Abstract
Treatments for patients suffering from severe temporomandibular joint (TMJ) dysfunction are limited, motivating the development of strategies for tissue regeneration. In this study, co-cultures of fibrochondrocytes (FC) and articular chondrocytes (AC) were seeded in agarose wells, and supplemented with growth factors, to engineer tissue with biomechanical properties and ECM composition similar to native TMJ fibrocartilage. In the first phase, growth factors were applied alone and in combination, in the presence or absence of serum, while in the second phase, the best overall treatment was applied at intermittent dosing. Continuous treatment of AC/FC co-cultures with TGF-β1 in serum-free medium resulted in constructs with GAG/WW (12.2%), instantaneous compressive moduli (790 kPa), relaxed compressive moduli (120 kPa), and Young’s moduli (1.87 MPa) that overlap with native TMJ disc values. Among co-culture groups, TGF-β1 treatment increased collagen deposition ~20%, compressive stiffness ~130%, and Young’s modulus ~170% relative to no growth factor controls. Serum supplementation, though generally detrimental to functional properties, was identified as a powerful mediator of FC construct morphology. Finally, both intermittent and continuous TGF-β1 treatment showed positive effects, though continuous treatment resulted in greater enhancement of construct functional properties. This work proposes a strategy for regeneration of TMJ fibrocartilage and its future application will be realized through translation of these findings to clinically viable cell sources.
Keywords: TMJ, fibrocartilage, tissue engineering, growth factors, scaffoldless
1. INTRODUCTION
Disorders of the temporomandibular joint (TMJ) affect millions in the United States.[1, 2] Many cases of temporomandibular dysfunction (TMD) are associated with malformations or displacements of the TMJ disc, a fibrocartilaginous tissue that assists in normal function of the joint, and/or injuries to the fibrous cartilage that covers the surface of the mandibular condyle.[3, 4] Sufferers experience pain and difficulty performing routine activities such as eating and talking. The majority of cases are best managed through conservative treatments and pain mitigation, though the most severe cases of joint degeneration require surgical intervention.[5] While current surgical methods, such as arthrocentesis, arthroscopic repositioning, discectomy, and joint replacement, can restore some function, these treatments do not fully address severe TMD.[6] Efforts to engineer tissues for repair or replacement are essential to provide the next generation of treatments and an ideal long-term solution.[4, 5]
An appropriate cell source for tissue engineering must be abundant, accessible, and functionally appropriate for the target tissue. Primary TMJ disc cells[7–9] and condylar cartilage cells[10, 11] have been examined for TMJ engineering, however, clinical success using these sources is improbable due to cell scarcity and their inability to produce sufficient matrix in vitro. Additionally, the availability of TMJ cells for in vitro studies is limited; therefore the pressing problems of TMD need to be addressed using alternative cell types.
The meniscus, the TMJ disc, and condylar cartilage are comprised of heterogeneous matrices containing predominantly collagen type I with lower levels of collagen type II, as well as glycosaminoglycans (GAGs), [12–15] and exhibit regional variation and anisotropy in material properties.[16–20] The cells of the knee meniscus, referred to as fibrochondrocytes (FCs), are a mixed population of rounded, chondrocyte-like cells and elongated, fibroblast-like cells similar to those found in the disc and condylar cartilage.[4, 21] While knee meniscus cells are not abundant or easily accessible, efforts are underway to differentiate stem cells toward this fibrocartilage phenotype.[10, 22, 23] When combined with articular chondrocytes (ACs) in a scaffoldless culture system, [24] these cells are capable of forming constructs comprised of collagen types I and II, and levels of GAGs similar to native fibrocartilage.[25–27] Importantly, by modulating the ratio of FCs to ACs, the composition of the resulting matrix could feasibly be tailored to more closely recapitulate the target tissue mechanical properties and ECM. As with fibrochondrocytes, abundant efforts are also underway in differentiating embryonic stem cells, [28, 29] bone marrow-derived mesenchymal stem cells, [30, 31] adipose-derived stem cells, [32, 33] and other cells[34, 35] toward a chondrocyte phenotype. Given the similarities in function, current progress in fibrocartilage tissue engineering, and the rapidly advancing field of stem cell differentiation, a co-culture of FCs and ACs is an attractive culture model for TMJ tissue engineering.
In addition to varying co-culture ratios, the use of anabolic agents can modulate matrix synthesis and subsequently affect biomechanical properties. Studies of several growth factors (GF) on fibrochondrocytes showed increases in collagen and GAG precursor uptake using TGF-β1 compared to IGF-1, bFGF, and PDGF, though IGF-1 also significantly increased collagen precursor uptake.[36, 37] In addition, previous studies using both IGF-1 and TGF-β1 on fibrochondrocytes led to significant increases in collagen and GAG synthesis over other GFs.[7, 38] Synergy between IGF-1 and TGF-β1 has been demonstrated in ACs in terms of aggrecan production, [39] and recent work suggests that intermittent dosing of GFs may enhance their efficacy relative to continuous treatment, likely the result of reduced receptor desensitization.[40] Though recent work has shown increases in functional properties using a serum-free medium in FC:AC co-cultures, [27] the use of fetal bovine serum (FBS) in conjunction with IGF-1 and TGF-β1 may be capable of accentuating functional differences between the resulting tissue. For meniscus tissue engineering, previous work in our laboratory has determined serum-free methods using a 50:50 FC:AC co-culture. Whether this can be achieved for other FC:AC ratios has yet to be determined.
The purpose of this study is two-fold. First, a ratio of FCs to ACs must be determined for engineering the TMJ disc and condylar cartilage. Second, a protocol for the use of bioactive factors in culturing these cells must be identified. Specifically, GF type, the contribution of serum to its effectiveness, whether different GFs need to be combined, and whether saturation occurs in their dosing are all variables that must be examined. To achieve these goals, two phases were employed. In the first phase of this study, the effects of TGF-β1, IGF-1, and serum, alone and in combination, on scaffoldless constructs of FCs and two co-cultures of FCs and ACs, were examined. In the second phase, the best overall treatments, as determined by quantitative assessments of matrix composition and biomechanical integrity, were applied continuously or intermittently. Based on previous experience, it was hypothesized that a spectrum of fibrocartilages displaying heterogeneous function and matrix composition would result from different co-culture combinations and GF/serum treatments, and that modulating the type and manner of application of anabolic agents would result in near recapitulation of native tissue material properties and matrix content.
2. MATERIALS AND METHODS
2.1. Cell isolation and seeding
Chondrocytes were harvested from the distal femoral condyle, and fibrochondrocytes were harvested from knee menisci of one-wk-old calves (Research 87, Boylston, MA) within 36 h of slaughter. Tissue was digested overnight in medium containing 0.2% collagenase type II (Worthington, Lakewood, NJ). Base medium consisted of DMEM with 4.5 g/L glucose and L-glutamine (Gibco, Grand Island, NY), 1% penicillin/streptomycin/fungizone, 1% non-essential amino acids, 0.4 mM L-proline, 5 mM ascorbate-2-phosphate, and 10 mM sodium pyruvate. Cells from multiple animals were pooled and frozen to obtain sufficient numbers for the experiments. Cylindrical agarose molds of 5 mm diameter were prepared as previously described.[41] Frozen ACs and FCs were thawed, resuspended in culture medium, and seeded at 5.5 × 106 cells/well in 150 μl of appropriate culture medium at specified FC:AC ratios. Cells maintained a spherical morphology throughout the isolation and seeding procedures as observed in our previous work.[24] Culture medium consisted of base medium with the addition of 1% ITS+ (BD Biosciences, San Jose, CA) and 100 nM dexamethasone. Constructs were given complete media changes of 500 μl on alternating days. After 10 days, constructs were excised from molds and placed in agarose coated 48 well plates and given 500 μl media changes on alternating days. Constructs were collected after 4 wks for gross measurements and partitioned to perform further analyses.
2.2. Phases I and II: GF/serum treatments and intermittent vs. continuous treatment
Constructs created from three combinations of FCs to ACs were examined (FC%:AC%): 100:0, 75:25, and 50:50. A full-factorial design was employed to test four levels of GF treatment (10 ng/ml TGF-β1, 5 ng/ml IGF-1, the combination of these, and no GF) and two levels of serum treatment (10% and none). The best overall GF/serum treatment for each culture ratio (that which resulted in the greatest mechanical integrity and matrix content) was carried forward to phase II to examine the effect of intermittent dosing. Intermittent groups were given GFs during the first and third wks, while continuous groups were supplemented throughout the 4 wk culture period.
2.3. Compressive and tensile biomechanics
Compression samples were prepared by removing a cylindrical portion from the center of the construct using a 2 mm biopsy punch. The sample was submerged in PBS and loaded into an Enduratec 3200 testing machine (Electroforce, Eden Prairie, MN). After determining initial sample thickness by applying a 0.02 N tare load, samples were preconditioned with 15 cycles of 5% strain at 1 Hz. A 10% step strain was applied for 20 min and stress relaxation data were recorded. Viscoelastic material properties were determined by fitting data to a Kelvin solid model in Matlab (MathWorks, Natick, MA). Equation 1 is a solution that describes stress relaxation in response to incremental strain application:[42]
| (1) |
where ui represents tissue deformation at the ith strain level, z represents tissue height, E r is the relaxation modulus, τσ and τε are time constants, and n represents the maximum strain level.
Tensile samples were dog-bone shaped pieces created using a biopsy punch. Samples were glued into paper frames and placed in grips of the Enduratec. Tension was applied at 1% strain/s until failure, and Young’s modulus was determined from the linear region of the stress vs. strain curve.
2.4. Quantitative biochemistry
Portions used for biochemical assessments were weighed, lyophilized, and digested in 1 ml papain at 65°C overnight. Total DNA content was determined in a fluorescence plate reader using Picogreen® Cell Proliferation Assay Kit (Molecular Probes, Eugene, OR). Total sulfated GAG content was determined using a dimethylmethylene blue (DMMB) dye-binding assay kit (Biocolor, Newtownabbey, Northern Ireland). Total collagen content was determined with a hydroxyproline assay using Sircol™ standards (Biocolor).[43]
2.5. Histology
Histology samples were frozen in tissue embedding medium and sectioned at 14 μm. Sections were fixed in 10% phosphate buffered formalin and stained with safranin-O/fast green to examine GAG distribution, and picrosirius red to examine collagen.
2.6. Statistical analyses
In phase I, a three-factor ANOVA (cell ratio, serum treatment, and GF treatment) was used to analyze quantitative data. For serum treatment, a Student’s t-test was performed post-hoc to determine significance between treatments, and a Tukey’s HSD was used for cell ratio and GF treatment assessments. In phase II, a two-factor ANOVA (cell ratio and GF treatment) was used, with a Student’s t-test or Tukey’s HSD test performed post-hoc for cell ratio and GF treatments, respectively. In both phases, a one-way blocked ANOVA was also performed to determine the best overall treatment for each of the cell ratios tested. Five samples were used for each experimental group, and all data are presented as mean ± S.D.
3. RESULTS
TGF-β1 in serum-free medium was determined to be best overall treatment for the 75:25 and 50:50 co-culture ratios and was therefore carried forward to phase II. Since GFs had little discernable effect on the 100:0 groups, this cell ratio was not examined in phase II.
3.1. Gross characteristics
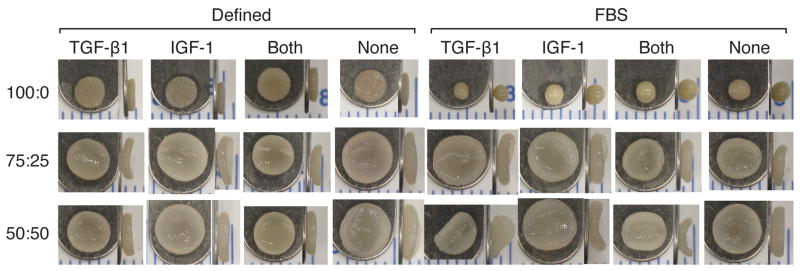
Construct gross morphology is shown in Figures 1 and 2. All constructs were disc-shaped, with the exception of serum treated 100% FC groups, which formed spherical constructs. In phase I, 100% FC groups had smaller diameters and wet weights (WW) than the co-culture groups, as demonstrated in Table 1, and 75:25 and 50:50 groups had similar WWs, diameters, and thicknesses for the respective GF and serum treatments. TGF-β1 and combined GF treatment led to 38% and 45% decreases in WW and 16% and 15% decreases in diameter relative to controls, respectively, while IGF-1 treatment did not have a significant effect. The presence of serum in culture medium led to a 12% decrease in WW and an 8% decrease in diameter relative to serum-free medium. In phase II, 75:25 and 50:50 groups had similar WWs, though 75:25 constructs were 5% smaller in diameter (Table 2). Continuous TGF-β1 supplementation caused more contraction and lower WW relative to intermittent and no TGF-β1, and intermittently treated constructs were also smaller and lighter than controls.
Figure 1.
Top and side views of phase I constructs after 4 wks of culture; scale markings are in mm. The 100:0 constructs contracted significantly from the original 5 mm diameter, while serum supplementation led to a spherical morphology. The 75:25 and 50:50 constructs were of similar size. TGF-β1 supplementation led to smaller constructs than IGF-1 supplementation alone or no GF treatment in co-culture constructs.
Figure 2.
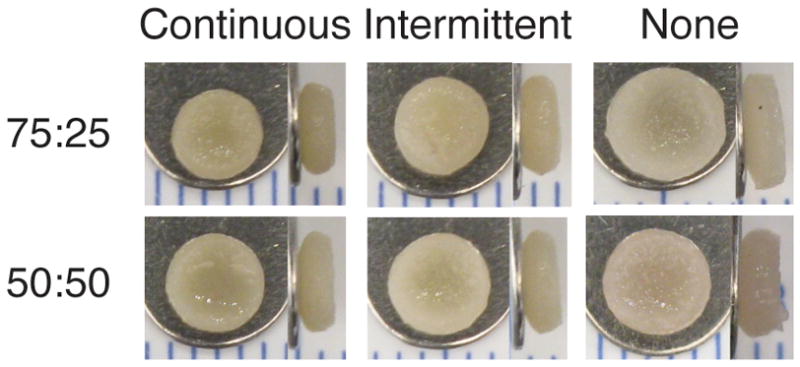
Top and side views of phase II constructs after 4 wks of culture; scale markings are in mm. Progressively greater contraction was observed with increased TGF-β1 supplementation.
Table 1.
Phase I construct characteristics after 4 wks of culture. Letters denote significance within each cell ratio, with a > b > c > d. Groups denoted by different letters are significantly different (p < 0.05)
| Cell ratio (%FC/AC) | Base medium | GF treatment | Wet Weight (mg) | Diameter (mm) | Thickness (mm) | Cells/construct (106) |
|---|---|---|---|---|---|---|
| 100:0 | Serum-free | TGF-β1 | 6.4 ± 0.7ab | 3.43 ± 0.12a | 0.76 ± 0.09b | 4.9 ± 0.1a |
| IGF-1 | 5.7 ± 0.3abc | 3.30 ± 0.24a | 0.75 ± 0.23b | 4.2 ± 0.1a | ||
| Both | 6.9 ± 0.5a | 3.59 ± 0.13a | 0.71 ± 0.05b | 4.9 ± 1.1a | ||
| None | 4.5 ± 0.1bc | 3.57 ± 0.02a | 0.69 ± 0.09b | 4.2 ± 0.6a | ||
| FBS | TGF-β1 | 3.7 ± 0.8c | 2.13 ± 0.16b | 1.78 ± 0.11a | 4.7 ± 0.7a | |
| IGF-1 | 5.2 ± 0.6abc | 2.29 ± 0.16b | 1.93 ± 0.14a | 4.2 ± 1.1a | ||
| Both | 5.3 ± 0.7abc | 2.20 ± 0.04b | 2.00 ± 0.03a | 5.0 ± 0.4a | ||
| None | 5.1 ± 0.1abc | 2.28 ± 0.05b | 1.95 ± 0.03a | 4.7 ± 0.6a | ||
| 75:25 | Serum-free | TGF-β1 | 10.5 ± 0.7d | 4.63 ± 0.14b | 0.87 ± 0.05ab | 3.9 ± 0.7ab |
| IGF-1 | 18.2 ± 2.0bc | 5.41 ± 0.15a | 0.87 ± 0.09ab | 4.0 ± 0.8ab | ||
| Both | 10.0 ± 1.0d | 4.50 ± 0.11b | 0.75 ± 0.10b | 4.6 ± 0.4a | ||
| None | 17.2 ± 1.3c | 5.51 ± 0.18a | 0.85 ± 0.07ab | 4.0 ± 0.6ab | ||
| FBS | TGF-β1 | 11.5 ± 0.3d | 4.71 ± 0.12b | 0.98 ± 0.10ab | 2.2 ± 0.9c | |
| IGF-1 | 23.1 ± 1.6a | 5.57 ± 0.11a | 0.99 ± 0.13a | 2.6 ± 0.8bc | ||
| Both | 11.9 ± 0.5d | 4.65 ± 0.13b | 0.86 ± 0.05ab | 2.6 ± 0.8bc | ||
| None | 21.8 ± 1.5ab | 5.64 ± 0.18a | 1.01 ± 0.13a | 3.0 ± 0.3bc | ||
| 50:50 | Serum-free | TGF-β1 | 10.7 ± 0.5d | 4.50 ± 0.10b | 0.92 ± 0.03a | 4.6 ± 0.7a |
| IGF-1 | 19.2 ± 1.0bc | 5.40 ± 0.41a | 1.00 ± 0.17a | 4.2 ± 0.8ab | ||
| Both | 10.4 ± 0.6d | 4.63 ± 0.05b | 0.86 ± 0.09a | 4.1 ± 0.5abc | ||
| None | 18.7 ± 0.4c | 5.40 ± 0.16a | 0.95 ± 0.08a | 4.7 ± 0.2a | ||
| FBS | TGF-β1 | 11.0 ± 0.9d | 5.00 ± 0.12b | 0.79 ± 0.01a | 2.3 ± 0.5d | |
| IGF-1 | 24.6 ± 1.4a | 5.75 ± 0.10a | 1.07 ± 0.23a | 3.2 ± 0.8bcd | ||
| Both | 11.3 ± 0.1d | 4.98 ± 0.11b | 0.78 ± 0.12a | 3.0 ± 0.4bcd | ||
| None | 22.6 ± 1.2ab | 6.09 ± 0.30a | 0.94 ± 0.08a | 2.8 ± 0.7cd | ||
Table 2.
Phase II construct characteristics after 4 wks in culture Letters denote significance within each cell ratio, with a > b > c. Groups denoted by different letters are significantly different (p < 0.05)
| Cell ratio (%FC:AC) | GF treatment | Wet weight (mg) | Diameter (mm) | Thickness (mm) | Cells/construct (106) |
|---|---|---|---|---|---|
| 75:25 | Continuous | 12.2 ± 0.4c | 3.98 ± 0.08b | 1.44 ± 0.11b | 5.2 ± 0.5a |
| Intermittent | 14.6 ± 1.0b | 4.21 ± 0.05b | 1.54 ± 0.08ab | 5.0 ± 0.3a | |
| None | 18.8 ± 1.3a | 4.78 ± 0.23a | 1.70 ± 0.15a | 4.0 ± 0.4b | |
| 50:50 | Continuous | 13.1 ± 0.5b | 4.28 ± 0.07b | 1.46 ± 0.06b | 5.8 ± 0.5a |
| Intermittent | 14.2 ± 0.5b | 4.42 ± 0.01b | 1.56 ± 0.06b | 5.6 ± 0.5ab | |
| None | 19.1 ± 1.1a | 4.94 ± 0.14a | 1.70 ± 0.05a | 5.0 ± 0.3b | |
3.2. Histology
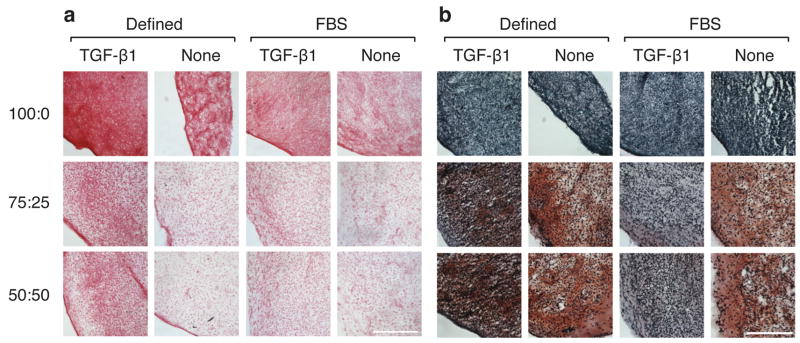
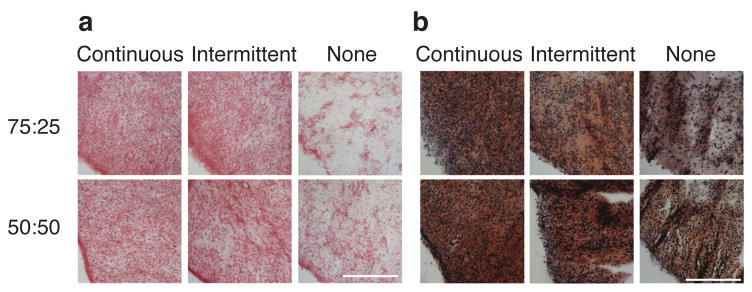
All groups stained positively for collagen (Figs. 3a and 4a). In phase I, staining was more intense in TGF-β1 and GF combination groups relative to IGF-1 and control groups for both co-culture ratios and base media. In phase II, continuous and intermittent groups stained more intensely than controls. The 75:25 and 50:50 groups stained positively for GAGs in both phases, while the 100:0 groups showed no GAG staining.
Figure 3.
Histological analysis of constructs from phase I. Picrosirius red stain for collagen (a) and safranin-o/fast green stain for GAG (b). IGF-1 and GF combination images were not shown due to their similarity to no-GF and TGF-β1 images. Bar represents 250 μm.
Figure 4.
Histological analysis of constructs from phase II. Collagen (a) and GAGs (b) were present throughout the constructs. Collagen staining was more intense in TGF-β1 treated constructs than in no GF constructs. Bar represents 250 μm.
3.3. Quantitative biochemistry
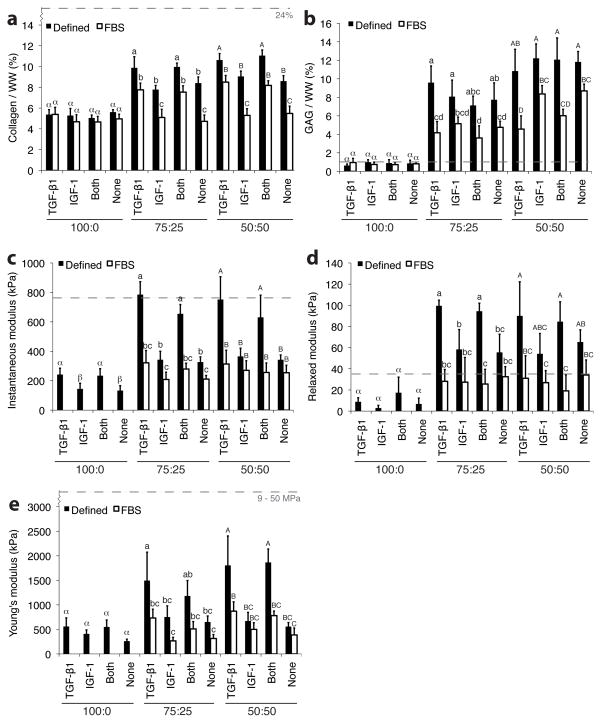
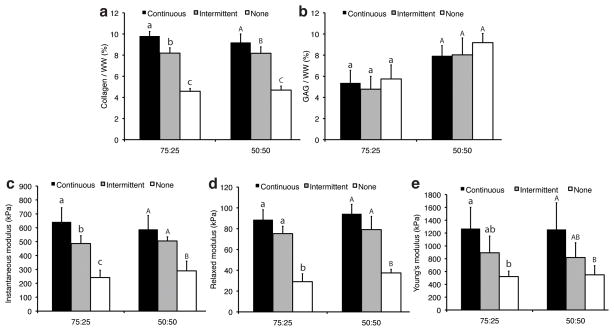
The 100% FC constructs contained more cells than co-culture constructs (Table 1), and the presence of serum in base medium coincided with a significant drop in cellularity relative to serum-free medium co-culture constructs. In phase I, collagen/WW was greatest in serum-free TGF-β1 and GF combination co-culture groups, with values ranging from 9.9 ± 1.1% to 11.0 ± 0.5%, while IGF-1 and no GF, serum-free co-culture groups ranged from 7.8 ± 0.4% to 9.0 ± 0.5% (Fig. 5a). Serum treated constructs yielded significantly less collagen relative to serum-free constructs, and similar collagen/WW values were obtained for 75:25 and 50:50 groups. Neither serum presence nor GF treatment had an effect on collagen content in 100% FC groups. In phase II, continuous TGF-β1 constructs had greater collagen/WW than intermittent and no GF groups (Fig 5b). GAG/WW was greatest in the 50:50 constructs, with values ranging from 10.8 ± 2.4% to 12.2 ± 1.6% for serum-free groups (Fig. 5b), while all 100:0 groups contained less than 1% GAG/WW. Serum treatment resulted in lowered GAG/WW for both co-culture ratios. In phase II, GF treatment did not significantly affect GAG/WW (Fig. 6b).
Figure 5.
Biochemical and biomechanical properties of constructs from phase I. Quantity of Collagen/wet weight (a) and GAG/weight (b) were higher for the co-culture groups than for the 100% FC groups. TGF-β1 treatment significantly increased instantaneous (c), relaxed (d), and Young’s moduli (e) relative to IGF-1 and no GF treatment. Dashed lines represent native human TMJ disc values obtained using similar equipment and testing and analysis methods.[53] Columns and error bars represent means and standard deviations. Letters denote significance among treatments within each cell ratio. Groups denoted by different letters are significantly different (p < 0.05).
Figure 6.
Biochemical and biomechanical properties of constructs from phase II. Collagen/WW (a) was greater with increasing TGF-β1 treatment, though GAG/WW (b) was not affected by TGF-β1 supplementation. Instantaneous (c), relaxed (d), and Young’s moduli (e) were higher with continuous and intermittent TGF-β1 supplementation than without GF treatment, and intermittent treatment led to increased biomechanical properties relative to no GF treatment. Letters denote significance within each cell ratio. Groups denoted by different letters are significantly different (p < 0.05).
3.4. Biomechanics
The serum-treated 100% FC groups were not mechanically testable. In phase I, treatment with TGF-β1 without serum increased instantaneous modulus two-fold relative to IGF-1 and no GF treatment in both co-culture groups (Fig. 5c). The greatest instantaneous modulus was 785 ± 88 kPa from the 75:25 TGF-β1 treated, serum-free group. The 100% FC groups were softer, with values between 133 ± 34 and 242 ± 43 kPa. Serum reduced compressive moduli relative to serum-free medium, and GF did not affect compressive moduli in the presence of serum. Overall, relaxed moduli trends across GF and serum treatments were similar to instantaneous moduli (Figs. 5c and 5d). In phase II, continuous and intermittent treatment increased compressive moduli relative to controls, and continuous groups were similar to intermittent groups, except for the 75:25 constructs upon instantaneous compression (Fig 6c and 6d).
TGF-β1 treatment dramatically increased Young’s modulus over IGF-1 and no GF controls (Fig. 5e). Young’s modulus values for co-culture groups treated with TGF-β1 in serum-free medium ranged from 1.49 ± 0.58 to 1.86 ± 0.27 MPa, while IGF-1 and no GF controls had values from 0.56 ± 0.08 to 0.75 ± 0.23 MPa. Overall, serum treatment reduced tensile stiffness 51% in the co-culture groups. In phase II, tensile stiffness was not significantly different among continuous and intermittent treatment groups within the 75:25 (p = 0.080) or 50:50 (p = 0.074) cell ratio groups, though the effect was significant (p = 0.009) for the combined groups (Fig 6e).
4. DISCUSSION
The overall aim of this work was to employ anabolic treatments to AC/FC co-cultures to develop an in vitro culturing regimen for regeneration of TMJ fibrocartilages. This study utilized a two-phased approach to elucidate 1) the effects of TGF-β1 and IGF-1 alone and in combination, and in serum-based and serum-free media, on the resulting construct functional properties, and 2) the effects of varying the supplementation regimen for the most effective anabolic treatments. Wide variation in morphological, biochemical, and biomechanical properties was found as a result of GF and serum treatments, and the results support the hypothesis that modulation of anabolic treatments would result in near recapitulation of native tissue functional properties.
Direct comparison of construct properties to native values provides validation of the methodologies explored in this study. Values for instantaneous modulus ranged from 130–790 kPa, while relaxed modulus ranged from 3–120 kPa, both of which approach or equal the native values. Under unconfined compression, a investigation of the porcine disc reported instantaneous moduli of 80–420 kPa and relaxed moduli of 17–38 kPa.[42] A similar investigation of porcine condylar cartilage reported elastic moduli from 800–1500 kPa and equilibrium moduli from 10–23 kPa.[16] Under tension, values of Young’s modulus from this study ranged from 0.3–1.9 MPa, significantly lower than native tissue values, which are between 9–28 MPa for the disc and 9–29 MPa for condylar cartilage.[20] Collagen/WW of constructs in this study (4–11%) were low compared with the native TMJ disc (~24%), [44] though GAG/WW values in this study (0.6-12%) actually exceeded those in the native disc (0.3–1.6%).[13, 44] The observation that constructs with a wide range of functional properties can be obtained by modulating anabolic treatments to FC/AC co-cultures, and that several properties overlap those seen in native values, are exciting findings. However, further optimization should be aimed at increasing collagen production and directing collagen organization to recapitulate higher end tensile values.
In agreement with our hypothesis, TGF-β1 supplementation significantly increased functional properties of co-culture constructs. In serum-free medium, collagen/WW increased 18% and 24% over no GF controls for 75:25 and 50:50 co-cultures, respectively. The effect of TGF-β1 on biomechanical properties was more robust, increasing instantaneous compressive modulus 140% and 120%, and increasing Young’s modulus 130% and 223% for 75:25 and 50:50 co-cultures, respectively. These findings reflect those of previous studies that show enhancement of cartilage construct properties using TGF-β1.[41, 45, 46] For example, in scaffoldless cultures of articular chondrocytes, 30 ng/ml TGF-β1 increased collagen/WW by 55%, compressive modulus by 73%, and tensile modulus by 58%.[41] In contrast to its effects on FC:AC co-cultures, the effects of TGF-β1 were not as evident on the 100% FC constructs. There were no differences in collagen/WW between GF treatments. While instantaneous compressive modulus was significantly higher than no GF controls (p = 0.0018), increases in relaxed modulus (p = 0.099) and Young’s modulus (p = 0.13) were not significant in TGF-β1 treated groups. These results were consistent with a recent study by Wilson et al.[46] that showed dramatic increases in dynamic compressive modulus in scaffoldless chondrocyte constructs with TGF-β1 supplementation compared with more modest increases in fibrochondrocyte constructs. As a result of its positive effects on construct functional properties, TGF-β1 treatment was selected for use in phase II on FC:AC co-cultures. The results showed benefits for both intermittent and continuous TGF-β1 treatment, although continuous treatment resulted in greater enhancement of construct functional properties. It is likely that the saturation threshold for TGF-β1 in this system was not met by the 10 ng/ml concentration. Further experimentation with higher concentrations and duty cycles of TGF-β1 treatment could yield further gains in functional properties.
IGF-1 did not appear to have an effect on construct properties in this study. In fact, groups supplemented with IGF-1 were not statistically different from controls in any functional assessment, either in the presence or absence of serum, and in addition, the combination of IGF-1 and TGF-β1 had statistically similar effects on constructs as TGF-β1 treatment alone for all functional assessments. This finding was surprising in light of previous work that shows increases in matrix synthesis in fibrochondrocyte constructs, [37] and increases in matrix synthesis and compressive properties in fibrochondrocyte and chondrocyte constructs with IGF-1 treatment.[7, 41] One explanation for this result is the presence of insulin in the base media. Insulin is considered an essential component of serum-free media due to its role in regulating glucose metabolism, though it also acts as a signaling molecule through receptor tyrosine kinases similar to those for IGF-1.[47, 48] A study by Bohme et al.[49] on chondrocytes in serum-free culture noted similar regulation of cell proliferation and collagen synthesis at identical insulin and IGF-1 concentrations. Considering that insulin is supplemented at 6.25 μg/ml in this study, compared with IGF-1 supplementation at 5 ng/ml, the effects of IGF-1 may be overshadowed by those of insulin.
Consistent with previous findings, [26, 27] 100% FC constructs contracted greatly from seeded dimensions, and it is therefore likely that a certain percentage of ACs is required to aid in maintenance of shape in cylindrical molds. However, when a nonadherent center post is incorporated into the mold, 100% FC constructs are better able to maintain their shape.[25] It is believed that cell-derived contraction forces, directed towards the center in cylindrical molds, are deflected circumferentially upon contact with the post, causing hoop stresses, which are likely responsible for the resultant circumferential fiber alignment.[25] The observed contraction could therefore be useful for TMJ fibrocartilage engineering assuming appropriate molds, capable of directing collagen into biomimetic orientations, can be designed for the TMJ.
For the co-cultures, the presence of serum had a detrimental effect shown by a decrease in all functional properties. For co-cultures of FCs and ACs on PLLA scaffolds, serum is necessary for maintenance of cellularity, [50] though in this study serum application actually reduced cellularity in the co-cultures by 57%. In addition, the combination of serum and TGF-β1 inhibited GAG production relative to either treatment alone. It is possible that serum application, in addition to the myriad of nutrients and signaling molecules in the serum-free medium, may have led to overstimulation of the chondrocytes. In the 100% FC groups, normalized matrix production was not affected by serum treatment, but obvious morphological differences were evident. This response suggests a role for serum in future engineering efforts in line with the above argument outlining the utility of contraction. Temporal application of serum could be used to induce desired morphological changes at appropriate stages in a tissue engineering process.
This study utilized a unique scaffoldless approach in which tissue develops from a high-density cell suspension.[24] Initial construct formation is facilitated by non-adherent agarose molds, which promote spherical cell morphology and cell-to-cell interactions.[51] However, within days of seeding the developing matrix is sufficiently robust to support the construct without the aid of the agarose, allowing constructs to be removed from the mold to grow in well plates while submerged in culture medium. This approach avoids a number of scaffold-related issues, including toxic degradation products and stress shielding.[24] In addition, there is evidence suggesting the progression of tissue maturation in this approach more closely follows known developmental processes for native cartilaginous tissue.[51] Though agarose was utilized in this study, similar results could be expected using other gels so long as they do not allow cell attachment.
As discussed in the introduction, this is a model system for translational use with clinically viable cells sources. Though it is possible that stem cells may respond differently to the combinatory treatments explored in this study, our expectation is that cells, differentiated along chondrocytic and fibrochondrocytic lineages, will respond in an analogous manner. Supporting this hypothesis is early results with differentiated human embryonic stem cells that suggest a serum-free, TGF-β1-containing medium best promotes fibrocartilaginous construct formation.[52] Further obstacles, such as attachment and integration in the TMJ, must also be addressed, and overcoming these remains a primary focus among the community of researchers involved with TMJ regeneration.[6]
In conclusion, this study assessed the effects of TGF-β1 and IGF-1, alone and in combination, in the presence or absence of serum, on three ratios of FCs and ACs, leading to construct functional properties approaching those of native TMJ fibrocartilages. Continuous treatment with TGF-β1 in the absence of serum was found to promote the greatest enhancement of ECM synthesis and biomechanical properties in FC/AC co-cultures. Future studies should use this treatment, in combination with mechanical stimulation such as hydrostatic pressure and direct compression, to further enhance collagen synthesis and tensile properties in fibrocartilage constructs. Direct translation of these findings towards the development of treatments for TMD will be realized through application of these strategies to clinically viable cells sources.
Acknowledgments
The authors would like to acknowledge funding from NIAMS 2R01 AR047839, NIDCR/NIAMS R01 DE019666, and NIDCR R01 DE015038. Additionally, the authors would like to thank the laboratory of Dr. K. Jane Grande-Allen for the use of their facilities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Solberg WK, Woo MW, Houston JB. Prevalence of mandibular dysfunction in young adults. J Am Dent Assoc. 1979;98:25. doi: 10.14219/jada.archive.1979.0008. [DOI] [PubMed] [Google Scholar]
- 2.NIDCR. TMJ Disorders. Vol. 2010. Bethesda, MD: NIDCR; 2006. [Google Scholar]
- 3.Wong ME, Allen KD, Athanasiou KA. Tissue Engineering of the Temporomandibular Joint. In: KBJ, editor. Tissue Engineering and Artificial Organs. Boca Raton, FL: CRC Press; 2006. [Google Scholar]
- 4.Wang L, Detamore MS. Tissue engineering the mandibular condyle. Tissue Eng. 2007;13:1955. doi: 10.1089/ten.2006.0152. [DOI] [PubMed] [Google Scholar]
- 5.Ingawale S, Goswami T. Temporomandibular joint: disorders, treatments, and biomechanics. Ann Biomed Eng. 2009;37:976. doi: 10.1007/s10439-009-9659-4. [DOI] [PubMed] [Google Scholar]
- 6.Detamore MS, Athanasiou KA, Mao J. A call to action for bioengineers and dental professionals: Directives for the future of TMJ bioengineering. Ann Biomed Eng. 2007;35:1301. doi: 10.1007/s10439-007-9298-6. [DOI] [PubMed] [Google Scholar]
- 7.Detamore MS, Athanasiou KA. Evaluation of three growth factors for TMJ disc tissue engineering. Ann Biomed Eng. 2005;33:383. doi: 10.1007/s10439-005-1741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almarza AJ, Athanasiou KA. Seeding techniques and scaffolding choice for tissue engineering of the temporomandibular joint disk. Tissue Eng. 2004;10:1787. doi: 10.1089/ten.2004.10.1787. [DOI] [PubMed] [Google Scholar]
- 9.Johns DE, Athanasiou KA. Improving culture conditions for temporomandibular joint disc tissue engineering. Cells Tissues Organs. 2007;185:246. doi: 10.1159/000102173. [DOI] [PubMed] [Google Scholar]
- 10.Bailey MM, Wang L, Bode CJ, Mitchell KE, Detamore MS. A comparison of human umbilical cord matrix stem cells and temporomandibular joint condylar chondrocytes for tissue engineering temporomandibular joint condylar cartilage. Tissue Eng. 2007;13:2003. doi: 10.1089/ten.2006.0150. [DOI] [PubMed] [Google Scholar]
- 11.Wang MSL, Lazebnik M, Detamore MS. Hyaline cartilage cells outperform mandibular condylar cartilage cells in a TMJ fibrocartilage tissue engineering application. Osteoarthritis Cartilage. 2008 doi: 10.1016/j.joca.2008.07.004. Epub. [DOI] [PubMed] [Google Scholar]
- 12.Eyre DR, Wu JJ. Collagen of fibrocartilage: a distinctive molecular phenotype in bovine meniscus. FEBS Lett. 1983;158:265. doi: 10.1016/0014-5793(83)80592-4. [DOI] [PubMed] [Google Scholar]
- 13.Detamore MS, Orfanos JG, Almarza AJ, French MM, Wong ME, Athanasiou KA. Quantitative analysis and comparative regional investigation of the extracellular matrix of the porcine temporomandibular joint disc. Matrix Biol. 2005;24:45. doi: 10.1016/j.matbio.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakano T, Scott PG. A quantitative chemical study of glycosaminoglycans in the articular disc of the bovine temporomandibular joint. Arch Oral Biol. 1989;34:749. doi: 10.1016/0003-9969(89)90082-4. [DOI] [PubMed] [Google Scholar]
- 15.Delatte M, Von den Hoff JW, van Rheden RE, Kuijpers-Jagtman AM. Primary and secondary cartilages of the neonatal rat: the femoral head and the mandibular condyle. Eur J Oral Sci. 2004;112:156. doi: 10.1111/j.0909-8836.2004.00108.x. [DOI] [PubMed] [Google Scholar]
- 16.Singh M, Detamore MS. Stress relaxation behavior of mandibular condylar cartilage under high-strain compression. J Biomech Eng. 2008 doi: 10.1115/1.3118776. Accepted. [DOI] [PubMed] [Google Scholar]
- 17.Allen KD, Athanasiou KA. Viscoelastic characterization of the porcine temporomandibular joint disc under unconfined compression. J Biomech. 2006;39:312. doi: 10.1016/j.jbiomech.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Sweigart MA, Zhu CF, Burt DM, DeHoll PD, Agrawal CM, Clanton TO, Athanasiou KA. Intraspecies and interspecies comparison of the compressive properties of the medial meniscus. Ann Biomed Eng. 2004;32:1569. doi: 10.1114/b:abme.0000049040.70767.5c. [DOI] [PubMed] [Google Scholar]
- 19.Detamore MS, Athanasiou KA. Tensile properties of the porcine temporomandibular joint disc. J Biomech Eng. 2003;125:558. doi: 10.1115/1.1589778. [DOI] [PubMed] [Google Scholar]
- 20.Singh M, Detamore MS. Tensile properties of the mandibular condylar cartilage. J Biomech Eng. 2008;130:011009. doi: 10.1115/1.2838062. [DOI] [PubMed] [Google Scholar]
- 21.Detamore MS, Hegde JN, Wagle RR, Almarza AJ, Montufar-Solis D, Duke PJ, Athanasiou KA. Cell type and distribution in the porcine temporomandibular joint disc. J Oral Maxillofac Surg. 2006;64:243. doi: 10.1016/j.joms.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connelly JT, Vanderploeg EJ, Mouw JK, Wilson C, Levenston ME. Tensile Loading Modulates BMSC Differentiation and the Development of Engineered Fibrocartilage Constructs. Tissue Eng Part A. doi: 10.1089/ten.tea.2009.0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoben GM, Willard VP, Athanasiou KA. Fibrochondrogenesis of hESCs: growth factor combinations and cocultures. Stem Cells Dev. 2009;18:283. doi: 10.1089/scd.2008.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu JC, Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006;12:969. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- 25.Aufderheide AC, Athanasiou KA. Assessment of a bovine co-culture, scaffold-free method for growing meniscus-shaped constructs. Tissue Eng. 2007;13:2195. doi: 10.1089/ten.2006.0291. [DOI] [PubMed] [Google Scholar]
- 26.Hoben GM, Hu JC, James RA, Athanasiou KA. Self-assembly of fibrochondrocytes and chondrocytes for tissue engineering of the knee meniscus. Tissue Eng. 2007;13:939. doi: 10.1089/ten.2006.0116. [DOI] [PubMed] [Google Scholar]
- 27.Hoben GM, Athanasiou KA. Creating a spectrum of fibrocartilages through different cell sources and biochemical stimuli. Biotechnol Bioeng. 2008;100:587. doi: 10.1002/bit.21768. [DOI] [PubMed] [Google Scholar]
- 28.Harkness L, Taipaleenmaki H, Mahmood A, Frandsen U, Saamanen AM, Kassem M, Abdallah BM. Isolation and differentiation of chondrocytic cells derived from human embryonic stem cells using dlk1/FA1 as a novel surface marker. Stem Cell Rev. 2009;5:353. doi: 10.1007/s12015-009-9099-4. [DOI] [PubMed] [Google Scholar]
- 29.Koay EJ, Hoben GM, Athanasiou KA. Tissue engineering with chondrogenically differentiated human embryonic stem cells. Stem Cells. 2007;25:2183. doi: 10.1634/stemcells.2007-0105. [DOI] [PubMed] [Google Scholar]
- 30.Dickhut A, Dexheimer V, Martin K, Lauinger R, Heisel C, Richter W. Chondrogenesis of human mesenchymal stem cells by local TGF-beta delivery in a biphasic resorbable carrier. Tissue Eng Part A. 2009 doi: 10.1089/ten.TEA.2009.0168. [DOI] [PubMed] [Google Scholar]
- 31.Felka T, Schafer R, Schewe B, Benz K, Aicher WK. Hypoxia reduces the inhibitory effect of IL-1beta on chondrogenic differentiation of FCS-free expanded MSC. Osteoarthritis Cartilage. 2009;17:1368. doi: 10.1016/j.joca.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 32.An C, Cheng Y, Yuan Q, Li J. IGF-1 and BMP-2 induces differentiation of adipose-derived mesenchymal stem cells into chondrocytes-like cells. Ann Biomed Eng. 38:1647. doi: 10.1007/s10439-009-9892-x. [DOI] [PubMed] [Google Scholar]
- 33.Diekman BO, Rowland CR, Caplan AI, Lennon D, Guilak F. Chondrogenesis of adult stem cells from adipose tissue and bone marrow: Induction by growth factors and cartilage derived matrix. Tissue Eng Part A. 2009 doi: 10.1089/ten.tea.2009.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng Y, Hu JC, Athanasiou KA. Isolation and chondroinduction of a dermis-isolated, aggrecan-sensitive subpopulation with high chondrogenic potential. Arthritis Rheum. 2007;56:168. doi: 10.1002/art.22300. [DOI] [PubMed] [Google Scholar]
- 35.Bilgen B, Ren Y, Pei M, Aaron RK, Ciombor DM. CD14-negative isolation enhances chondrogenesis in synovial fibroblasts. Tissue Eng Part A. 2009;15:3261. doi: 10.1089/ten.tea.2008.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pangborn CA, Athanasiou KA. Effects of growth factors on meniscal fibrochondrocytes. Tissue Eng. 2005;11:1141. doi: 10.1089/ten.2005.11.1141. [DOI] [PubMed] [Google Scholar]
- 37.Pangborn CA, Athanasiou KA. Growth factors and fibrochondrocytes in scaffolds. J Orthop Res. 2005;23:1184. doi: 10.1016/j.orthres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 38.Detamore MS, Athanasiou KA. Effects of growth factors on temporomandibular joint disc cells. Arch Oral Biol. 2004;49:577. doi: 10.1016/j.archoralbio.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 40.Lieb E, Vogel T, Milz S, Dauner M, Schulz MB. Effects of transforming growth factor beta1 on bonelike tissue formation in three-dimensional cell culture. II: Osteoblastic differentiation. Tissue Eng. 2004;10:1414. doi: 10.1089/ten.2004.10.1414. [DOI] [PubMed] [Google Scholar]
- 41.Elder BD, Athanasiou KA. Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis Cartilage. 2009;17:114. doi: 10.1016/j.joca.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allen KD, Athanasiou KA. A surface-regional and freeze-thaw characterization of the porcine temporomandibular joint disc. Ann Biomed Eng. 2005;33:951. doi: 10.1007/s10439-005-3872-6. [DOI] [PubMed] [Google Scholar]
- 43.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 44.Almarza AJ, Bean AC, Baggett LS, Athanasiou KA. Biochemical analysis of the porcine temporomandibular joint disc. Br J Oral Maxillofac Surg. 2006;44:124. doi: 10.1016/j.bjoms.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Elder BD, Athanasiou KA. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS ONE. 2008;3:e2341. doi: 10.1371/journal.pone.0002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson CG, Nishimuta JF, Levenston ME. Chondrocytes and meniscal fibrochondrocytes differentially process aggrecan during de novo extracellular matrix assembly. Tissue Eng Part A. 2009;15:1513. doi: 10.1089/ten.tea.2008.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alberts B. Molecular biology of the cell. New York: Garland Science; 2002. [Google Scholar]
- 48.Gaissmaier C, Koh JL, Weise K. Growth and differentiation factors for cartilage healing and repair. Injury. 2008;39 (Suppl 1):S88. doi: 10.1016/j.injury.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 49.Bohme K, Conscience-Egli M, Tschan T, Winterhalter KH, Bruckner P. Induction of proliferation or hypertrophy of chondrocytes in serum-free culture: the role of insulin-like growth factor-I, insulin, or thyroxine. J Cell Biol. 1992;116:1035. doi: 10.1083/jcb.116.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gunja NJ, Uthamanthil RK, Athanasiou KA. Effects of TGF-beta1 and hydrostatic pressure on meniscus cell-seeded scaffolds. Biomaterials. 2009;30:565. doi: 10.1016/j.biomaterials.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ofek G, Revell CM, Hu JC, Allison DD, Grande-Allen KJ, Athanasiou KA. Matrix development in self-assembly of articular cartilage. PLoS ONE. 2008;3:e2795. doi: 10.1371/journal.pone.0002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koay EJ, Athanasiou KA. Development of serum-free, chemically defined conditions for human embryonic stem cell-derived fibrochondrogenesis. Tissue Eng Part A. 2009;15:2249. doi: 10.1089/ten.tea.2008.0320. [DOI] [PubMed] [Google Scholar]
- 53.Kalpakci KN, Willard VP, Wong ME, Athanasiou KA. An Interspecies Comparison of the Temporomandibular Joint Disc. J Dent Res. doi: 10.1177/0022034510381501. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]