Abstract
Biomodification of existing hard tissue structures, specifically tooth dentin, is an innovative approach proposed to improve the biomechanical and biochemical properties of tissue for potential preventive or reparative/regenerative therapies. The objectives of the study were to systematically characterize dentin matrices biomodified by proanthocyanidin-rich grape seed extract (GSE) and glutaraldehyde (GD). Changes to the biochemistry and biomechanical properties were assessed by several assays to investigate the degree of interactions, biodegradation rates, proteoglycans interaction, and effect of collagen fibril orientation and environmental conditions on the tensile properties. The highest degree of agent-dentin interaction was observed with GSE which exhibited the highest denaturation temperature, regardless of the agent concentration. Biodegradation rates remarkably decreased following biomodification of dentin matrices after 24hs collagenase digestion. A significant decreased in the proteoglycans content of GSE treated samples was observed using a micro-assay for glycosaminoglycans and histological electron microscopy, while no changes were observed for GD and control. Tensile strength properties of GD biomodified dentin matrices were affected by dentin tubule orientation, most likely due to the orientation of the collagen fibrils. Higher and/or increased stability of the tensile properties of GD and GSE-treated samples were observed following exposure to collagenase and 8 month water storage. Biomodification of dentin matrices using chemical agents not only affects the collagen biochemistry; it also involves interaction with proteoglycans. Tissue biomodifiers interact differently with dentin matrices and may provide the tissue with enhanced preventive and restorative/reparative abilities.
Keywords: collagen, dentin, glutaraldehyde, grape seed extract, biomechanical properties
1. Introduction
Fibrillar type I collagen represents the major component of the hard tissue organic matrix. It plays a number of structural roles: it defines compartments within the tissue that become impregnated with mineral; it provides a periodic 3-dimensional template for the orderly deposition and packing of mineral crystals; visco-elastic properties; and it serves as a substrate for other matrix molecules, either for the initiation and/or inhibition of mineralization [1–5]. Type I collagen is a heterotrimeric molecule composed of two α1 chains and one α2 chain that are compromised of three domains: the NH2-terminal non-triple helical (N-telopeptide), the central triple helical, and the COOH-terminal non-triple helical (C-telopeptide) domains. The inter-molecular cross-linking, representing the final post-translational modification of collagen, is the basis for the stability, tensile strength and viscoelasticity of the collagen fibrils. Endogenous cross-linking chemistry is controlled by a number of factors such as lysine hydroxylation, glycosylation, turnover rate, molecular packing, and external forces [6]. The reactions result in the formation of covalent intra- and inter-molecular cross-links [7,8]. Exogenous cross-linking can be induced by sources such as chemical agents, UV-light, heat and others. The induction of exogenous collagen cross-links has been proposed for maintenance, restoration or improvement of tissue function, and also the production of a mechanically and enzymatically resistant collagen scaffold.
Tooth hard tissue regeneration is challenging due to its inability to heal when tissue is lost in consequence of dental caries, tooth wear and injury. The biomodification of existing hard tissue structures, specifically tooth dentin, is a novel approach proposed to improve the biomechanical and biochemical properties of the tissue for preventive or reparative/restorative purposes. Well-known synthetic agents such as glutaraldehyde and most recently, nature-derived agents such as proanthocyanidin-rich extracts have been shown to effectively modify the mechanical properties of dentin organic matrix [9,10], most likely by covalent and non-covalent bonds within collagen. Their potential interaction with non-collagenous proteins such as proteoglycans and matrix metalloproteinases [11] may affect the characteristics of the tissue and therefore the term dentin matrix biomodifiers is more suited to the chemical agents. The present study thoroughly characterizes biomodified demineralized dentin matrices related to changes to its biochemistry and biomechanical properties, specifically, the degree on interactions, biodegradation rates, proteoglycans interaction, impact of fibril orientation, and long and short term mechanical stability. The specific aims explored herein were (1) To evaluate the degree of agent-matrix interactions on the thermal properties of biomodified dentin matrices; (2) to assess biodegradation rates of biomodified dentin matrices exposed to proteolytic challenge; (3) examine interactions of dentin biomodifiers with proteoglycans assessed by a quantitative glycosaminoglycans assay and electron microscopy; (4) to evaluate the effects of collagen fibrils orientation on the tensile properties of biomodified dentin matrices; and (5) to evaluate the mechanical properties following short and long-term evaluations of the tensile strength properties of biomodified dentin matrices.
2. Material & Methods
Extracted human molars kept frozen for no longer than 6 months were used under a protocol approved by the Institutional Review Board committee from the University of Illinois at Chicago. The human molars had their roots removed and the crown was sectioned in the occlusal-cervical direction into approximately 0.2 mm or 0.5 mm thick slabs. The slabs were further trimmed (#557D - Brasseler, Savannah, GA, USA) with maximum flow water spray at mid-coronal dentin (Figure 1) according to each experiment. After preparation, specimens were fully demineralized [9] with 10% phosphoric acid solution for 5 hours.
Figure 1.
Illustration of sample preparation: A –Dentin section obtained from sectioning crowns at either 0.5 or 0.2 thickness; B – Dentin sample obtained for temperature denaturation analysis (dimensions 0.5 × 2.0 × 2.0 mm); and C – Sample for ultimate tensile strength testing prepared (dimensions 0.5 × 0.5 mm neck) according to the tubule orientation (parallel or perpendicular).
The following agents were used in this study: 95% proanthocyanidin rich grape seed extract – GSE (MegaNatural gold grape seed extract, Polyphenolics, Madera, CA, USA), 25% glutaraldehyde solution – GD (Fisher Scientific, Fair Lawn, NJ, USA), and bacterial collagenase from Clostridium histolyticum - type I, >125 CDU/mg solid (Sigma-Aldrich, St. Louis, MO, USA).
2.1. Degree of biomodifiers-dentin matrix interaction
Denaturation Temperature
Specimens obtained from 5 molars (Figure 1A and B) were randomly divided into 5 treatments (n = 4 per group): 0.65% and 6.5% GSE, 5% and 25% GD (positive controls) and distilled water (negative control). Agents were prepared in distilled water and pH adjusted to 7.2. Samples were treated for 60 minutes, thoroughly rinsed for 30 minutes, placed in high pressure stainless steel pans, weighted and denaturation temperature (Td) [12] was assessed using a differential scanning calorimeter (Pyris 1, Perkin-Elmer, USA) scanned from 25 °C to 130 °C at a rate of 5 °C/min. An empty sealed pan was used as a reference pan. Cooling and reheating confirmed that the collagen denaturation was irreversible. The endothermic transition was recorded as peak denaturation temperature.
Biodegradation rates - Hydroxyproline (HYP)content in supernatant
The HYP assay followed a standard protocol [13] with minor modifications. Specimens obtained from 5 molars (Figure 1A and B) were randomly divided into 5 treatments (n = 5 specimens per group): control (no treatment), 0.65% GSE, 6.5% GSE, 5% GD and 25% GD. Samples were treated for 30 minutes, thoroughly rinsed for 30 minutes and exposed for 24 hr to 100 µg/mL bacterial collagenase in 0.2 M ammonium bicarbonate buffer (pH=7.9). Samples treated in the same manner were kept in the buffer without collagenase as a negative control. A 0.5 mL aliquot of the supernatant was collected and hydrolyzed in 6 N HCl at 90 °C overnight. Aliquots were mixed with water, transferred to glass tubes and adjusted to pH 7.0. A 500 uL aliquots was added to 1 mL Chloramine T buffer, followed by addition of 1 mL 3.15 M perchloric acid; and then 1 mL p-dimethylaminobenzaldehyde buffer was mixed at 60 °C for 20 min. Absorbance was measured at 557 nm in a spectrophotometer 96 well plate reader (Spectramax Plus, Molecular devices, Sunnyvale, CA, USA). Standard curves for HYP quantities (0, 1, 2, 3, 4, 5 ug/mL) were generated using 100 ug/mL OH-L-proline and 25 mg L-hydroxyproline in 250 mL of 0.001 N HCl. HYP content for each specimen was averaged from duplicate measurements.
2.2. Biomodifiers-proteoglycans interactions – micro-assay for glycosaminoglycans detection and electron microscopy histological detection
A 100 µL aliquot of the buffer solution (without collagenase), obtained from dentin matrices prepared and treated in the same manner described on section 2.2., was used for a micro-assay for sulfated glycosaminoglycans chains (GAGs). The content of sulphated chains in the supernatant were determined using DMMB solutions (1,9-dimethylmethylene blue) according to protocol from Barbosa et al. [14]. Briefly, the aliquot was added to 1 mL working DMMB solution, vortexed for 30 min to promote complete complexation of the GAGs with DMMB. The GAG-DMMB complex was separated from the soluble material by centrifugation, including DMMB excess. The supernatant was discarded; the pellet was dissolved in a 500 µL decomplexation solution. Samples were analyzed in duplicates at 656 nm absorbance using a spectrophotometer (Spectramax Plus, Molecular devices). Shark chondroitin sulfate sodium salt was used to determine standard curves for sulphated GAGs quantities (0.5, 1, 2, 4, 8, 16, 32, 64 µg/mL). Chondroitin-sulfate GAGs chains are the most predominant GAGs in dentin [15,16].
Qualitative histological detection of proteoglycans in dentin matrices using transmission electron microscope (TEM) was performed using the samples described above (n = 3). Samples were prepared for visualization of GAGs, using cupromeronic blue (CB) stain according to protocol from Scott et al. [17]. Briefly, specimens were fixed in 2.5 % GD/25 mM sodium acetate solution (pH = 5.8) for 30 min and then washed with 25 mM sodium acetate solution (pH = 5.8). Specimens were immersed in 0.1 M MgCl2, 25 mM NaAc, 2.5 % GD, and 0.05 % w/v CB for 3 h at 37 °C. After three washes in the same solution without staining, specimens were further stained in 0.034 M sodium tungstate for 30 min. Specimens were dehydrated in ethanol and embedded in epon resin. Ultra thin sections (70 nm thick) were obtained with a diamond knife in ultramicrotome (Ultracut UCT Leica Bannockburn, IL, USA). Sections were mounted on nickel grids and no further staining was used for analysis and imaging (TEM, Jeol 2010F, Peabody, MA, USA) at 5 Kv.
2.3. Tensile properties - Effect of dentinal tubule orientation
Specimens obtained from 20 molars (Figure 1A and C) were randomly divided according to tubule orientation (parallel - PR or perpendicular – PE to the tensile forces) (Figure 1 and 2) and 5 treatments (n = 12 per group) treatment: Control (distilled water)/PR; Control/PE, 6.5% GSE/PR, 6.5% GSE/PE; 0.65% GSE/PR; 0.65% GSE/PE; 25% GD/PR; 25% GD/PE; 5% GD/PR; 5% GD/PE. Specimens were kept in their respective solutions for 1 hour, rinsed for 30 minutes, glued with a cyanoacrylate adhesive to a tensile testing jig, which was inserted to a microtensile tester (Bisco Co, Schaumburg, IL, USA) and subjected to tensile forces at a crosshead speed of 1mm/min. Specimens were kept hydrated during testing and ultimate tensile strength (UTS) data were presented in MPa.
Figure 2.
High resolution field emission scanning electron micrograph (left image) of demineralized dentin showing the orientation of collagen fibrils from inter-tubular dentin surrounding the dentinal tubules and the tensile force orientation for the ultimate tensile strength evaluation. Schematic picture on the right shows the presence and location of intra-, inter-molecular and inter-microfibrillar cross-links.
2.4. Tensile properties - Effect of short and long term challenges
Specimens obtained from 18 molars (Figure 1A and C, parallel to the tensile forces) were randomly divided into 3 experimental groups: Control - no treatment (C); 6.5% GSE; 25% GD. Specimens were kept in their respective solutions for 1 hour, thoroughly rinsed for 10 minutes and subdivided according to the storage media: 24 hours in 0.2 M ammonium bicarbonate buffer, pH=7.9 (no treatment), 24 hours in collagenase/0.2 M ammonium bicarbonate buffer, and 8 months water storage (distilled water at 37°C). After elapsed time, specimens were tested as previously described (section 2.3.).
2.5. Statistical analysis
Data analyses were performed using two- and one- way ANOVA followed by post-hoc Fisher’s PLSD tests at a 5% confidence level. When variances inter-groups were found to be non-homogenous, data were converted into log10.
3. Results
The Td results are shown in Table 1. One-way ANOVA showed that there were statistically significant differences between groups (p < 0.0001). Control group presented the lowest Td that was significantly lower than all other groups (p < 0.05). Samples treated with GSE exhibit the highest Td values, which were statistically different than GD groups (p < 0.05). Different concentrations of GD or GSE did not affect the Td values (p > 0.05). Interestingly, multiple peaks up to 106.80 °C were observed for GSE, where the most defined peak was selected to characterize the Td for each sample (Figure 3).
Table 1.
Assessment of thermal properties, biodegradation rates and glycosaminoglycans release of biomodified dentin matrices.
| Characterization of biomodified dentin matrices [mean (standard deviation)] | |||
|---|---|---|---|
| Treatment |
§Td (°C) n = 4 |
¥Supernatant HYP content after collagenase digestion (ug/mL per mg demineralized dentin) n = 5 |
¥Supernatant GAGs content 24 hrs rinsing solution (ug/mL per mg demineralized dentin) n = 5 |
| 0.65% GSE | 86.33 (4.81) A | 4.83 (0.61) B | 9.43 (4.23) B |
| 6.5% GSE | 85.62 (1.60) A | 0 A | 22.50 (6.85) A |
| 5% GD | 78.46 (3.06) B | 0 A | 1.14 (1.27) DC |
| 25%GD | 81.13 (1.91) B | 1.08 (0.61) A | 0 D |
| Control (no treat) | 56.20 (1.92) C | 38.01 (3.65) C | 5.92 (3.16) BC |
Td: Collagen denaturation temperature; HYP: Hydroxyproline; GAGs: sulphated glycosaminoglycans chains; GSE: Grape seed extract, GD: Glutaraldehyde.
Letters indicate statistically significant (p < 0.05) differences between groups for each column.
Samples were immersed in treatment solution for 1 hour.
Samples were immersed in treatment solution for 30 minutes.
Figure 3.
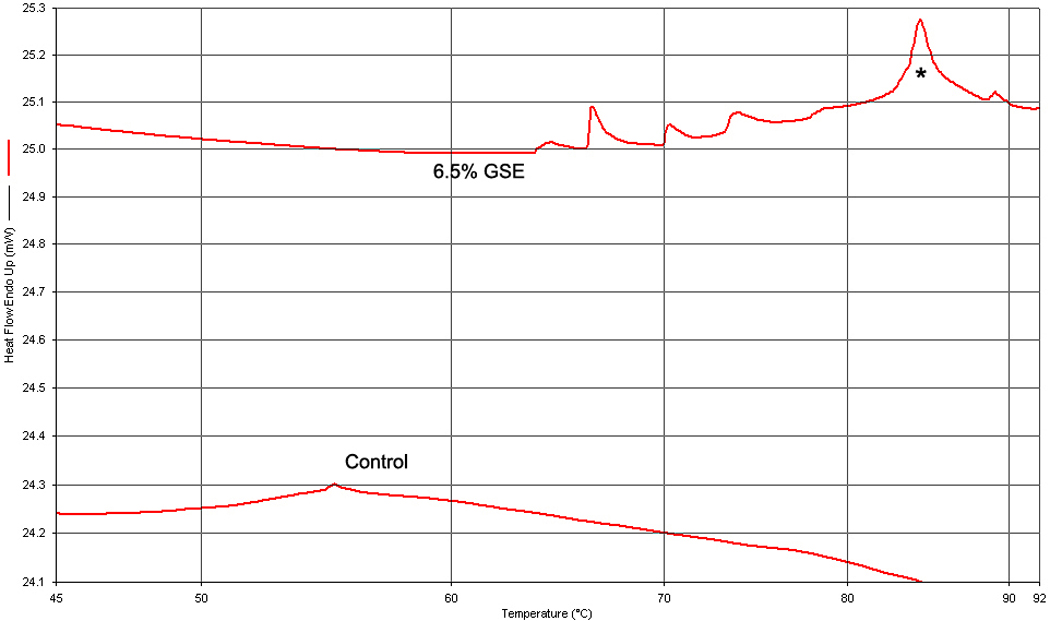
Representative standard DSC graphs (Temperature °C × heat flow mW) of specimens biomodified with 6.5% GSE and untreated (control). Denaturation temperature (Td), is detected by abrupt changes in the heat flow. Note the multiple peaks for GSE; the most defined peak (asterisk) was selected to characterize the tissue.
Biodegradation rates significantly decreased following biomodification of dentin matrices as depicted by hydroxyproline content in supernatant following collagenase digestion (Table 1). Hydroxyproline detection in the supernatant following 24hs collagenase digestion was significantly reduced for 0.65% GSE and 25% GD groups and undetectable for 6.5% GSE and 5% GD groups when compared to the control. The higher concentration of GSE was more effective in reducing biodegradation rates, while both concentrations of GD affected the biodegradation rates similarly (p > 0.05). No hydroxyproline was detected in the negative controls (data not shown).
High concentration of sulfated GAGs was detected in the supernatant of dentin specimens biomodified by GSE; where 6.5% GSE significantly increased GAGs release from dentin matrix when compared to control group (p < 0.05, Table 1). The results were confirmed by transmission electron microscopy where few or no GAGs were observed in dentin matrices samples treated with GSE (Figure 4). High concentration of GD resulted in decreased presence of GAGs in supernatant when compared to control group (p < 0.05). No qualitative histological (TEM) differences were observed between GD and control group.
Figure 4.

Representative transmission electron images of demineralized dentin matrix: A – dentin matrices biomodified with 5% GD depicted dark filaments (arrows) indicative of proteoglycans throughout the sample; B – dentin matrices biomodified with 6.5% GSE showed a remarkable decrease in the presence of filaments, no filaments were observed in most sections; C – control samples shows similar morphology as GD groups, where proteoglycans (arrows) are abundant throughout the sample. (scale bar = 200µm)
The effect of dentinal tubule orientation on the UTS of demineralized dentin is depicted in Table 2. Data were transformed into log10 for homogeneity and two-way ANOVA revealed statistically significant interaction between the factors (tensile force orientation vs. agent concentration; p= 0.0125). Orientation of dentin tubule and treatment significantly affected the UTS (p < 0.0001). Samples oriented perpendicular to the dentin tubule presented statistically significant higher UTS values when compared to parallel, except for 0.65% GSE. All treatments significantly affected the UTS values of samples oriented perpendicular to the tensile forces, except for 0.65% GSE. Whereas 6.5% GSE increased UTS values for both dentinal tubules orientations, GD concentrations did not affect the UTS in perpendicular dentin tubule direction ( p> 0.05).
Table 2.
Results for the ultimate tensile strength (mean ± standard deviation) evaluation of demineralized dentin: effect of tubule orientation and treatment.
| Ultimate tensile strength (MPa) of demineralized dentin: effect of tubule orientation | |||||
|---|---|---|---|---|---|
| Dentinal Tubule Orientation/tensile forces |
Treatment | ||||
| Control | 25% GD | 5% GD | 6.5% GSE | 0.65% GSE | |
| Parallel | 9.8±2.8B,b | 10.7±2.7B,b | 9.8±1.5B,b | 15.7±3.6A,b | 11.1±2.6B,a |
| Perpendicular | 12.44±2.4D,a | 18.6±5.7AB,a | 15.7±4.9BC,a | 20.0±4.9A,a | 13.1±3.2CD,a |
Different upper and lower case letters indicate statistically significant (p < 0.05) differences on rows and columns, respectively.
GD: glutaraldehyde; GSE: grape seed extract
The effect of environmental challenges on the UTS of demineralized dentin is shown in Table 3. Data were transformed into log10 for homogeneity and two-way ANOVA revealed statistically significant differences between agents (p < 0.0001), environmental challenges (p < 0.0001) and a significant interaction between both factors (p < 0.0001). The different environmental conditions significantly affected the control (un-treated) and GSE groups, while no differences were observed for GD. Stability of the UTS values were observed for GD and GSE following exposure to collagenase, while control groups samples were completely digested. Following 8 month water storage UTS values of the control and GSE significantly decreased when compared to 24 hour results, however the GSE values were statistically higher than GD and control after 8 month water storage (p< 0.05).
Table 3.
Results for the ultimate tensile strength evaluation (mean ± standard deviation) of biomodified demineralized dentin: effect of environmental challenges and treatment.
| Ultimate tensile strength (MPa) of demineralized dentin: effect of environmental challenge | |||
|---|---|---|---|
| Environmental challenge |
Dentin treatment |
||
| Control | 25% GD | 6.5% GSE | |
| 24 hours | 12.67 ± 4.92 B,a | 8.98 ± 2.94C,a | 16.78 ±3.58A,a |
| 24 hours/collagenase | 0.0 ± 0.0 C,c | 10.54 ± 3.94B,a | 17.12 ± 3.96A,a |
| 8 months DW | 4.73 (2.25)C,b | 7.85 (3.22)B,a | 12.13 (4.22)A,b |
Different upper and lower case letters indicate statistically significant differences (p < 0.05) on each row and column, respectively.
DW: distilled water; GD: glutaraldehyde; GSE: grape seed extract
4. Discussion
The mechanism of interactions of specific protein biomodifiers with dentin was initially believed to be mainly associated with changes to collagen biochemistry, but evidence presented herein shows interactions of GSE agent with proteoglycans. In the present study, demineralized dentin samples were successfully modified by exposure to GD and GSE (proanthocyanidin rich) that increased resistance to biodegradation and improved mechanical properties of dentin matrices. GD increases type I collagen covalent bonds by bridging amino groups of lysine or hydroxylysine residues of different collagen polypeptide chains by monomeric or ologomeric cross-links, while proanthocyanidin-rich agents have been shown to interact with proline rich proteins by covalent interactions [18], ionic interactions [19], hydrogen and hydrophobic bonding interactions [20].
The increase in the Td may be related to the degree of cross-linking of treated tissue [21] and therefore increased stability of the biomodified matrix. GSE-treated samples showed higher Td than GD-treated samples, regardless of the agent concentration. Higher Td peaks and also the presence of multiple peaks observed for GSE treated samples (Figure 3) indicate different degrees of cross-linking and therefore an increased resistance to heat diffusion through the demineralized dentin that can be explained by the multi-interactions of GSE with the organic matrix. Heat diffusion could be held back by newly induced covalent and non-covalent bonds in collagen and also removal of highly negatively charged GAGs. Decreased degradability of the matrix observed using a HYP assay supports the protective and strengthening mechanisms of GD and GSE. The concentration of GSE affects the biodegradation rates after collagenase treatment and indicates an inverse relationship between concentration of GSE and collagen solubilization. The same was not observed for GD most likely due to the limited covalent interactions with dentin collagen. Once the availability of free amino groups is diminished, no supplementary effect of glutaraldehyde is observed.
The GAGs content was greatly diminished in GSE extract treated matrix as seen by increased detection in the supernatant and remarkable reduction of GAGs visualization in the dentin matrix (Figure 4). GAGs are covalently bounded to the core protein that attaches to the collagen fibrils by protein-protein non-covalent multi-interactions [22]. PGs can be removed from the extracellular matrix by specific proteases and chemicals such as guanidinum hydrochloride [23]. While the exact mechanisms of removal of GAGs by GSE need to be further investigated, it can be speculated that the decrease in GAGs may play a role in the increased mechanical properties and perhaps biodegradation rates of biomodified dentin matrices tissues by changing the hydration rates and molecule diffusivity through the tissue [24]. GD group released less GAGs than control; which can be explained by the well-known tissue fixative properties of the agent. Further studies must be conducted to elucidate the roles of PGs on dentin matrix degradation and mechanical properties associated or not with biomodifiers agents, especially given that GAGs may affect the restorative therapies of dentin using resin-based materials [25;26;27;28].
Despite its high cytotoxicity, lower concentration of glutaraldehyde (around 5%) has been used in dental biomaterials as tooth desensitizers or component of restorative bonding systems. GD should be discouraged for clinical use and was included in this study as a positive control. Effective formation of covalent bonds has been reported following treatment of bovine dentin matrix with GD [29]; and preliminary ultimate tensile strength data of human dentin matrix were not significantly affected by treatment [9]. It is well known that the tensile properties of mineralized and demineralized dentin are affected by the dentinal tubule orientation [30;31]. The present findings show that the tubule orientation had a significant effect on the UTS of dentin, as was especially evident when samples were treated with both dentin biomodifiers. Changes to the strength of GD-treated dentin were observed when dentin matrices were tested with tensile forces perpendicular to the tubule orientation, while only the high concentration of GSE increased UTS for specimens were tested parallel to the dentinal tubules. The type, quantity and orientation of exogenous and endogenous collagen cross-links may play a role on the tensile properties of dentin matrices. While dentin type I collagen fibrils do not display a steady parallelism to each other, they follow a pattern of relative parallelism along the dentinal tubule (Figure 2). Exogenous cross-links can be induced in individual molecular strands (intra-molecular), between adjacent different molecules (inter-molecular cross-links) and between two adjacent fibrils (inter-microfibrillar cross-links) [21;32]. The direction of tensile forces exerted in the collagen fibrils may more easily rupture covalent bonds, which are more susceptible to breakage in presence of shear forces [33] (Figure 2). We hypothesize that when samples are oriented perpendicular to the dentinal tubules, the reduced strength of the covalent bonds and the presence on newly induced inter and intra-molecular cross-links were not substantial enough to influence the UTS values of GD treated samples. On the other hand, the tissue presented increased strength when subjected to tensile forces perpendicular to the dentinal tubule where increased shear forces between collagen molecules and fibrils are reduced. Dentin matrices biomodified by GSE increased strength in both dentinal tubules orientations are a result of multi-mechanisms of GSE-collagen interactions and also the ability of the material to induce inter-microfibrilar cross-links by the presence of large proanthocyanidins complexes (oligomers). Therefore the orientation of the tensile forces may play important role on the characterization of the mechanical matrices, and may not be affected solely on the type of cross-links as previously proposed [24]. Dentin-restoration interfaces are continuously subjected to mastication forces along different dentin tubule orientations (due to shape of the tooth preparation and remaining tooth structure). The findings may indicate broader applicability of GSE treated dentin when compared to GD due to enhancement of biomechanical properties of dentin-resin interfaces, regardless of the dentin surface to be restored.
Changes to the UTS of biomodified dentin matrices following collagenase exposure and water storage showed remarkable ability of the modified dentin to sustain its biomechanical properties over-time when compared to an untreated group. The sustainability of the tensile strength of biomodified dentin following collagenase treatment is confirmed by the HYP assay. Direct physical protective ability of the agents by hiding cleavage sites, impairment of collagenase infiltration, and inhibitory effect of residual agents may have resulted in UTS preservation. While the GSE-treated specimens showed decreased UTS after 8 months water storage, most likely due to decrease in non-covalent bonds, the values were 4 times higher than the control group and were not statistically different than the GD-treated group. The findings indicate that great portion of the chemical bonds introduced by both collagen biomodifiers remain and are durable under a hydrated condition. Over-time decrease in the UTS of the control indicates solubilization of the collagen by endogenous proteases [34]. The superior biomechanical properties of biomodified dentin under simulated proteolytic challenge indirectly underlines the inhibitory effects of GSE and GD on endogenous proteases [11,35,36] present in dentin/saliva [37,38,39]. Endogenous proteases inhibition have been shown to affect durability of dentin-resin bonds [40,41] and dental caries [42,43,44,45].
Innovative preventive and reparative therapies using dentin biomodifiers can result in dentin surfaces with higher strength and lower biodegradation rates than that of the natural tissue. The long term effects of dentin biomodifiers may be applicable for root caries prevention in vulnerable populations (such as elderly and xerostomic patients) by protecting the root surfaces against organic degradation. The modification of remaining dentin structure could generate a substrate more suitable for reparative procedures by increasing the biomechanical properties and decreasing collagen solubilization of tooth-biomaterial interfaces. The use of naturally occurring proanthocyanidin-rich extracts (GSE) presents many advantages over GD, including biocompatibility, enhanced biomechanical properties and comparable biodegradation rates.
5. Conclusion
Biomodification of dentin matrices using chemical agents not only affects the collagen biochemistry but also the proteoglycans, a major group of non-collagenous proteins in dentin. Dentin biomodifiers interact differently with dentin matrices and provide tissues with distinctive biomechanical and biochemical properties that can be applied for specific preventive and restorative/reparative therapies.
Acknowledgment
The study was supported by research grant DE017740 from the National Institute of Dental and Craniofacial Research, National Institutes of Health (NIH-NIDCR). We greatly appreciate the assistant provided by Linda Juarez (UIC-Research Resources Center) for TEM’s sample processing and imaging; and Noor Obaisi and Katie Yoo for sample preparation and testing. The grape seed extract was provided by Polyphenolics Inc.
Abbreviation
- GSE
grape seed extract
- GD
glutaraldehyde
- PA
proanthocyanidins
- Td
denaturation temperature
- UTS
ultimate tensile strength
- Hyp
Hydroxyproline
- GAGs
glycosaminoglycans chains
- PGs
proteoglycans
- DMMB
1,9-dimethylmethylene blue
- PE
perpendicular
- PR
parallel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Butler WT. Dentin matrix proteins and dentinogenesis. Connect Tissue Res. 1995;33:59–65. doi: 10.3109/03008209509016983. [DOI] [PubMed] [Google Scholar]
- 2.Scott JE. Extracellular matrix, supramolecular organisation and shape. J Anat. 1995;187(Pt 2):259–269. [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng H, Caterson B, Neame PJ, Lester GE, Yamauchi M. Differential distribution of lumican and fibromodulin in tooth cementum. Connect Tissue Res. 1996;34:87–96. doi: 10.3109/03008209609021494. [DOI] [PubMed] [Google Scholar]
- 4.Beniash E, Traub W, Veis A, Weiner S. A transmission electron microscope study using vitrified ice sections of predentin: structural changes in the dentin collagenous matrix prior to mineralization. J Struct Biol. 2000;132:212–225. doi: 10.1006/jsbi.2000.4320. [DOI] [PubMed] [Google Scholar]
- 5.Veis S, Sabsay B, Wu CB. Surface Reactive Peptides and Polymers. Washington, DC: 1991. [Google Scholar]
- 6.Yamauchi M. Advances in Tissue Banking. New Jersey, NJ: World Scientific Publishing; 2000. Collagen biochemistry: an overview; pp. 455–500. [Google Scholar]
- 7.Eyre DR, Koob TJ, Van Ness KP. Quantitation of hydroxypyridinium crosslinks in collagen by high-performance liquid chromatography. Anal Biochem. 1984;137:380–388. doi: 10.1016/0003-2697(84)90101-5. [DOI] [PubMed] [Google Scholar]
- 8.Yamauchi MMG. Cross-linking of collagen. Boca Raton: CRC Press; 1988. [Google Scholar]
- 9.Bedran-Russo AK, Pereira PN, Duarte WR, Drummond JL, Yamauchi M. Application of crosslinkers to dentin collagen enhances the ultimate tensile strength. J Biomed Mater Res B Appl Biomater. 2007;80:268–272. doi: 10.1002/jbm.b.30593. [DOI] [PubMed] [Google Scholar]
- 10.Bedran-Russo AK, Pashley DH, Agee K, Drummond JL, Miescke KJ. Changes in stiffness of demineralized dentin following application of collagen crosslinkers. J Biomed Mater Res B Appl Biomater. 2008;86B:330–334. doi: 10.1002/jbm.b.31022. [DOI] [PubMed] [Google Scholar]
- 11.Nandakumar V, Singh T, Katiyar SK. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett. 2008;269:378–387. doi: 10.1016/j.canlet.2008.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong SR, Jessop JL, Winn E, Tay FR, Pashley DH. Effects of polar solvents and adhesive resin on the denaturation temperatures of demineralised dentine matrices. J Dent. 2008;36:8–14. doi: 10.1016/j.jdent.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woessner J. The determination of hydroxyproline in tissue and protein samples containing small proportions of the imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 14.Barbosa I, Garcia S, Barbier-Chassefière V, Caruelle JP, Martelly I, Papy-García D. Improved and simple micro assay for sulfated glycosaminoglycans quantification in biological extracts and its use in skin and muscle tissue studies. Glycobiology. 2003;13:647–653. doi: 10.1093/glycob/cwg082. [DOI] [PubMed] [Google Scholar]
- 15.Embery G, Hall R, Waddington R, Septier D, Goldberg M. Proteoglycans in dentinogenesis. Crit Rev Oral Biol Med. 2001;12:331–349. doi: 10.1177/10454411010120040401. [DOI] [PubMed] [Google Scholar]
- 16.Waddington RJ, Hall RC, Embery G, Lloyd DM. Changing profiles of proteoglycans in the transition of predentine to dentine. Matrix Biol. 2003;22:153–161. doi: 10.1016/s0945-053x(03)00019-2. [DOI] [PubMed] [Google Scholar]
- 17.Scott JE. Proteoglycan histochemistry--a valuable tool for connective tissue biochemists. Coll Relat Res. 1985;5:541–575. doi: 10.1016/s0174-173x(85)80008-x. [DOI] [PubMed] [Google Scholar]
- 18.Pierpoint WS. o-Quinones formed in plant extracts. Their reactions with amino acids and peptides. Biochem J. 1969;112:609–616. doi: 10.1042/bj1120609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loomis WD. Overcoming problems of phenolics and quinones in the isolation of plant enzymes and organelles. Methods Enzymol. 1974;31(Pt A):528–544. doi: 10.1016/0076-6879(74)31057-9. [DOI] [PubMed] [Google Scholar]
- 20.Nimni ME. The cross-linking and structure modification of the collagen matrix in the design of cardiovascular prosthesis. J Card Surg. 1988;3:523–533. doi: 10.1111/j.1540-8191.1988.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 21.Sung HS, Chang WH, Ma CY, Lee MH. Crosslinking of biological tissues using genipin and/or carbodiimide. J Biomed Mater Res. 2003;64A:427–438. doi: 10.1002/jbm.a.10346. [DOI] [PubMed] [Google Scholar]
- 22.Parfitt GJ, Pinali C, Young RD, Quantock AJ, Knupp C. Three-dimensional reconstruction of collagen-proteoglycan interactions in the mouse corneal stroma by electron tomography. J Struct Biol. 2010;170:392–397. doi: 10.1016/j.jsb.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 23.Oegema TR, Jr, Hascall VC, Eisenstein R. Characterization of bovine aorta proteoglycan extracted with guanidine hydrochloride in the presence of protease inhibitors. J Biol Chem. 1979;254:1312–1318. [PubMed] [Google Scholar]
- 24.Torzilli PA, Arduino JM, Gregory JD, Bansal M. Effect of proteoglycan removal on solute mobility in articular cartilage. J Biomech. 1997;30:895–902. doi: 10.1016/s0021-9290(97)00059-6. [DOI] [PubMed] [Google Scholar]
- 25.Pereira PN, Bedran-de-Castro AK, Duarte WR, Yamauchi M. Removal of noncollagenous components affects dentin bonding. J Biomed Mater Res B Appl Biomater. 2007;80:86–91. doi: 10.1002/jbm.b.30572. [DOI] [PubMed] [Google Scholar]
- 26.Bedran-Russo AK, Pereira PN, Duarte WR, Okuyama K, Yamauchi M. Removal of dentin matrix proteoglycans by trypsin digestion and its effect on dentin bonding. J Biomed Mater Res B Appl Biomater. 2008;85:261–266. doi: 10.1002/jbm.b.30944. [DOI] [PubMed] [Google Scholar]
- 27.Mazzoni A, Pashley DH, Ruggeri A, Jr, Vita F, Falconi M, Di Lenarda R, Breschi L. Adhesion to chondroitinase ABC treated dentin. J Biomed Mater Res B Appl Biomater. 2008;86:228–236. doi: 10.1002/jbm.b.31010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boukpessi T, Menashi S, Camoin L, Tencate JM, Goldberg M, Chaussain-Miller C. The effect of stromelysin-1 (MMP-3) on non-collagenous extracellular matrix proteins of demineralized dentin and the adhesive properties of restorative resins. Biomaterials. 2008;29:4367–4373. doi: 10.1016/j.biomaterials.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 29.Ritter AV, Swift EJ, Jr, Yamauchi M. Effects of phosphoric acid and glutaraldehyde-HEMA on dentin collagen. Eur J Oral Sci. 2001;109:348–353. doi: 10.1034/j.1600-0722.2001.00088.x. [DOI] [PubMed] [Google Scholar]
- 30.Bedran-de-Castro AK, Pereira PN, Thompson JY. Influence of load cycling and tubule orientation on ultimate tensile strength of dentin. J Adhes Dent. 2004;6:191–194. [PubMed] [Google Scholar]
- 31.Carvalho RM, Fernandes CA, Villanueva R, Wang L, Pashley DH. Tensile strength of human dentin as a function of tubule orientation and density. J Adhes Dent. 2001;3:309–314. [PubMed] [Google Scholar]
- 32.Zeeman R, Dijkstra PJ, van Wachem PB, van Luyn MJ, Hendriks M, Cahalan PT, Feijen J. Successive epoxy and carbodiimide cross-linking of dermal sheep collagen. Biomaterials. 1999;20:921–931. doi: 10.1016/s0142-9612(98)00242-7. [DOI] [PubMed] [Google Scholar]
- 33.Buehler MJ. Nature designs tough collagen: explaining the nanostructure of collagen fibrils. Proc Natl Acad Sci U S A. 2006;103:12285–12290. doi: 10.1073/pnas.0603216103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carrilho MR, Tay FR, Donnelly AM, Agee KA, Tjäderhane L, Mazzoni A, Breschi L, Foulger S, Pashley DH. Host-derived loss of dentin matrix stiffness associated with solubilization of collagen. J Biomed Mater Res B Appl Biomater. 2009;90:373–380. doi: 10.1002/jbm.b.31295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.La VD, Bergeron C, Gafner S, Grenier D. Grape seed extract suppresses lipopolysaccharide-induced matrix metalloproteinase (MMP) secretion by macrophages and inhibits human MMP-1 and -9 activities. J Periodontol. 2009;80:1875–1882. doi: 10.1902/jop.2009.090251. [DOI] [PubMed] [Google Scholar]
- 36.Calero P, Jorge-Herrero E, Turnay J, Olmo N, López de Silanes I, Lizarbe MA, Maestro MM, Arenaz B, Castillo-Olivares JL. Gelatinases in soft tissue biomaterials. Analysis of different crosslinking agents. Biomaterials. 2002;23:3473–3478. doi: 10.1016/s0142-9612(02)00054-6. [DOI] [PubMed] [Google Scholar]
- 37.Chaussain-Miller C, Fioretti F, Goldberg M, Menashi S. The role of matrix metalloproteinases (MMPs) in human caries. J Dent Res. 2006;85:22–32. doi: 10.1177/154405910608500104. [DOI] [PubMed] [Google Scholar]
- 38.Hannas AR, Pereira JC, Granjeiro JM, Tjäderhane L. The role of matrix metalloproteinases in the oral environment. Acta Odontol Scand. 2007;65:1–13. doi: 10.1080/00016350600963640. [DOI] [PubMed] [Google Scholar]
- 39.Tersariol IL, Geraldeli S, Minciotti CL, Nascimento FD, Pääkkönen V, Martins MT, Carrilho MR, Pashley DH, Tay FR, Salo T, Tjäderhane L. Cysteine cathepsins in human dentin-pulp complex. J Endod. 2010;36:475–481. doi: 10.1016/j.joen.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 40.Hebling J, Pashley DH, Tjäderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res. 2005 Aug;84(8):741–746. doi: 10.1177/154405910508400811. Erratum in: J Dent Res 2006;85:384. [DOI] [PubMed] [Google Scholar]
- 41.Breschi L, Mazzoni A, Nato F, Carrilho M, Visintini E, Tjäderhane L, Ruggeri A, Jr, Tay FR, Dorigo Ede S, Pashley DH. Chlorhexidine stabilizes the adhesive interface: a 2-year in vitro study. Dent Mater. 2010;26:320–325. doi: 10.1016/j.dental.2009.11.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tjäderhane L, Larjava H, Sorsa T, Uitto VJ, Larmas M, Salo T. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J Dent Res. 1998;77:1622–1629. doi: 10.1177/00220345980770081001. [DOI] [PubMed] [Google Scholar]
- 43.Sulkala M, Wahlgren J, Larmas M, Sorsa T, Teronen O, Salo T, Tjäderhane L. The effects of MMP inhibitors on human salivary MMP activity and caries progression in rats. J Dent Res. 2001;80:1545–1549. doi: 10.1177/00220345010800061301. [DOI] [PubMed] [Google Scholar]
- 44.van Strijp AJ, Jansen DC, DeGroot J, ten Cate JM, Everts V. Host-derived proteinases and degradation of dentine collagen in situ. Caries Res. 2003;37:58–65. doi: 10.1159/000068223. [DOI] [PubMed] [Google Scholar]
- 45.Vitorino R, de Morais Guedes S, Ferreira R, Lobo MJ, Duarte J, Ferrer-Correia AJ, Tomer KB, Domingues PM, Amado FM. Two-dimensional electrophoresis study of in vitro pellicle formation and dental caries susceptibility. Eur J Oral Sci. 2006;114:147–153. doi: 10.1111/j.1600-0722.2006.00328.x. [DOI] [PubMed] [Google Scholar]




