Abstract
Methylglyoxal (MG) is a biologically reactive byproduct of glucose metabolism levels of which increase in diabetes. MG modification of protein generates neutral hydroimidazolone adducts on arginine residues which can alter functional active sites. We investigated the site-specificity of MG adduction to human serum albumin (HSA) using multiple reaction monitoring (MRM) of 13 MG modified tryptic peptides, each containing an internal arginine. Seven new sites for MG modification (R257 >R209 >R222 >R81 >R485 >R472 >R10) are described. Analysis of MG-treated HSA showed substantial R257 and R410 modification, with MG-modified R257 (at 100 μM MG) in drug site I causing significant inhibition of prostaglandin catalysis. The MG hydroimidazolone (MG-H1) adduct was modeled at R257, and molecular dynamics simulations and affinity docking revealed a decrease of 12.8–16.5 kcal/mol (S and R isomers, respectively) for warfarin binding in drug site I. Taken together, these results suggest that R257 is a likely site for MG modification in vivo, which may have functional consequences for prostaglandin metabolism and drug bioavailability.
Keywords: 15-keto PGE2, arginine damage, carbonyl stress, diabetic complications, dicarbonyl adduction, human serum albumin, methylgloxal, MG-H1, multiple reaction monitoring, prostaglandin, warfarin
1. Introduction
Dicarbonyls represent a class of biologically reactive glucose metabolites that are implicated in the pathogenesis of diabetic complications. Such dicarbonyls are electrophillic reactive intermediates that covalently adduct to proteins, reactions which may induce structural and functional changes at critical amino acid residues. Moreover, these modifications may represent an important mechanistic link between poor glucose metabolism and vascular complications.
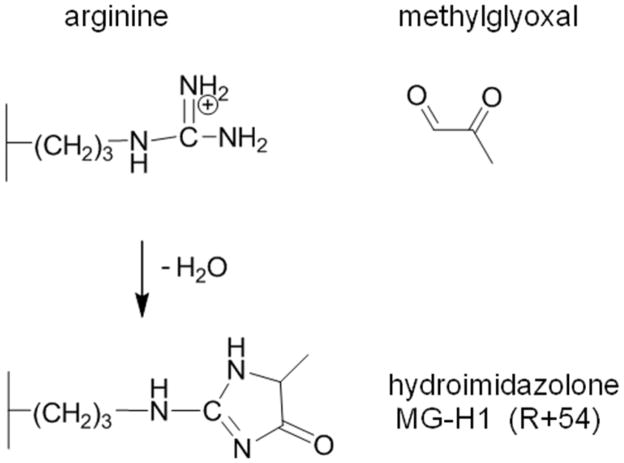
One of the most reactive dicarbonyls is methylglyoxal (MG), an ∝-oxo aldehyde that is generated by the spontaneous degradation of triose phosphates and autoxidation of Amadori products [1]. As MG is a highly reactive intermediate, the majority does not exist free in solution and some estimates claim that MG is as much as 99% protein bound [2]. Depending on the analytical platform, measurements of unbound plasma MG range from 40 nM to 4.5 μM [3, 4]. At physiological concentrations of free MG (< 5 μM), the primary amino acid residue target of protein adduction is arginine, and the major adduct formed is the MG-H1 hydroimidazolone (Scheme 1) [5]. This reaction produces a net loss of positive charge, with potentially severe consequences for arginine residues positioned at critical sites for protein function. For example, a hotspot for MG adduction on human serum albumin (HSA) at R410 impacts ketoprofen binding and esterase activity in drug binding site I [6]. While this is a major site for MG modification in vitro, studies of human albumin mutants revealed that modification of R410 results in rapid elimination of HSA in mice [7]. MG modification at this hotspot may be both susceptible to adduction and elimination, and thus, may not accumulate to appreciable levels as a result of protein turnover.
Scheme 1. MG modification of arginine.
MG adducts to arginine, and upon condensation, forms a neutral hydroimidazolone ring. MG-H1 is the methylglyoxal-derived hydroimidazolone isomer 1 (Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine)
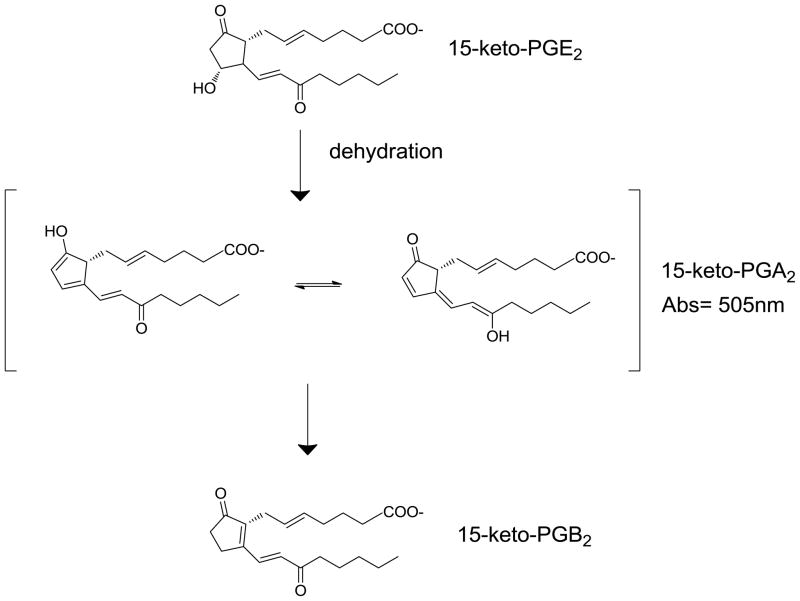
In addition to esterase activity at drug binding site II, HSA also plays a significant role in the breakdown of prostaglandins (PGs), and stabilization of other eicosanoids [8, 9]. A spectrophotometric assay was developed to measure the catalytic dehydration of 15-keto PGE2 to 15-keto PGA2 which undergoes keto-enol tautamerization, and absorbs light at 505 nm (Scheme 2) [8]. Site-directed mutagenesis and expression of fragments of HSA demonstrated that the R257M mutant and expression of albumin fragments other than domain II (containing drug binding site I) had the most profound decrease in formation of 15-keto PGA2. It was determined that drug site I in HSA is an important regulator of PG metabolism, due especially to the high concentrations of albumin in blood. Drug site I is also known as the warfarin binding site, and the integrity of this site is critical because warfarin has a narrow therapeutic range [10]. Approximately 99% of plasma warfarin is bound to HSA, and slight changes in the affinity between HSA and warfarin can have profound effects on bioavailability [11].
Scheme 2. Conversion of 15-keto PGE2 to 15-keto PGB2.
Dehydration of 15-keto PGE2 in drug site I of human serum albumin yields a keto-enol tautomer hybrid (15-keto PGA2) that absorbs light at 505 nm. Further rearrangement of the chromophore eventually decays to 15-keto PGB2, which does not absorb light.
In this report, we describe the use of quantitative mass spectrometry using multiple reaction monitoring (MRM) to determine the relative reactivity of sites of MG adduction on human serum albumin. We synthesized tryptic peptides containing MG modified arginine sites, and obtained optimal mass spectral transitions between parent and expected fragment masses which allowed us to relatively quantify these adducts (i.e., multiple reaction monitoring). HSA was treated with physiological concentrations of MG, followed by tryptic digestion and MRM analysis using a triple quadrupole mass spectrometer. The discovery of a MG-arginine hotspot at R257 in drug site I led to the study of the impact of MG treatment upon PG catalysis. Finally, molecular modeling of MG modification at R257 showed a decrease in warfarin binding at drug site I of HSA relative to unmodified R257.
2. Materials and Methods
2.1. Chemicals
All reagents, including methylglyoxal and fatty acid-free human serum albumin (catalog # A3782) were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. Sequencing-grade trypsin was purchased from Promega (Madison, WI). Synthetic peptides were purchased from New England Peptide (Gardner, MA). 15-keto Prostaglandin E2 (catalog #14720) was purchased from Cayman Chemical (Ann Arbor, MI). Lipidex-1000 was purchased from PerkinElmer (Waltham, MA).
2.2. Derivatization of MG-modified peptides and transition optimization
Lyophilized tryptic peptides (Table 1) containing an internal arginine (R) and a C-terminal lysine (K) were synthesized, HPLC purified, lyophilized, and resuspended in 18Ω Milli-Q grade water to 5 mg/ml. A 150 μl aliquot was used for each reaction. In peptides that also contained cysteine residues (ie., peptides containing R257, R485, R98, R81), 200 mM iodoacetamide (20 μl) in 20 mM ammonium bicarbonate pH 7.4 was added and the solution was incubated in the dark at room temperature for 1 hour. MG (150 μl of 10 mM) in 2X PBS pH 7.4 was added to each peptide solution, and the reactions were incubated at 37°C for 2 hours. The reactions were stopped with the addition of formic acid (10 μl), and the modified peptides were desalted with 50 mg Hypersep C18 RP cartridges (Thermo Scientific) and eluted with 80% acetonitrile containing 0.1% TFA. The peptides were diluted four-fold with a solution of 0.5% formic acid in water prior to infusion.
Table 1. MRM transition list of MG-modifed and unmodified peptides.
Peptide name refers to site of MG modification, arginine in bold denotes hydroimidazolone (R+54). All peptides containing cysteines were treated with iodoacetamide (C+57) prior to MRM. Unmodified control peptides are shown in italics. Q1 and Q3 masses for parent ions and fragment ions are listed. Charge state of parent ion is indicated, and fragment ion is listed.
| peptide name | peptide sequence | Parent (Q1) | Fragment (Q3) | CE | charge | ion |
|---|---|---|---|---|---|---|
| R10 | SEVAHR(54)FK | 514.2 | 712.3 | 32 | 2 | y5 |
| R81 | LC(57)TVATLR(54)ETYGEMADC(57)C(57)AK | 801.7 | 1065.4 | 35 | 3 | y18 +2 |
| R98 | QEPER(54)NEC(57)FLQHK | 590.3 | 525.3 | 33 | 3 | y4 |
| R186 | LDELR(54)DEGK | 565 | 1015.3 | 34 | 2 | y8 |
| 565 | 796.4 | 32 | 2 | b6 | ||
| 376.9 | 508.2* | 18 | 3 | y8 +2 | ||
| R209 | FGER(54)AFK | 303.6 | 381.2* | 17 | 3 | y6 +2 |
| 454.8 | 615.2 | 30 | 2 | b5 | ||
| R218 | AWAVAR(54)LSQR | 606.6 | 784.4* | 30 | 2 | y6 |
| 606.6 | 954.5 | 30 | 2 | y8 | ||
| R222 | LSQR(54)FPK | 465.3 | 816.2 | 29 | 2 | y6 |
| R257 | VHTEC(57)C(57)HGDLLEC(57)ADDR(54)ADLAK | 660.6 | 801.5 | 31 | 4 | y20 +3 |
| 660.6 | 635.6 | 32 | 4 | y21 +4 | ||
| 660.6 | 237.1* | 35 | 4 | b2 | ||
| 660.6 | 109.6 | 90 | 4 | y2 +2 | ||
| 528.7 | 605.2 | 19 | 5 | b10 +2 | ||
| R348 | HPDYSVVLLLR(54)LAK | 560 | 331.2 | 50 | 3 | y3 |
| R410 | FQNALLVR(54)YTK | 469.6 | 566.3* | 21 | 3 | y9 +2 |
| 469.6 | 622.2 | 24 | 3 | y4 | ||
| 469.6 | 120.1 | 55 | 3 | y1 | ||
| R428 | KVPQVSTPTLVEVSR(54)NLGK | 702.8 | 627.2 | 35 | 3 | y17+3 |
| 702.8 | 940.3 | 34 | 3 | y17 +2 | ||
| 527.3 | 683.8* | 20 | 4 | y12 +2 | ||
| R472 | TPVSDR(54)VTK | 528.9 | 759.4 | 33 | 2 | y6 |
| 352.9 | 478.2* | 18 | 3 | y8 +2 | ||
| 352.9 | 429.8 | 18 | 3 | y7 +2 | ||
| 352.9 | 759.4 | 21 | 3 | y6 | ||
| R485 | R(54)PC(57)FSALEVDETYVPK | 655.6 | 244.2 | 23 | 3 | y2 |
| K51 unmodified | LVNEVTEFAK | 575.3 | 937.5* | 25 | 2 | y8 |
| R257 unmodified | VHTEC(57)C(57)HGDLLEC(57)ADDR | 696.5 | 925.8 | 34 | 3 | y15 +2 |
| R410 unmodified | FQNALLVR | 481.4 | 685.4 | 30 | 2 | y6 |
| R218 unmodified | AWAVAR | 337.2 | 416.2 | 18 | 2 | y4 |
| R218 unmodified | AWAVAR(10) | 342.2 | 426.3 | 18 | 2 | y4 |
Transition used for relative quantitation
CE: Collision Energy
R114, R117 peptide: DDNPNLPRLVRPEVDVMCTAFHDNEETFLK
Peptide solutions were infused at 3 μl/min into a 4000 QTRAP (Applied Biosystems/MDS Sciex) equipped with a Turbo Spray ion source and the manual transition optimization was performed as follows. The source temperature was set at 200°C, source voltage was 5000 volts, GS1 and GS2 were set to 0 and 25 psi, respectively, and the declustering potential was set to 70 volts for all parent ions.
Theoretical fragmentation spectra were obtained using the MS-Product function in Protein Prospector [12], where R+54 was used to account for the MG modification. While the individual peptide solutions were infused into the QTRAP, mass spectra of parent ions for each charge state (+2, +3, +4) were analyzed using enhanced MS (EMS) mode in Analyst v. 1.4 (AB Sciex). Parent ions from each charge state above were fragmented in enhanced product ion (EPI) mode and a list of potential MRM transitions was generated. In MRM mode, transitions were monitored as the collision energy was ramped from 5 to 100 eV and 1–5 transitions were chosen for each modified peptide.
2.3. MG-modification of HSA
Lipidex-1000 slurry in methanol was buffer exchanged five times into an equal volume of 1X PBS, pH 7.4. HSA (100 mg) was dissolved in 10 ml of 1XPBS pH 7.4 and was delipidated with aqueous Lipidex-1000 for 1 hour on a rotator at room temperature. A 2-fold dilution series of MG in 1XPBS pH 7.4 from 200 μM was prepared and this series was added to an equal volume of delipidated HSA at 10 mg/ml for a final HSA concentration of 5 mg/ml (75 μM). The reactions were incubated at 37°C for 18 hours. MG and PBS were removed via centrifugation using Amicon Ultra 10K MWCO (Millipore) filters, and MG-modified HSA was buffer exchanged into 50 mM ammonium bicarbonate pH 7.4.
2.4. Trypsin digestion and peptide purification
An aliquot (250 μg) of MG-modified HSA in 50 mM ammonium bicarbonate pH 7.4 from section 2.3 was reduced with 100 μl of 20 mM TCEP (tris(2-carboxyethyl)phosphine) in 0.4 M ammonium bicarbonate pH 7.4, and was incubated at 55°C for 30 min. Once the reactions reached room temperature, 100 μl of 25 mM iodoacetamide in 50 mM ammonium bicarbonate pH 7.4 was added, and the reactions incubated for 30 minutes in the dark at room temperature. Trysin was added at a 1:50 (w/w; trypsin: HSA) ratio, and the reactions incubated for 18 hours at 37°C. Formic acid (5 μl ) was added to terminate the reactions, and the tryptic digests were desalted as described in section 2.2. The digests, in 80% acetonitrile, were frozen and lyophilized to dryness. The peptides were resuspended in 100 μl of a 1% formic acid solution containing the R218 stable isotope labeled peptide internal standard at 5 μg/ml prior to LC-MS/MS.
2.5 Relative quantitation of MG hotspots using MRM
Aliquots of HSA digest (25 μg in 10 μl) were loaded onto a ZORBAX 300SB-C18 capillary column (5 μm, 0.5 × 150 mm, Agilent). Peptides were eluted from the column using an LC Packings Ultimate II HPLC (Dionex) into a 4000 QTRAP using a flow rate of 40 μl/min of solvent A (0.01% TFA, 0.5% formic acid) with a 30 min linear gradient from 5% to 45% of solvent B (acetonitrile containing 0.01% TFA, 0.5% formic acid). The 4000 QTRAP was operated in MRM mode with the optimal transitions listed in Table 1. Dwell time was set to 40 ms, Q1 resolution was set to low, and Q3 resolution was set to unit. Data analysis and peak integration was performed with Multiquant software (AB Sciex).
2.6 15-keto Prostaglandin E2 conversion assay
Lipidex was buffer exchanged as described above into 1X PBS, pH 7.4. HSA (50 mg) was dissolved in 4.96 mL 1X PBS, pH 7.4 to a final concentration of 10 mg/mL. HSA was delipidated with Lipidex for 30 min. MG dilutions were created from 200 mM stock to concentrations of 2, 1.5, 1 and 0.5 mM. These dilutions were then used as stock for a 10 fold series dilution yielding 13 separate concentrations of MG in PBS. The concentrations used for the assay were 2, 1.5, 1, 0.5, 0.2, 0.15, 0.1, 0.05, 0.02, 0.015, 0.01, 0.005, and 0.002 mM. HSA solution (100 μL) was incubated with 100 μL of MG solution, yielding protein concentration of 5 mg/mL and MG concentration half of those reported above, for 20 hours at 37°C. 15-keto PGE2 (5 mg) was dissolved in 1 mL EtOH. Fifty aliquots of 20 μL (each containing 100 μg) were taken, the EtOH was evaporated to dryness, and the dried aliquots were stored at −20°C. 15-keto PGE2 was resuspended in 200 μL 1X PBS, pH7.4. Following incubation, each of the 13 HSA/MG solutions was treated with 16 μL of PGE2. The absorbance of each reaction at 505 nm (A505) was measured immediately following the addition of PGE2 giving a time point of t=0 using a blank containing 200 μL 1X PBS and 16 μL PGE2. Measurements of A505 were taken at time points t= 0.5, 1, 1.5, 2, 4 and 5 hours.
2.7 Affinity docking and molecular dynamics simulations
We have previously reported the use of molecular modeling as a tool to simulate changes of protein conformation following electrophile adduction [13]. The X-ray crystal structure coordinates for the HSA complexed with warfarin (PDB code: 2bxd) were used as a starting model [14]. MG-modified hydroimidazolone (MG-H1) was built using Insight II – builder module (Accelrys Inc., San Diego, CA). The charges were assigned using consistent valence force field (CVFF) parameters. Docking studies were carried out using Affinity docking program within Insight II 2005L modeling software. Twenty different conformations were generated for each ligand using Metropolis algorithm and distance dependent dielectric = 4.00 during docking. Best docking poses were refined using 105 steps of Discover 3.0 minimization in case of methylglyoxal. MG-modified adduct was formed on R257 of HSA. The modified structure was then subjected to 105 steps of minimization using Discover 3.0. R-(+) warfarin and human serum albumin (native and MG-modified) complexes were then soaked with a 10 Å layers of TIP3P water molecules. This assembly was then subjected to dynamic simulations for 250 ps. Trajectories were collected every 0.1 ps. The lowest-potential energy structure was selected and then minimized using 105 steps of minimization. The final minimized structure was then used for the analysis.
3. Results
3.1. Validation of MG-modified peptides
MG-modification of arginine induces a missed tryptic cleavage site. Of the 24 arginine sites in HSA, the 13 sites listed in Table 1 were considered good candidates for MRM because they lie on tryptic peptide fragments, are of an appropriate length (5–26 residues), and because they contain a C-terminal lysine. The exception to this is the R218 peptide, which contains a C-terminal arginine. The synthetic tryptic peptide containing R218 was not chosen for MG modification because it contains two arginines, and modification with MG would likely target both arginines. Instead, LC-LC-MS/MS of a tryptic digest of MG modified HSA (data not shown) was used to estimate the optimal transitions for the MG modified R218 peptide. For example, it was determined in this manner that the doubly charged peptide containing R218 at m/z=606.6 resulted in strong y6 and y8 fragments at 30eV collision energy. Other sites identified from LC-LC-MS/MS of MG-treated HSA were R257, R410, R485, and R428. The spectra identifying the R428 peptide contained a missed tryptic cleavage at the N-terminus, and the synthetic peptide used for this study has a N-terminal lysine. The remaining 8 arginine containing peptides (R10, R81, R98, R186, R209, R222, R348, R472) were identified from a theoretical tryptic digest that assumed zero missed cleavages, other than the internal arginine.
Twelve synthetic peptides containing an internal arginine (all except R218) were modified with MG and the transitions that gave the most intense fragment ions are reported in Table 1. Transitions for each modified peptide were considered valid if they had overlapping peaks for multiple transitions at identical retention times. In some cases, (R186, R209, R257, R428, R472) parent ions from more than one charge state produced fragments with intense signals, and monitoring transitions from more than one charge state provides further validation of transition specificity. In other cases (R10, R81, R98, R222, R348, R485) a single transition was chosen as this was the sole dominant fragment and/or the other fragments were nonspecific.
Unmodified HSA peptides were used as controls to monitor assay conditions and are shown in italics in Table 1. The “K51 unmodified” peptide was validated in a previous study [15] and this peptide does not overlap with any of the arginines monitored in this study. Three unmodified sites were monitored: R218, R257, and R410. Unmodified R257 peptide contains cysteines, and this peptide was used as a control for reduction and cysteine alkylation. Unmodified R218, unmodified R410, and unmodified K51 peptides were used to monitor digestion and C18 RP cartridge purification. Stable isotope-labeled unmodified R218 was used to assess autosampler run-to-run variability, and 50 ng was injected per 10 ul injection loop fill volume.
3.2. Relative reactivity of arginine sites to MG
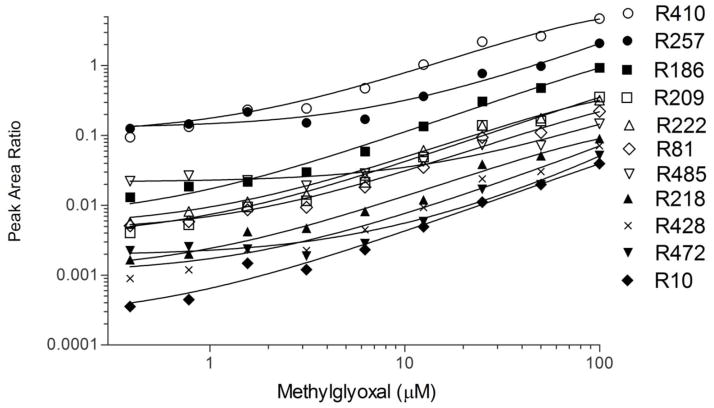
This is the first report of MG-modification at R257, a critical residue in drug binding site I in domain II of HSA. Figure 1 shows the relative reactivity of different sites toward MG modification, expressed as a ratio of the peak areas to the “K51 unmodified” internal standard. The other unmodified peptides gave similar results in terms of relative order of reactivity when the data was normalized to their peak areas. MG-modification at R257 (filled circles) and R186 (filled squares) are significant hotspots in terms of MS signal at low MG concentrations, with the peak area ratios to the unmodified peptide at approximately 10% and 1%, respectively. R186 has previously been reported as a MG site [6] when 5-fold higher concentrations of MG were used (500 μM). We chose a cut-off of 100 μM for the MG incubations because we noticed incomplete digestion at higher MG concentrations.
Figure 1. Relative modification of arginine sites by methyglyoxal.
Human serum albumin (75 μM final) was treated with a 2-fold dilution series (0.39–100 μM final) of MG from 100 μM in 1X PBS pH 7.4. MG-modified albumin was reduced, alkylated, digested, and analyzed by LC-MS/MS in MRM mode using the transitions in Table 1. Peak AUC’s were normalized to the unmodified tryptic albumin peptide LVNEVTEFAK.
Our findings corroborate previous work [6] using peptide mapping that identifies R410 (open circles) as a prominent hotspot in HSA for MG modification in vitro. MG modification of R98 and R348 containing peptides was not observed at any of the MG concentrations. Of the arginine sites that yielded signal, R485 and R472 appear to be the least responsive to MG modification (open down triangles, closed down triangles, respectively), showing detectable peak area increases above 12.5 μM MG. R209 (open squares), R222 (open triangles), and R81 (open diamonds) exhibited intermediate signal intensities, yet had dynamic ranges of peak area ratios that spanned ~1.5 orders of magnitude. R218 (closed triangles) and R428 (X’s) had a similar response, but had lower initial signal intensities. R10 (closed diamonds) had the lowest initial signal intensity, but this transition was responsive to MG treatment that spanned nearly two orders of magnitude.
3.3. MG-induced inhibition of PGE2 catalysis
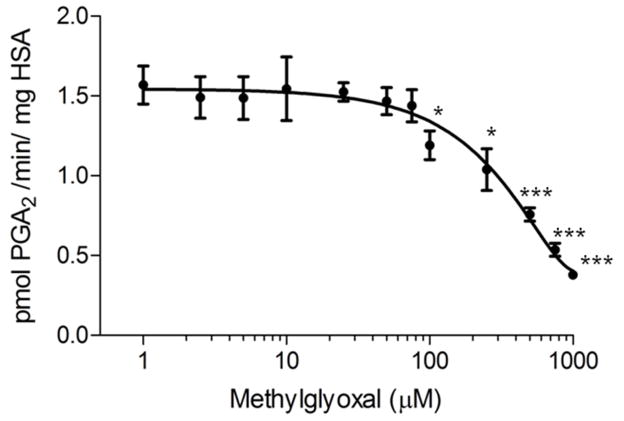
MG inhibits the HSA-mediated conversion of 15-keto PGE2 to 15-keto PGA2 (Figure 2). Absorbance vs. time was plotted and abs/min was calculated from the slope using linear regression. Dividing by the extinction coefficient (ε=35000 M−1) absorbance was converted to concentration. The coefficient .000216 L (volume of the reaction in L) was applied to yield units of mol/min. Because 1 mg of HSA was used in the assay, and the mol unit can be converted to pmol by application of a coefficient, the final units for the assay become pmol/min*mg HSA. These activities were plotted vs. log[MG]. The HSA concentration in this reaction was 75 μM, and MG modification was found to inhibit the dehydration reaction significantly (p<.05) at 100 μM. This is near-equimolar inhibition (1.33:1 MG:albumin), suggesting that MG could play a significant role in PG metabolism in vivo.
Figure 2. Inhibition of prostaglandin catalysis by methylglyoxal-modified human serum albumin.
Human serum albumin was modified with MG at various concentrations and 15-keto PGA2 formation was monitored by absorbance at 505 nm. Slope of the initial rate was converted to activity using a molar extinction coefficient of 35,000 M−1 cm−1. Values represent Means ± SE (n=3). Data are statistically significant at the * p<0.05 and *** p<0.001 respectively when compared to control (HSA without MG treatment).
3.4. Molecular dynamics and affinity docking of MG-modified R257
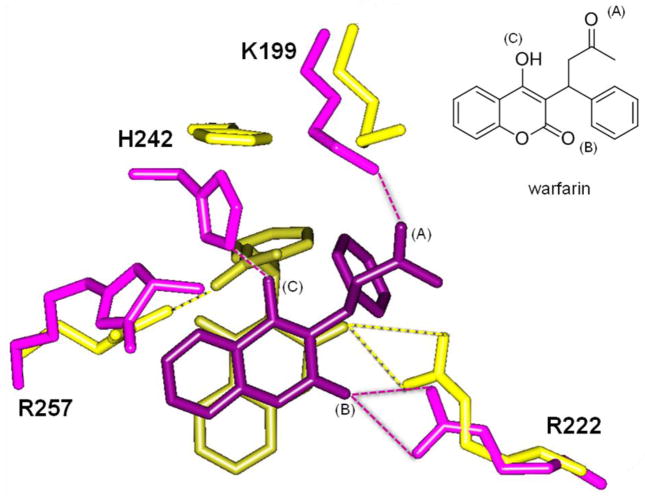
Changes in drug site I pocket of unmodified R257 (yellow) to MG-modified R257 (purple), and the energy-minimized conformations of warfarin (dark yellow for unmodified R257, dark purple for MG-modified R257) are illustrated in Figure 3. Atomic distances consistent with hydrogen bonding interactions are indicated with dashed lines. Oxygen atoms that participate in hydrogen bonding interactions are designated in the warfarin structure (inset). In its unmodified form (yellow), R257 participates in hydrogen bonding with the aliphatic ketone (A) of warfarin. Another important interaction of warfarin and the drug site I pocket is between R222 and the lactone carbonyl (B). These two key arginine interactions (R257 and R222) help stabilize and orient warfarin in this drug binding site.
Figure 3. Methylglyoxal modification of human serum albumin R257 changes warfarin binding conformation in drug site I.
Structure coordinates from PDB 2BXD were used as a starting model for molecular dynamics and energy minimization. Drug site I residues are overlaid for unmodified R257 (yellow) and MG-modified R257 (purple). Energy minimized warfarin conformation is colored dark yellow for unmodified R257, and dark purple for MG-modified R257. Dashed lines indicate hydrogen bonds. Inset shows warfarin structure labeled at three oxygen atoms (A), (B), and (C) that participate in hydrogen bonding. Oxygen atoms on warfarin docked after MG modification (purple) are labeled.
MG-modified R257 (purple) is not able to form hydrogen bonding interactions with warfarin, and this causes a shift that frees warfarin to rotate and undergo additional interactions with H242 and K199. These two residues (H242, K199) do not form hydrogen bonding interactions with warfarin when R257 is in its unmodified, positively charged form. MG modification at R257 essentially pulls K199 into the binding pocket to replace this lost interaction, which is between the ketone (A) of warfarin and the ε-amino side chain of K199. H242 also picks up an interaction, and forms a hydrogen bond between the imidazole nitrogen of the histidine side chain and the keto-enol oxygen (C) of warfarin. The overall result of MG modification at R257 is that warfarin loses this arginine interaction, and this effect is compensated by a change in pocket conformation that pulls in H242 and K199, thus creating a tighter pocket. One interaction that remains unchanged, however, is between R222 and the lactone ketone (B) of warfarin.
Although warfarin has a chiral center and is clinically available as a racemic mixture, the R-(+) and S-(−) enantiomers of warfarin bind HSA in similar positions as one another [14]. Figure 3 shows the R-(+) enantiomer, and this isomer was selected because the available crystal structure is based on this form. The hydroimidazolone formed from MG modification on arginine also has a chiral center, and the (R) enantiomer is shown. It is not known which hydroimidazolone enantiomer is formed predominantly in vivo, so we docked warfarin at both (R) and (S) isomers. Interaction energy values for R-(+) warfarin binding to drug site I were: R257 (unmodified) - 77.1 kcal/mol, R257-MG adduct (R isomer) - 60.6 kcal/mol, R257-MG adduct (S isomer) - 64.3 kcal/mol. The increase in free energy (+ΔG) as a result of modification indicates that MG modification at R257 decreases HSA affinity for warfarin, because a more negative interaction energy value indicates better binding.
4. Discussion
We present seven new sites for MG modification in human serum albumin. At low MG concentration (100 μM) the preferential sites, in decreasing order of affinity, are R257 >R209 >R222 >R81 >R485 >R472 >R10. Moreover it was noted that the slope of concentration dependent MG modification varies at different sites, the reason for which is unclear. The present study verifies four sites described previously (R410, R186, R218, R428) [6], but did not identify R114. The tryptic peptide that spans R114 is 30 residues long and contains two arginine sites (Table 1 footnote). As with the R218 peptide, we did not synthesize peptides containing two potential sites for modification. It is anticipated that development of solid phase peptide chemistry to synthesize MG-H1 modifications [16] will generate site-specific modifications for peptides that contain more than one arginine.
All of the arginine sites have varying degrees of solvent exposure [6], yet there is not an apparent correlation between solvent exposure and degree of modification. Likely, hydroimidazolone formation is governed by the arginine microenvironment where neighboring residues facilitate the condensation of MG on arginine. It should be noted that normalization by peak area ratio to the “K51 unmodified” control peptide does not necessarily equal % modification, because different peptides will have differences in ionization efficiency. However, signal intensity is related to peptide abundance and may be used to show when a particular peptide is increased or decreased relative to other peptides [17].
Of the 13 internal arginine peptides that were monitored, the peptides that contain R98 and R348 did not show any modification. It is likely that the R348 peptide is too hydrophobic for the assay conditions, as the synthetic peptide was not readily soluble. It is possible that trypsin does not produce the R98 peptide as predicted from the theoretical digest. If there are missed cleavages at the N or C terminus of this peptide, then all of the masses would shift. Another possible explanation for the lack of signal at R98 is that glutamine (Q) at the N-terminus may undergo gas phase deamination (Q-17), which would also shift the masses.
Identification of the R257 MG modification led us to examine this modification in an assay that is sensitive to R257 function. Site-directed mutagenesis revealed that R257 is critical for HSA-mediated prostaglandin catabolism [8]. Although 15-keto PGE2 and its derivatives are not found in nature, their spectral properties are useful in monitoring the integrity of drug site I. MG-modification at R257 may decrease the base-catalyzed elimination reaction, as the neutral hydromidazolone is not able to abstract a proton from carbon 10 of PGE2. The potential link between MG adduction and altered PG metabolism is intriguing, and certainly merits further investigation.
Molecular dynamics simulations based on the available crystal structures were useful to illustrate the impact of MG-modified R257. The interaction energy value estimate is based on computer algorithms used in in silico drug design, and is useful for ranking drug candidates based on docking interactions with an active site. Therefore, the approximate decrease of 15 kcal/mol in interaction energy as a result of R257 MG modification could result in a significant change in warfarin bioavailability. Decreased protein binding would further increase the proportion of free drug, thus altering warfarin pharmacokinetics. Future studies will investigate the impact of MG modification on warfarin and other drugs known to bind to site I of HSA.
Acknowledgments
This work was supported by RO1GM070890 (SSL), R24DK083948 (SSL, CS), TRIF-BIO/Biodesign Collaborative Project, P30 ES006694 Pilot Project (CS, SSL). NIH/NCRR 1S10RR022384-01 (GT) and T32 ES016652 (MJK). Mass spectrometric data was acquired by the Arizona Proteomics Consortium supported by NIEHS grant P30ES06694 to the Southwest Environmental Health Sciences Center (SWEHSC), NIH/NCI grant P30CA023074 to the Arizona Cancer Center (AZCC) and by the BIO5 Institute of the University of Arizona. We would like to thank Dr. Vijay Gokhale of the AZCC/SWEHSC supported Molecular Modeling and Synthetic Chemistry Facility Core for his assistance with computational modeling of the MG adducts.
Footnotes
Conflicts of interest
The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thornalley PJ. Pharmacology of methylglyoxal: Formation, modification of proteins and nucleic acids, and enzymatic detoxification - A role in pathogenesis and antiproliferative chemotherapy. Gen Pharmacol. 1996;27(4):565–573. doi: 10.1016/0306-3623(95)02054-3. [DOI] [PubMed] [Google Scholar]
- 2.Chaplen FWR, Fahl WE, Cameron DC. Evidence of high levels of methylglyoxal in cultured Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1998;95(10):5533–5538. doi: 10.1073/pnas.95.10.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khuhawar MY, Zardari LA, Laghari AJ. Capillary gas chromatographic determination of methylglyoxal from serum of diabetic patients by precolumn derivatization with 1,2-diamonopropane. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;873(1):15–19. doi: 10.1016/j.jchromb.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 4.Dhar A, Desai K, Liu J, Wu L. Methylglyoxal, protein binding and biological samples: Are we getting the true measure? Journal of Chromatography B. 2009;877(11–12):1093–1100. doi: 10.1016/j.jchromb.2009.02.055. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed N, Argirov OK, Minhas HS, Cordeiro CA, Thornalley PJ. Assay of advanced glycation endproducts (AGEs): surveying AGEs by chromatographic assay with derivatization by 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and application to Nepsilon-carboxymethyl-lysine- and Nepsilon-(1-carboxyethyl)lysine-modified albumin. Biochem J. 2002;364(Pt 1):1–14. doi: 10.1042/bj3640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed N, Dobler D, Dean M, Thornalley PJ. Peptide mapping identifies hotspot site of modification in human serum albumin by methylglyoxal involved in ligand binding and esterase activity. J Biol Chem. 2005;280(7):5724–5732. doi: 10.1074/jbc.M410973200. [DOI] [PubMed] [Google Scholar]
- 7.Iwao Y, Anraku M, Yamasaki K, Kragh-Hansen U, Kawai K, Maruyama T, Otagiri M. Oxidation of Arg-410 promotes the elimination of human serum albumin. BBA-Proteins. Proteomics. 2006;1764(4):743–749. doi: 10.1016/j.bbapap.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Yang JS, Petersen CE, Ha CE, Bhagavan NV. Structural insights into human serum albumin-mediated prostaglandin catalysis. Protein Sci. 2002;11(3):538–545. doi: 10.1110/ps.28702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzpatrick FA, Liggett WF, Wynalda MA. Albumin-eicosanoid interactions. A model system to determine their attributes and inhibition. J Biol Chem. 1984;259(5):2722–2727. [PubMed] [Google Scholar]
- 10.Ha CE, Petersen CE, Park DS, Harohalli K, Bhagavan NV. Investigations of the effects of ethanol on warfarin binding to human serum albumin. J Biomed Sci. 2000;7(2):114–121. doi: 10.1007/BF02256617. [DOI] [PubMed] [Google Scholar]
- 11.Petersen CE, Ha CE, Curry S, Bhagavan NV. Probing the structure of the warfarin-binding site on human serum albumin using site-directed mutagenesis. Proteins. 2002;47(2):116–125. doi: 10.1002/prot.10068. [DOI] [PubMed] [Google Scholar]
- 12.Clauser KR, Baker P, Burlingame AL. Role of accurate mass measurement (+/− 10 ppm) in protein identification strategies employing MS or MS MS and database searching. Anal Chem. 1999;71(14):2871–2882. doi: 10.1021/ac9810516. [DOI] [PubMed] [Google Scholar]
- 13.Fisher AA, Labenski MT, Malladi S, Gokhale V, Bowen ME, Milleron RS, Bratton SB, Monks TJ, Lau SS. Quinone electrophiles selectively adduct “electrophile binding motifs” within cytochrome c. Biochemistry. 2007;46(39):11090–11100. doi: 10.1021/bi700613w. [DOI] [PubMed] [Google Scholar]
- 14.Ghuman J, Zunszain PA, Petitpas I, Bhattacharya AA, Otagiri M, Curry S. Structural basis of the drug-binding specificity of human serum albumin. Journal of Molecular Biology. 2005;353(1):38–52. doi: 10.1016/j.jmb.2005.07.075. [DOI] [PubMed] [Google Scholar]
- 15.Kuzyk MA, Smith D, Yang JC, Cross TJ, Jackson AM, Hardie DB, Anderson NL, Borchers CH. Multiple Reaction Monitoring-based, Multiplexed, Absolute Quantitation of 45 Proteins in Human Plasma. Mol Cell Proteomics. 2009;8(8):1860–1877. doi: 10.1074/mcp.M800540-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gruber P, Hofmann T. Chemoselective synthesis of peptides containing major advanced glycation end-products of lysine and arginine. J Pept Res. 2005;66(3):111–124. doi: 10.1111/j.1399-3011.2005.00279.x. [DOI] [PubMed] [Google Scholar]
- 17.Steen H, Jebanathirajah JA, Springer M, Kirschner MW. Stable isotope-free relative and absolute quantitation of protein phosphorylation stoichiometry by MS. Proc Natl Acad Sci U S A. 2005;102(11):3948–3953. doi: 10.1073/pnas.0409536102. [DOI] [PMC free article] [PubMed] [Google Scholar]