Abstract
Rationale
Delayed matching-to-position and nonmatching-to-position procedures are widely used to model working memory in rodents. Mediating behavior—which enhances performance but is not explicitly required by the task—is generally considered an obstacle to the measurement of memory, but often occurs despite attempts to prevent it. The ubiquitous nature of mediating behavior suggests it might be analogous to rehearsal, an important component of learning and memory in humans.
Objectives
The aim was to study an easily recordable, rehearsal-like mediating response in rats under baseline conditions and after treatment with amnestic drugs [scopolamine (0.1–0.3 mg/kg) and delta-9-tetrahydrocannabinol (THC; 1–5.6 mg/kg)].
Methods
Lighted nosepoke holes were used to present position cues and record delayed matching or nonmatching responses. Performance of a distractor task was required to prevent simply waiting at the correct choice, but the nosepoke holes were left accessible during the delay.
Results
Each rat trained with the nonmatching task exhibited one of two mediating “strategies” that increased the odds of a correct choice: responding in the to-be-correct hole during the delay or responding in the opposite hole during the delay. Rats trained with the matching task all showed the former strategy. Treatment with scopolamine disrupted performance of the mediating response. Scopolamine and THC both decreased the effectiveness of the mediating response, increasing errors even on trials when the “appropriate” mediating behavior did occur.
Conclusions
The procedures and data analysis approach used here provide an objective, automated means of measuring mediating behavior, which might be useful as an animal model of memory rehearsal.
Keywords: Delayed spatial matching, Mediating response, Working memory, Rehearsal, Scopolamine, THC
Introduction
Delayed matching-to-position (DMTP) procedures have been used extensively to model working memory in rats. In a typical DMTP procedure, rats are trained in a chamber with two retractable levers. At the start of a trial, one of the two levers is extended as the sample and then retracted when it is pressed by the rat. After a delay period, both levers are extended, and the rat receives a food pellet if it presses the correct lever (i.e., the same one that had been presented as the sample). If the incorrect lever is pressed instead, no food is delivered and the trial ends. This can be repeated many times during a daily session with the delay value varied across trials to provide a within-session function relating accuracy to delay. The procedure for delayed nonmatching to sample (DNMTP) is similar, except the rat receives a food pellet only when it chooses the lever that was not presented as the sample.
DMTP and DNMTP procedures are modified in various ways to prevent the rat from simply remaining in front of the to-be-correct choice throughout the delay. The rat may be required to press a panel or poke its nose into an aperture during the delay, or food pellets may be presented to draw the rat away from the retracted choice levers until they are extended. However, rats still tend to exhibit specific mediating behaviors that can facilitate choosing the correct lever but are not required by the procedure (Pontecorvo et al. 1996). For example, when rats can obtain food by nosepoking during the delay, they still repeatedly approach and sometimes even depress the retracted levers (Bushnell 1988). When rats are required to press a panel during the delay, they often press on the side of the panel closest to the to-be-correct lever or press the panel with a paw while keeping their head oriented toward the to-be-correct lever (Chudasama and Muir 1997; see also Stanhope et al. 1995).
Mediating behavior has been recognized since the earliest delayed matching experiments (Hunter 1913), and it is not restricted to procedures in which position is the relevant property of the sample. Blough (1959) found that some pigeons trained with a visual matching to sample task developed differential patterns of behavior during the delay, such as slowly waving the head from side to side when the sample had been a flickering light versus rapidly pecking the unlit central sample bar when the sample had been a steady light. Hunter and Blough both suggested that subjects that did not demonstrate overt mediating behavior might have used analogous covert responses. These overt and covert responses might be analogous to memory rehearsal in humans (Grant 1982, 1998), a normal component of working memory that is worth studying in its own right (D’Esposito 2007; Hasher and Zacks 1979; Smith and Jonides 1998; Unsworth and Engle 2007).
Rather than viewing overt mediating behavior as a confounding variable that clouds the measurement of working memory in DMTP and DNMTP procedures, it might be productive to treat it as an observable manifestation of rehearsal (Dudchenko and Sarter 1992; Jans and Catania 1980). Visual observation with a structured rating system and verified inter-rater reliability provides the most comprehensive means of measuring behavior, but this approach can be time- and labor-intensive. Therefore, the study of overt mediating behavior might be facilitated by the availability of an objective, automated recording method.
To investigate mediating behavior in DMTP and DNMTP procedures in rats, we used illumination of nose-poke holes rather than extension of levers as the position stimuli. Responding was required in a separate hole during the delay to prevent the rat from simply remaining in front of one of the choice holes. However, the darkened choice holes remained accessible throughout the delay period to allow responding in these holes as an overt mediating response. Responses during the delay were analyzed to determine whether they predicted the accuracy of the choice response. Since amnestic drugs might alter mediating behavior in working memory tasks (Chudasama and Muir 1997; Dudchenko and Sarter 1992; Herremans et al. 1996), we also examined the effects of two drugs that have been used extensively as amnestic agents in working memory research: the muscarinic-receptor antagonist scopolamine and the cannabinoid-receptor partial agonist Δ9-tetrahydrocannabinol (THC).
Materials and methods
Subjects
Male Sprague–Dawley and Long–Evans hooded rats were maintained in individual cages with a 12-h light/dark cycle (lights on at 0645 hours). Procedures were conducted Monday through Friday between 0900 and 1300 hours. Rats were fed approximately 15 g of food per day to maintain stable body weights. The facilities were fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and all experiments were conducted in accordance with the guidelines of the Animal Care and Use Committee of the National Institute on Drug Abuse Intramural Research Program and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 1996).
Apparatus
Each of eight individually enclosed training chambers (model MED-NPW-9L; MED Associates, St. Albans, VT) had nine response holes (2 cm high×2 cm wide×2 cm deep) in a horizontal array on one wall. Only the 3rd, 5th, and 7th holes were used; the rest were covered by metal plates. The side holes were 2.75 cm from the center hole. Each open hole could be illuminated from within by a yellow LED at the back of the hole. Food pellets (45 mg; type F0021; Bio-Serv, Frenchtown, NJ, USA) were dispensed into a food trough mounted on the wall opposite to the wall with the response holes.
Drugs
Scopolamine hydrobromide (Sigma-Aldrich, St. Louis, MO) in a vehicle of saline solution was given 20 min before the session. THC (Research Triangle Institute, Research Triangle Park, NC) in a vehicle of 40% cyclodextrin (Sigma-Aldrich, St. Louis, MO) and 60% saline was given 30 min before the session. Drugs were injected i.p (1 ml/kg) and were given up to two times per week, usually on Tuesday or Friday.
Procedure
Magazine training and response shaping
Rats were trained to eat from the food trough and respond in one of the nosepoke holes during two sessions in which a food pellet was presented automatically about every 45 s (range, 30–60 s) or when a response occurred in the center hole. The center hole was lit and the houselight was off throughout the session except for 15 s after each pellet delivery, when the hole light was extinguished, the houselight was lit, and responses had no programmed effect. Once the rat had produced 20 pellets by nosepoking within a session, automatic presentation of pellets was discontinued for the remainder of the session. Each session lasted until 150 pellets were delivered. All rats produced at least 100 pellets by nosepoking during the second session.
Preliminary response-chain training
For the next five sessions, rats were trained with a discrete-trial procedure that required a chain of responding in two holes. At the start of a trial, the center hole was lit. Two responses in the center hole extinguished the center-hole light and immediately lit a randomly selected side hole. Then, a single response in the lit side hole delivered a food pellet, extinguished the side-hole light, and turned on the houselight for a 15-s intertrial interval during which further responses had no programmed effect. These sessions lasted for 120 min or 100 pellets, whichever came first.
Matching and nonmatching to position
During all subsequent sessions, each trial started with the houselight off and one of the two side holes being lit as the sample. Two responses in the sample hole were required to extinguish the side-hole light and turn on the center-hole light, starting the delay period. After a delay of 0, 7, 14, 21, or 28 s, the next response in the center hole extinguished the center-hole light and lit both side holes, starting the choice phase of the trial. Depending on whether a rat was trained with the matching or nonmatching task, either the sample hole or the opposite side hole, respectively, was considered the correct choice. A response in the correct hole immediately produced a food pellet, extinguished the hole lights, and turned on the houselight for a 15-s intertrial interval. An incorrect choice response produced no pellet, extinguished the hole lights, and caused the houselight to flash (5 Hz) for 5 s, after which the houselight remained on for the 15-s intertrial interval. Under the final training parameters, the delay value for each trial was selected by drawing without replacement from a list in which each of the five possible values appeared once. When each of the values had been used once, the list was replenished. The side used for the sample hole was chosen in a similar manner, using a list in which each side (left and right) appeared twice. During initial training with the matching or nonmatching procedure, only the 0-s delay was used; longer delays were added gradually over 30 sessions. Each session lasted for 90 min or 100 food pellets, whichever came first. One group of Sprague–Dawley rats (n=6) was trained with the matching procedure, one group of Sprague–Dawley rats (n=8) was trained with the nonmatching procedure, and one group of Long–Evans rats (n=9) was trained with the nonmatching procedure. Although drug effects could not be compared across strains, the use of both Sprague–Dawley and Long–Evans rats in the nonmatching task allowed an assessment of strain-related differences in baseline performance under this task.
Drug testing
When the accuracy of choice responding was consistent (with over 90% correct at the 0-s delay and <10 percentage point difference in accuracy at each given delay over consecutive sessions), drug testing was conducted. First, dose-effect functions were obtained with scopolamine (0, 0.1, and 0.3 mg/kg) in both of the Sprague–Dawley groups and with THC (0, 1, 3, and 5.6 mg/kg) in the Long–Evans group by giving each dose at least once, in counterbalanced order. Since some rats showed little or no effect at lower doses during this initial phase, in the next phase more extensive testing was conducted to compare vehicle with 0.3 m/kg scopolamine in the Sprague–Dawley rats and with 5.6 mg/kg THC in the Long–Evans rats; during this phase, each rat was given scopolamine or THC three to six times to ensure a sufficient number of trials for analysis of mediating responses. One rat failed to respond in the nosepoke holes when given the high dose of THC and was dropped from this phase.
Data analysis
Data for each group were analyzed separately. For each subject, data from all sessions at a specific dose were pooled. All analyses except logistic regression were performed using restricted maximum likelihood estimation (Proc Mixed; SAS Institute, Cary, NC), using Tukey–Kramer correction to maintain a .05 significance level for post-hoc comparisons. Arcsine-root transformation was used for percentage measures. To assess accuracy of choice responding, the percentage of trials with a correct response was analyzed using drug dose and delay value as factors. To assess mediating behavior during the delay periods, side-hole responses in each trial were characterized as occurring in either the hole that would be correct at choice time or the hole that would be incorrect at choice time. To determine whether behavior during the delay predicted the accuracy of the choice response, logistic regression was performed on data under vehicle conditions for each rat, relating trial outcome (correct vs. incorrect) to three factors: whether there was at least one response in the to-be-correct hole, whether there was at least one response in the to-be-incorrect hole, and the length of the delay (excluding the nominal 0-s delay, for which there were few side-hole responses). This analysis provided odds ratios for each rat describing the influence of side-hole responding, taking the effect of delay into account. In certain rats, a response in the to-be-correct hole during the delay increased the odds of a correct outcome, but in other rats a response in the to-be-incorrect hole increased the odds of a correct outcome. Therefore, in all subsequent analyses, the “appropriate” mediating hole was defined for each rat as the hole associated with the higher odds ratio, and the “inappropriate” mediating hole was defined as the hole associated with the lower odds ratio (The labels “appropriate” and “inappropriate,” defined by the subject’s established pattern of behavior, were chosen to be distinct from “correct” and “incorrect,” which are defined by the procedure). Four “trial types” were defined in terms of whether there was at least one response during the delay (1) in the appropriate hole only, (2) in the inappropriate hole only, (3) in both side holes, or (4) in neither side hole. To determine whether drugs altered behavior during the delay, Proc Mixed was used to assess the effects of drug treatment and delay value on response rates in the three holes and on the relative frequency of the four trial types. To determine whether drugs altered the relationship between behavior during the delay and the subsequent outcome of the trial, Proc Mixed was used to assess the effects of trial type, drug treatment, and delay value on the accuracy of choice responding.
Results
Effects of drugs on accuracy of matching or nonmatching
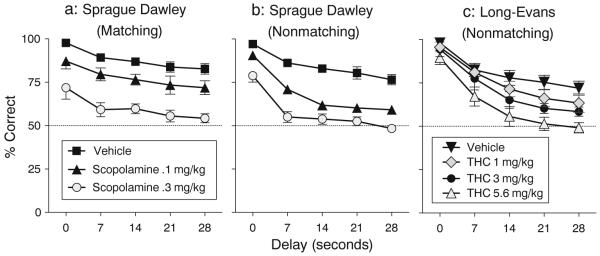
For all three groups of rats, accuracy of the choice response under vehicle treatment was about 97% at the shortest delay and decreased monotonically at longer delays, with accuracy of 82% at the longest delay in the matching group and about 75% in the nonmatching groups (Fig. 1). Accuracy in vehicle-treatment sessions was about the same as during baseline sessions in which no injection was given (data not shown). Length of the delay significantly affected accuracy in all three groups [main effect of delay; matching group, Fig. 1a: F(4,20)=8.62, p<.0003; Sprague–Dawley nonmatching group, Fig. 1b: F(4,28)=75.4, p<.0001; Long–Evans nonmatching group, Fig. 1c: F(4,32)=129.18, p<.0001].
Fig. 1.
Accuracy of matching or nonmatching to position under vehicle and drug conditions in the three groups: a Sprague–Dawley rats trained with the matching task and tested with scopolamine, b Sprague–Dawley rats trained with the nonmatching task and tested with scopolamine, and c Long–Evans rats trained with the nonmatching task and tested with THC. Data represent mean (±s.e.m.) percentage of trials with a correct outcome as a function of dose and delay value
Scopolamine significantly affected accuracy [main effect of scopolamine dose; matching, Fig. 1a: F(2,10)=95.52, p< .0001; nonmatching, Fig. 1b: F(2,14)=166.65, p<.0001], with the nonmatching group showing greater sensitivity to this effect. That is, (1) both doses of scopolamine produced significant deficits at all delay values in the nonmatching task, and (2) the high dose (0.3 mg/kg) produced significant deficits at all delay values in the matching task, but (3) the low dose (0.1 mg/kg) did not significantly affect accuracy at any delay in the matching group.
THC, which was only tested with the nonmatching task, also produced a dose-dependent decrease in accuracy [main effect of THC treatment, Fig. 1c: F(3,23)=38.54, p<.0001]. The highest dose (5.6 mg/kg) significantly decreased accuracy at all delays. The intermediate dose (3 mg/kg) produced significant effects at delays greater than 7 s. The lowest dose (1 mg/kg) did not have any significant effect on accuracy.
In all three groups, accuracy was significantly higher at the 0-s delay than at the other delays under vehicle conditions. In the matching group, under both doses of scopolamine accuracy no longer differed between the 0-s delay and the longer delays; but, in the nonmatching groups, both doses of scopolamine and all three doses of THC shifted the accuracy curves in such a way that the superiority of accuracy at the 0-s delay was maintained.
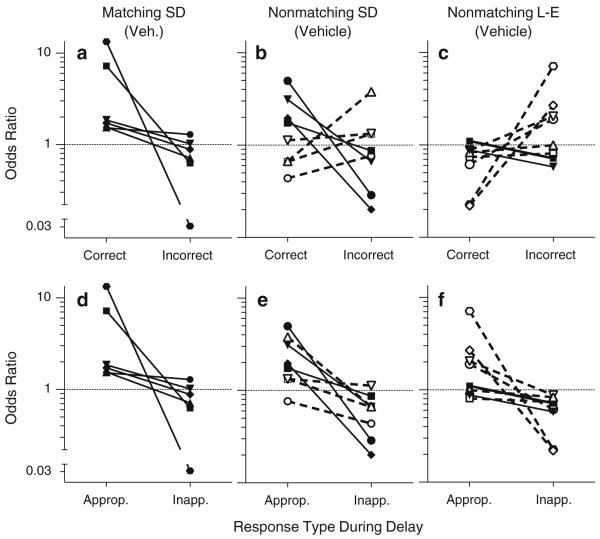
Odds ratios
For all of the rats in the matching group (Fig. 2a), the odds of a correct choice were increased when there was a response in the to-be-correct hole during the delay (as indicated by a odds ratio greater than 1), but the odds were either increased to a lesser extent (as indicated by a value closer to but still greater than 1) or even decreased (as indicated by a value less than 1) when there was a response in the to-be-incorrect hole. Thus, the functions in Fig. 2a slope downward for all rats in the matching group. In contrast, only half the rats in the Sprague–Dawley nonmatching group (Fig. 2b) and two of the nine rats in the Long–Evans nonmatching group (Fig. 2c) show this pattern; the remaining rats show the opposite pattern, with a higher odds ratio associated with the to-be-incorrect hole than the to-be-correct hole. Based on these results, the hole associated with the higher odds ratio for each individual rat was defined as “appropriate” (as depicted in Fig. 2d, e, f) for all subsequent analyses of delay responding presented below.
Fig. 2.
Odds ratios for each subject in each of the three groups, based on data obtained under vehicle treatment. The upper panels (a, b, c) show the ratios when at least one response was made in the to-be-correct hole or in the to-be-incorrect hole during the delay period. The lower panels (d, e, f) show the same data, but with “appropriate” defined for each subject as the hole with the higher odds ratio and “inappropriate” defined as the hole with the lower odds ratio. Dashed lines represent subjects for which the “appropriate” hole was the to-be-incorrect hole. In all panels, odds ratios higher than 1 indicate improved odds of a correct trial outcome, and odds ratios lower than 1 indicate decreased odds of a correct trial outcome
Rates of delay responding
Response rates for the matching group [Fig. 3a; F(2,10)=6.14, p<.02] and the Sprague–Dawley nonmatching group [Fig. 3b; F(2,14)=5.57, p<.02] showed significant hole by drug treatment interactions, but only the main effect of hole was significant for the Long–Evans nonmatching group [Fig. 3c; F(2,16)=89.85, p<.0001]. Delay value did not significantly affect response rates in any group, regardless of hole or drug treatment. All three groups responded significantly more in the center hole than in either side hole under all conditions (vehicle and drug). Both of the Sprague–Dawley groups (matching and nonmatching) had significantly higher response rates in the appropriate hole than in the inappropriate hole under vehicle conditions but not under scopolamine; in the nonmatching group, this was due to a significant 44.3% increase in inappropriate-hole responding. Response rates during the delay did not differ significantly between the appropriate and inappropriate holes for the Long–Evans nonmatching group under vehicle conditions or under THC.
Fig. 3.
Response rates in the center, “appropriate,” and “inappropriate” holes during the delay period. For each group, data represent mean (±s.e.m.) responses per second as a function of treatment (vehicle or drug) and delay value
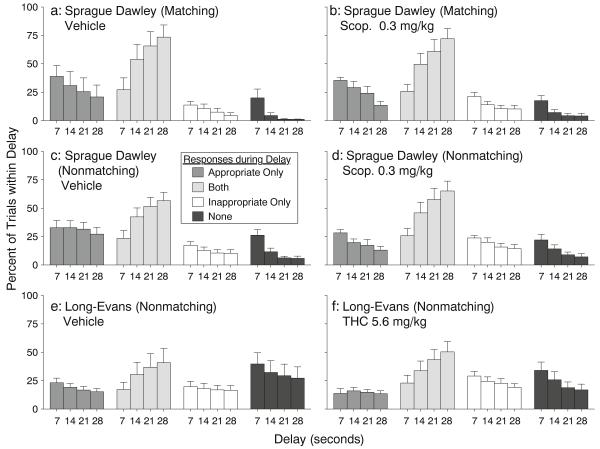
Distribution of mediating-response trial types
In all three groups, the relative frequency of the four trial types (i.e., the percentage of trials at each delay value that were categorized as “appropriate only,” “inappropriate only,” “both,” or “neither”) was significantly affected by the interaction of trial type and delay value [matching group, Fig. 4a, b: F(9,45)=8.23, p<.0001; Sprague–Dawley nonmatching group, Fig. 4c, d: F(9,63)=9.76, p<.0001; Long–Evans nonmatching group, Fig. 4e, f: F(9,63)=3.84, p<.002]. The frequency of “none” trials tended to be higher in the Long–Evans group than the other groups. For all three groups, post-hoc comparisons revealed that the distribution of “both” trials (i.e., trials with delay responses in both side holes) was delay dependent, with significantly fewer “both” trials at the 7-s delay than at longer delays. “Both” trials were significantly more frequent than other trial types at delays of 14, 21, and 28 s for the Sprague–Dawley groups but only at 28 s for the Long–Evans group. The distribution of trial types was only affected significantly by drug treatment in the Sprague–Dawley nonmatching group [Fig. 4c, d, interaction of trial type and dose: F(3,21)=5.0, p<.009], which showed a scopolamine-induced decrease of 36.8% in the frequency of “appropriate only” trials.
Fig. 4.
Distribution of trials with at least one response during the delay period in the “appropriate” hole only, both holes, the “inappropriate” hole only, or neither hole. For each group, data represent mean (±s.e.m.) percentage of trials of each type within each delay value, as a function of treatment (vehicle or drug) and delay value
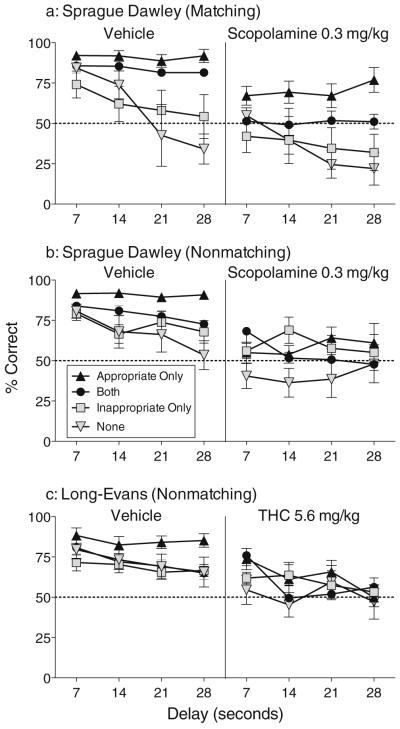
Effects of mediating behavior on accuracy
When accuracy was analyzed as a function of mediating behavior (i.e., trial type), delay value, and drug treatment, it was found that in trials with only appropriate responses during the delay, accuracy was quite high and did not change as a function of delay (see Fig. 5). In the matching group, accuracy varied as a function of trial type [Fig. 5a; F(3,15)= 3.42, p<.05] and drug treatment [Fig. 5a; F(1,5)=59.98, p<.0006]. In the nonmatching groups, accuracy was significantly affected by the interaction of trial type and drug treatment [Sprague–Dawley, Fig. 5b: F(3,21)=4.34, p<.02; Long–Evans, Fig. 5c: F(3,21)=4.58, p<.02]. Post-hoc comparisons revealed that accuracy in the matching group was higher in “appropriate only” trials than in each other trial type; this was true under both vehicle and scopolamine. In the two nonmatching groups, accuracy was also significantly higher in “appropriate only” trials than all other trial types under vehicle treatment, but unlike the matching group, accuracy in the nonmatching groups no longer differed between the trial types when rats received scopolamine or THC. Furthermore, accuracy of the matching group was only significantly decreased by scopolamine treatment in “both” trials, while in the nonmatching groups, scopolamine or THC significantly decreased accuracy in all trial types except “inappropriate only”.
Fig. 5.
Accuracy as a function of behavior during the delay (i.e., trial type). For each group, data represent mean (±s.e.m.) percentage of trials with a correct outcome as a function of trial type, delay value, and treatment (vehicle or drug)
Discussion
The baseline accuracy of delayed matching and nonmatching behavior in the present study was comparable to the performance of rats in other DMTP and DNMTP studies that involved retractable levers (e.g., Bushnell 1988; Harper et al. 2006) or illuminable nose-poke holes (Etherington et al. 1987; McAlonan et al. 1995). Treatment with amnestic drugs produced dose-dependent decreases in accuracy that were comparable to those seen in previous studies with scopolamine (Bushnell 1990; Chudasama and Muir 1997; Dunnett 1985) or THC (Mallet and Beninger 1998; Hampson and Deadwyler 2000; Heyser et al. 1993). Analysis of nose-poke responding during the delay period suggests that this behavior served a mediating function, enhancing the accuracy of performance even though it was not explicitly required by the task. This automatically recorded mediating behavior appears to be analogous to the mediating behavior observed visually in earlier studies (Bushnell 1988; Chudasama and Muir 1997; Herremans et al. 1996).
In all rats trained with the matching procedure, responding in the to-be-correct hole during the delay increased the odds of a correct outcome in the trial; but surprisingly, in rats trained with the nonmatching procedure, there were two distinct patterns of mediating behavior. Some of these rats performed better when they responded in the sample hole during the delay, while the others performed better when they responded in the nonmatching hole during the delay. The essential difference between these two patterns seems to be in the timing of the switch from responding in the sample hole to responding in the nonmatching hole. That is, after the sample-hole response that started the delay period, some rats switched to the to-be-correct hole during the delay, but the other rats continued to respond in the sample hole during the delay, then switched to the correct hole when the delay ended.
It should be noted that this mediating behavior was interspersed with responding in the center hole, which was required to end the delay. All rats responded much more in the center hole than in either side hole during the delay. Although the two Sprague–Dawley groups had significantly higher response rates in the “appropriate” hole than the “inappropriate” hole, the magnitude of these differences was small, and the Long–Evans group’s response rates were about the same in both side holes. When trials were sorted according to whether there was at least one response in the appropriate hole only, the inappropriate hole only, both holes, or neither hole, it was found that “both” trials were most frequent in all three groups, especially at longer delay values, but each of the other three trial types also occurred at a substantial frequency.
The most striking finding regarding mediating behavior is that accuracy under vehicle conditions was consistently high in trials that included at least one mediating response in the appropriate hole and none in the inappropriate hole. Accuracy in these “appropriate only” trials did not decrease at even the longest delay value (Fig. 5, left panels). Thus, the fact that accuracy decreased as a function of delay when trials of all types were averaged together (Fig. 1) must be attributed to trials of the other three types, in which at least one response occurred in the inappropriate hole (“both” or “inappropriate only” trials) or there were no side-hole responses at all (“none” trials). Unlike “appropriate only” trials, in which all three groups had consistently high accuracy, the matching group differed from the nonmatching groups with regard to the other three trial types. In the matching group, the delay curve showing accuracy of “both” trials was almost as high and flat as the “appropriate only” curve, and it was clearly distinguishable from the “inappropriate only” and “none” curves. In contrast, the “both” curve was similar to the “inappropriate only” and “none” curves in the nonmatching groups.
The matching and nonmatching groups also differed with respect to the differential effects of amnestic drugs on accuracy in the four trial types (Fig. 5, right panels). When the matching group was given scopolamine, accuracy was only decreased significantly in “both” trials; “appropriate only” trials maintained clear superiority over the other trial types. In the nonmatching groups, scopolamine and THC decreased significantly accuracy in all trial types except “inappropriate only”, and “appropriate only” trials were no longer superior. Thus, scopolamine disrupted the effectiveness of rehearsal in both the matching and nonmatching tasks, but the nonmatching task was more sensitive to this disruption. This might be due to the fact that the nonmatching task itself and the mediating behavior that develops in this task are more complex, involving a switch from the sample hole to the nonmatching hole at either the beginning or end of the delay.
In addition to producing a greater disruption in the effectiveness of mediating behavior in the nonmatching task compared to the matching task, scopolamine also produced a more distinct change in the distribution of mediating behavior in the nonmatching task. Although scopolamine effectively disrupted the response pattern (i.e., the tendency to respond at a higher rate in the appropriate hole than in the inappropriate hole) under both DMTP and DNMTP, only in the nonmatching task did it significantly increase the rate of inappropriate responses and thereby decrease the frequency of “appropriate only” trials.
In a previous study, Bushnell (1988) used a retractable-lever DMTP procedure in which food pellets were presented for nosepoking into a food cup during the delay. All rats were visually observed to repeatedly explore the retracted levers during the delay. Half of the rats pressed the retracted lever hard enough for responses to be counted, and 90% of these responses were on the to-be-correct lever. This effect is qualitatively similar to the finding that the matching group in the present study had significantly higher response rates in the appropriate hole than in the inappropriate hole. Based on an analysis somewhat similar to the logistic regression of the present study—but in which the effects of delay value were not accounted for, trial outcome was analyzed on a response-by-response basis rather than a trial-by-trial basis, and “both” and “none” categories were not considered—it was concluded that responding during the delay did not predict the outcome of the trial. The discrepancy between this conclusion and that of the present study is probably due to these differences in how the data were analyzed and the fact that not all rats in the Bushnell study fully depressed the retracted lever. The especially low frequency of inappropriate-lever pressing in the Bushnell study seems to indicate that approaching and pressing the appropriate lever actually did serve a mediating function.
Chudasama and Muir (1997) visually observed behavior during the delay in a retractable-lever DNMTP task. They found that mediating responses could take several different forms and that scopolamine had a disruptive effect, abolishing some established responses and causing the appearance of others that did not occur in the absence of scopolamine. These results are consistent with the present finding that scopolamine decreased the incidence of the highly propitious “appropriate only” trial type in the nonmatching task. However, accuracy in the present study was also decreased by scopolamine in the matching task and by THC in the nonmatching task even though the incidence of “appropriate only” trials was not significantly altered. Thus, it appears that these drugs can decrease accuracy by altering either the incidence or the effectiveness of the mediating response.
Gutnikov et al. (1994) studied DMTP using five-hole chambers similar to the three-hole chambers used in the present study. When the positions of samples and choices were varied, accuracy depended on the distance between the samples (rather than the distance between the choices) and on the relative positions (rather than absolute positions) of the choices. Although they did not report visual observations of the rats, Gutnikov et al. concluded that these findings can be explained most simply by assuming that rats maintained a biased body position toward the sample hole during the delay and that, therefore, the task did not require working memory. Since matching accuracy was reduced by scopolamine, they suggested that scopolamine’s effects in this and other delayed matching tasks might not reflect amnesic effects, although they might still be a valid measure of dysfunction in particular neurochemical or anatomical systems.
The present results and those of Chudasama and Muir (1997) demonstrate that drugs can induce amnesia by disrupting rehearsal. Specifically, they cause the appearance of inappropriate responding. Although it may not be possible to demonstrate it directly, it is likely that in studies where overt mediating responses are not observed, some amnestic effects are due to disruption of covert mediating response. However, it is unlikely that all the amnestic effects obtained here were due to disruption of rehearsal. Scopolamine and THC decreased accuracy in the memory task even when the appropriate mediating response did occur. If it is assumed that covert rehearsal is functionally equivalent to overt rehearsal, the present study suggests that scopolamine and THC can produce amnestic effects even when performance of the mediating response (whether overt or covert) is not affected.
Many attempts have been made to eliminate mediating behavior in order to obtain a more direct measure of memory, but even if obvious forms of mediation can be eliminated, covert forms cannot be ruled out (Pontecorvo et al. 1996). Procedures that do not allow the animal to simply wait at the correct choice but that do allow an overt, rehearsal-like mediating response such as that in the present study might provide a useful model of rehearsal in humans. The results of the present study indicate that overt mediating behavior can be recorded automatically and that drugs can induce amnesia by disrupting rehearsal or by reducing the effectiveness of rehearsal.
Acknowledgement
This research was supported by the Intramural Research Program of the NIH, National Institute on Drug Abuse. Thanks to Mark Good for comments on the manuscript.
Contributor Information
Leigh V. Panlilio, Preclinical Pharmacology Section, Behavioral Neuroscience Research Branch, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, 251 Bayview Blvd., Baltimore, MD 21224, USA
Sevil Yasar, Division of Geriatric Medicine and Gerontology, Johns Hopkins University School of Medicine, Baltimore, MD 21224, USA.
Eric B. Thorndike, Preclinical Pharmacology Section, Behavioral Neuroscience Research Branch, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, 251 Bayview Blvd., Baltimore, MD 21224, USA
Steven R. Goldberg, Preclinical Pharmacology Section, Behavioral Neuroscience Research Branch, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, 251 Bayview Blvd., Baltimore, MD 21224, USA
Charles W. Schindler, Preclinical Pharmacology Section, Behavioral Neuroscience Research Branch, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, 251 Bayview Blvd., Baltimore, MD 21224, USA
References
- Blough DS. Delayed matching in the pigeon. J Exp Anal Behav. 1959;2:151–160. doi: 10.1901/jeab.1959.2-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell PJ. Effects of delay, intertrial interval, delay behavior and trimethyltin on spatial delayed response in rats. Neurotoxicol Teratol. 1988;10:237–244. doi: 10.1016/0892-0362(88)90023-2. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ. Modeling working and reference memory in rats: effects of scopolamine on delayed matching-to-position. Behav Pharmacol. 1990;1:419–427. [PubMed] [Google Scholar]
- Chudasama Y, Muir JL. A behavioural analysis of the delayed non-matching to position task: the effects of scopolamine, lesions of the fornix and of the prelimbic region on mediating behaviours by rats. Psychopharmacology. 1997;134:73–82. doi: 10.1007/s002130050427. [DOI] [PubMed] [Google Scholar]
- D’Esposito M. From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci. 2007;362:761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudchenko P, Sarter M. Behavioral microanalysis of spatial delayed alternation performance: rehearsal through overt behavior, and effects of scopolamine and chlordiazepoxide. Psychopharmacology. 1992;107:263–270. doi: 10.1007/BF02245146. [DOI] [PubMed] [Google Scholar]
- Dunnett SB. Comparative effects of cholinergic drugs and lesions of nucleus basalis or fimbria-fornix on delayed matching in rats. Psychopharmacology. 1985;87:357–363. doi: 10.1007/BF00432721. [DOI] [PubMed] [Google Scholar]
- Etherington R, Mittleman G, Robbins TW. Comparative effects of nucleus basalis and fimbria-fornix lesions on delayed matching and alternation tests of memory. Neurosci Res Commun. 1987;1:135–143. [Google Scholar]
- Grant DS. Stimulus control of information processing in rat short-term memory. J Exp Psychol Anim Behav Processes. 1982;8:154–164. [Google Scholar]
- Grant DS. Directed forgetting in pigeons. In: Golding JM, MacLeod C, editors. Intentional forgetting: Interdisciplinary approaches. Erlbaum; Hillsdale: 1998. pp. 239–264. [Google Scholar]
- Gutnikov SA, Barnes JC, Rawlins JN. Working memory tasks in five-choice operant chambers: use of relative and absolute spatial memories. Behav Neurosci. 1994;108:899–910. doi: 10.1037//0735-7044.108.5.899. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Cannabinoids reveal the necessity of hippocampal neural encoding for short-term memory in rats. J Neurosci. 2000;20:8932–8942. doi: 10.1523/JNEUROSCI.20-23-08932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper DN, Hunt M, Schenk S. Attenuation of the disruptive effects of (+/−)3, 4-methylene dioxymethamphetamine (MDMA) on delayed matching-to-sample performance in the rat. Behav Neurosci. 2006;120:201–205. doi: 10.1037/0735-7044.120.1.201. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Automatic and effortful processes in memory. J Exp Psychology General. 1979;108:356–388. [Google Scholar]
- Herremans AH, Hijzen TH, Welborn PF, Olivier B, Slangen JL. Effects of infusion of cholinergic drugs into the prefrontal cortex area on delayed matching to position performance in the rat. Brain Res. 1996;711:102–111. doi: 10.1016/0006-8993(95)01404-7. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Hampson RE, Deadwyler SA. Effects of delta-9-tetrahydrocannabinol on delayed match to sample performance in rats: alterations in short-term memory associated with changes in task specific firing of hippocampal cells. J Pharmacol Exp Ther. 1993;264:294–307. [PubMed] [Google Scholar]
- Hunter WS. The delayed reaction in animals and children. Behav Monogr. 1913;2:1–86. [Google Scholar]
- Jans JE, Catania AC. Short-term remembering of discriminative stimuli in pigeons. J Exp Anal Behav. 1980;34:177–183. doi: 10.1901/jeab.1980.34-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet PE, Beninger RJ. The cannabinoid CB1 receptor antagonist SR141716A attenuates the memory impairment produced by delta9-tetrahydrocannabinol or anandamide. Psychopharmacology. 1998;140:11–19. doi: 10.1007/s002130050733. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Dawson GR, Wilkinson LO, Robbins TW, Everitt BJ. The effects of AMPA-induced lesions of the medial septum and vertical limb nucleus of the diagonal band of Broca on spatial delayed non-matching to sample and spatial learning in the water maze. Eur J Neurosci. 1995;7:1034–1049. doi: 10.1111/j.1460-9568.1995.tb01091.x. [DOI] [PubMed] [Google Scholar]
- Pontecorvo MJ, Sahgal A, Steckler T. Further developments in the measurement of working memory in rodents. Brain Res Cogn Brain Res. 1996;3:205–213. doi: 10.1016/0926-6410(96)00007-9. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proc Natl Acad Sci USA. 1998;95:12061–12068. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanhope KJ, McLenachan AP, Dourish CT. Dissociation between cognitive and motor/motivational deficits in the delayed matching to position test: effects of scopolamine, 8-OH-DPAT and EAA antagonists. Psychopharmacology. 1995;122:268–280. doi: 10.1007/BF02246548. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Engle RW. On the division of short-term and working memory: an examination of simple and complex span and their relation to higher order abilities. Psychol Bull. 2007;133:1038–1066. doi: 10.1037/0033-2909.133.6.1038. [DOI] [PubMed] [Google Scholar]