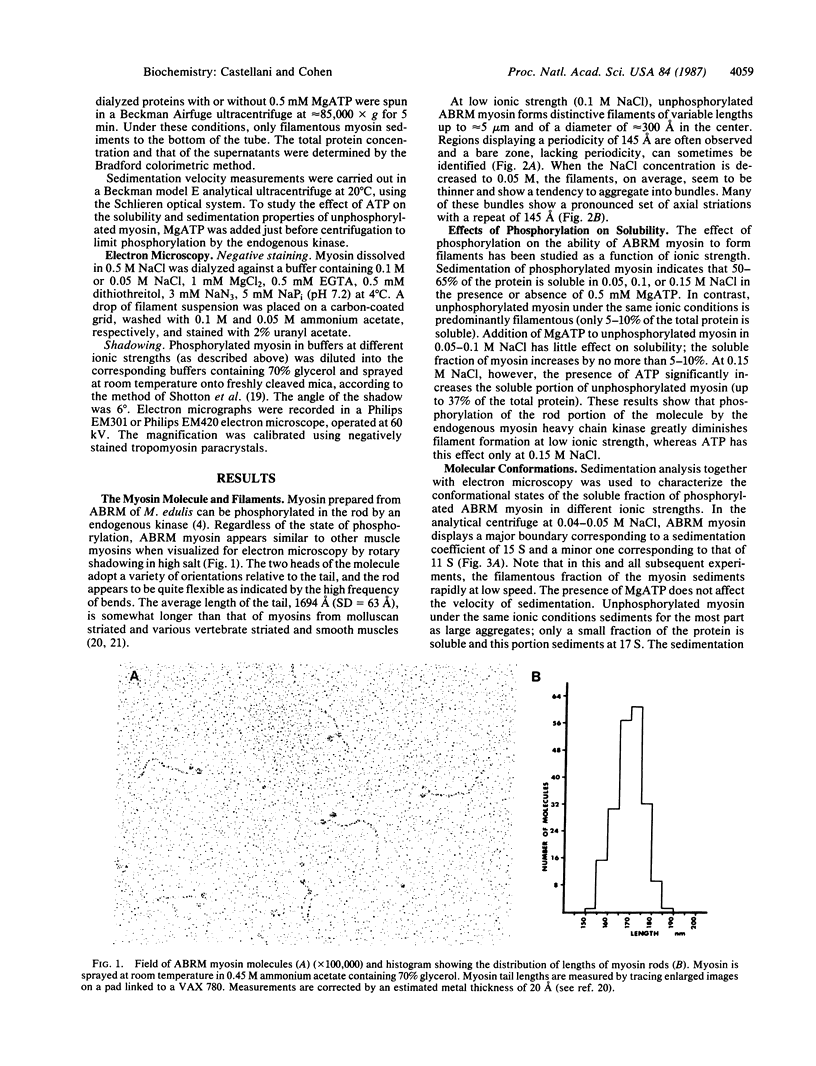
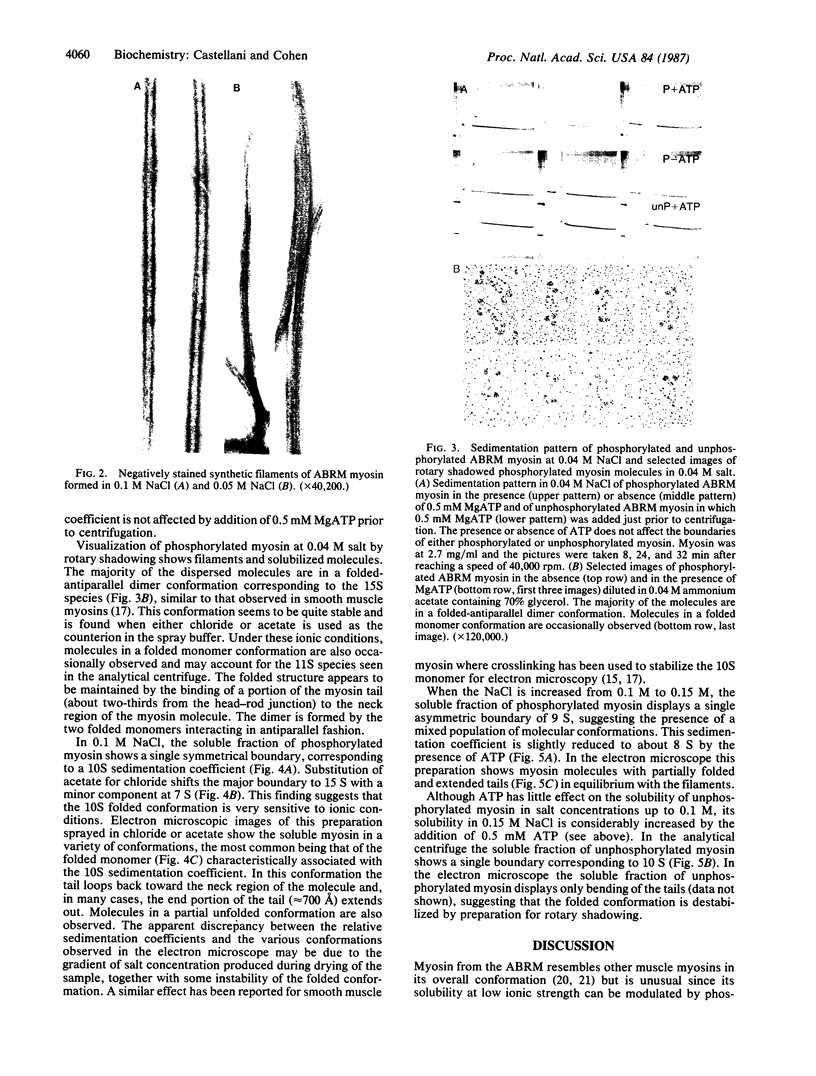
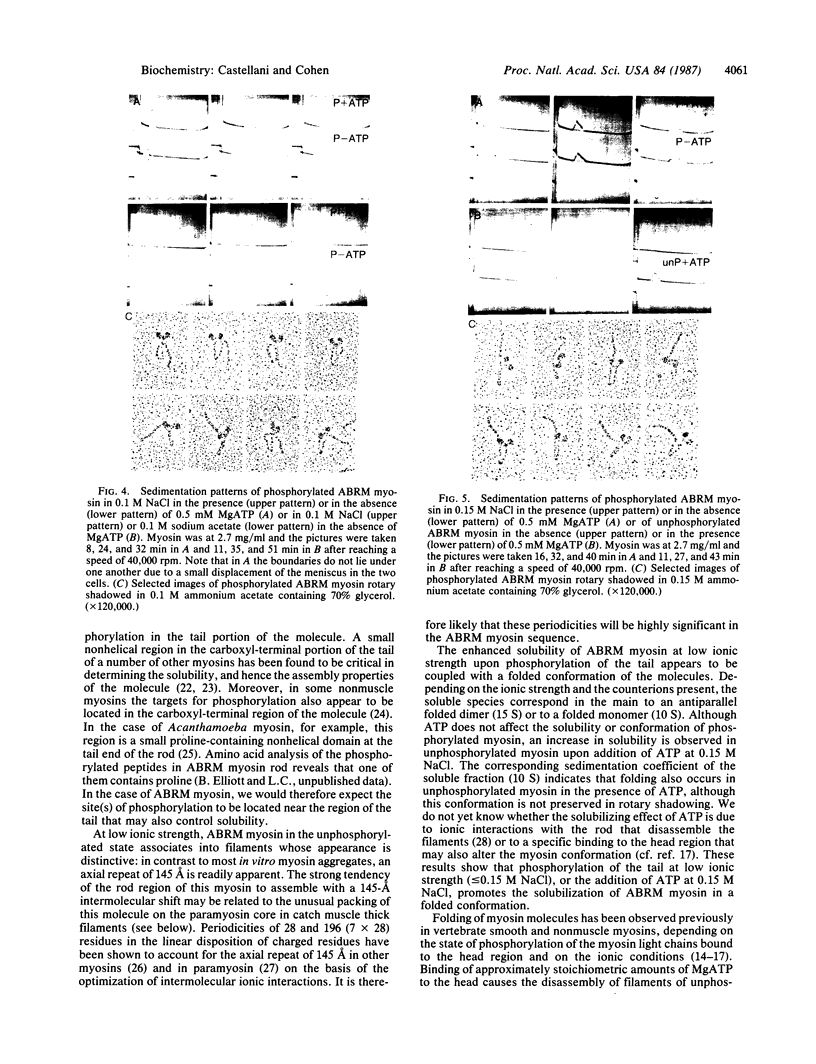
Abstract
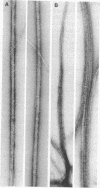
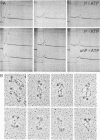
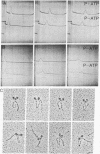
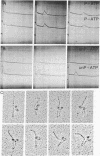
Myosin from a molluscan catch muscle is unusual in being phosphorylated in the rod by an endogenous heavy chain kinase. The overall structure of the molecule resembles that of other muscle myosins, although the tail is somewhat longer (approximately equal to 1700 A). At low ionic strength the unphosphorylated molecules associate in filaments that display a striking axial repeat of 145 A. Phosphorylation of the rod enhances myosin solubility in the range of NaCl between 0.05 and 0.15 M. Depending on the ionic strength and the counterions present, the soluble species corresponds to an antiparallel folded dimer (15 S) or to a folded monomer (10 S). Unphosphorylated myosin can also be partially solubilized into folded monomers by addition of ATP in 0.15 M NaCl. A similar molecular folding has also been observed in smooth muscle and nonmuscle myosins that depends, however, on the state of phosphorylation of the light chains in the myosin head. We discuss these results in relation to possible mechanisms for control of catch contraction.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett P. M., Elliott A. The structure of the paramyosin core in molluscan thick filaments. J Muscle Res Cell Motil. 1981 Mar;2(1):65–81. doi: 10.1007/BF00712062. [DOI] [PubMed] [Google Scholar]
- Castellani L., Cohen C. Myosin rod phosphorylation and the catch state of molluscan muscles. Science. 1987 Jan 16;235(4786):334–337. doi: 10.1126/science.3026049. [DOI] [PubMed] [Google Scholar]
- Cohen C. Matching molecules in the catch mechanism. Proc Natl Acad Sci U S A. 1982 May;79(10):3176–3178. doi: 10.1073/pnas.79.10.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. H., Côté G. P., Korn E. D. Localization of the three phosphorylation sites on each heavy chain of Acanthamoeba myosin II to a segment at the end of the tail. J Biol Chem. 1982 Apr 25;257(8):4529–4534. [PubMed] [Google Scholar]
- Collins J. H., Korn E. D. Actin activation of Ca2+-sensitive Mg2+-ATPase activity of Acanthamoeba myosin II is enhanced by dephosphorylation of its heavy chains. J Biol Chem. 1980 Sep 10;255(17):8011–8014. [PubMed] [Google Scholar]
- Collins J. H., Kuznicki J., Bowers B., Korn E. D. Comparison of the actin binding and filament formation properties of phosphorylated and dephosphorylated Acanthamoeba myosin II. Biochemistry. 1982 Dec 21;21(26):6910–6915. doi: 10.1021/bi00269a045. [DOI] [PubMed] [Google Scholar]
- Cornelius F. Tonic contraction and the control of relaxation in a chemically skinned molluscan smooth muscle. J Gen Physiol. 1982 May;79(5):821–834. doi: 10.1085/jgp.79.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R., Smith R., Kendrick-Jones J. Light-chain phosphorylation controls the conformation of vertebrate non-muscle and smooth muscle myosin molecules. 1983 Mar 31-Apr 6Nature. 302(5907):436–439. doi: 10.1038/302436a0. [DOI] [PubMed] [Google Scholar]
- Côté G. P., Robinson E. A., Appella E., Korn E. D. Amino acid sequence of a segment of the Acanthamoeba myosin II heavy chain containing all three regulatory phosphorylation sites. J Biol Chem. 1984 Oct 25;259(20):12781–12787. [PubMed] [Google Scholar]
- Elliott A., Offer G. Shape and flexibility of the myosin molecule. J Mol Biol. 1978 Aug 25;123(4):505–519. doi: 10.1016/0022-2836(78)90204-8. [DOI] [PubMed] [Google Scholar]
- Flicker P. F., Wallimann T., Vibert P. Electron microscopy of scallop myosin. Location of regulatory light chains. J Mol Biol. 1983 Sep 25;169(3):723–741. doi: 10.1016/s0022-2836(83)80167-3. [DOI] [PubMed] [Google Scholar]
- Frearson N., Focant B. W., Perry S. V. Phosphorylation of a light chain component of myosin from smooth muscle. FEBS Lett. 1976 Mar 15;63(1):27–32. doi: 10.1016/0014-5793(76)80187-1. [DOI] [PubMed] [Google Scholar]
- Harrington W. F., Himmelfarb S. Effect of adenosine di- and triphosphates on the stability of synthetic myosin filaments. Biochemistry. 1972 Aug 1;11(16):2945–2952. doi: 10.1021/bi00766a004. [DOI] [PubMed] [Google Scholar]
- Kuczmarski E. R., Spudich J. A. Regulation of myosin self-assembly: phosphorylation of Dictyostelium heavy chain inhibits formation of thick filaments. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7292–7296. doi: 10.1073/pnas.77.12.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznicki J., Côté G. P., Bowers B., Korn E. D. Filament formation and actin-activated ATPase activity are abolished by proteolytic removal of a small peptide from the tip of the tail of the heavy chain of Acanthamoeba myosin II. J Biol Chem. 1985 Feb 10;260(3):1967–1972. [PubMed] [Google Scholar]
- Matsumura S., Murakami N., Yasuda S., Kumon A. Phosphorylation of the bovine brain myosin. Biochem Biophys Res Commun. 1982 Oct 29;108(4):1595–1600. doi: 10.1016/s0006-291x(82)80090-9. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D. Structural implications of the myosin amino acid sequence. Annu Rev Biophys Bioeng. 1984;13:167–189. doi: 10.1146/annurev.bb.13.060184.001123. [DOI] [PubMed] [Google Scholar]
- Nonomura Y. Fine structure of the thick filament in molluscan catch muscle. J Mol Biol. 1974 Sep 15;88(2):445–455. doi: 10.1016/0022-2836(74)90494-x. [DOI] [PubMed] [Google Scholar]
- Nyitray L., Mocz G., Szilagyi L., Balint M., Lu R. C., Wong A., Gergely J. The proteolytic substructure of light meromyosin. Localization of a region responsible for the low ionic strength insolubility of myosin. J Biol Chem. 1983 Nov 10;258(21):13213–13220. [PubMed] [Google Scholar]
- Onishi H., Suzuki H., Nakamura K., Takahashi K., Watanabe S. Adenosine triphosphatase activity and "thick filament" formation of chicken gizzard myosin in low salt media. J Biochem. 1978 Mar;83(3):835–847. doi: 10.1093/oxfordjournals.jbchem.a131980. [DOI] [PubMed] [Google Scholar]
- Shotton D. M., Burke B. E., Branton D. The molecular structure of human erythrocyte spectrin. Biophysical and electron microscopic studies. J Mol Biol. 1979 Jun 25;131(2):303–329. doi: 10.1016/0022-2836(79)90078-0. [DOI] [PubMed] [Google Scholar]
- Stafford W. F., 3rd, Szentkiralyi E. M., Szent-Györgyi A. G. Regulatory properties of single-headed fragments of scallop myosin. Biochemistry. 1979 Nov 27;18(24):5273–5280. doi: 10.1021/bi00591a002. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi A. G., Cohen C., Kendrick-Jones J. Paramyosin and the filaments of molluscan "catch" muscles. II. Native filaments: isolation and characterization. J Mol Biol. 1971 Mar 14;56(2):239–258. doi: 10.1016/0022-2836(71)90462-1. [DOI] [PubMed] [Google Scholar]
- Trybus K. M., Huiatt T. W., Lowey S. A bent monomeric conformation of myosin from smooth muscle. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6151–6155. doi: 10.1073/pnas.79.20.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trybus K. M., Lowey S. Conformational states of smooth muscle myosin. Effects of light chain phosphorylation and ionic strength. J Biol Chem. 1984 Jul 10;259(13):8564–8571. [PubMed] [Google Scholar]