cytometry analysis thrombin induced platelet activation in β3−/− platelets pre-treated CCR3 inhibitor SB328437 and control (nil). (n=3) (a). Summary of flow cytometry result of thrombin induced platelet activation in β3−/− platelets pre-treated CCR3 inhibitor SB328437 and control (nil) (b).
Flow cytometry analysis of platelets treated with CCL5 with or without PGE1 or EGTA. Control (nil) n=3. Histogram of flow cytometry analysis CD154 (a) and CD62P (b).