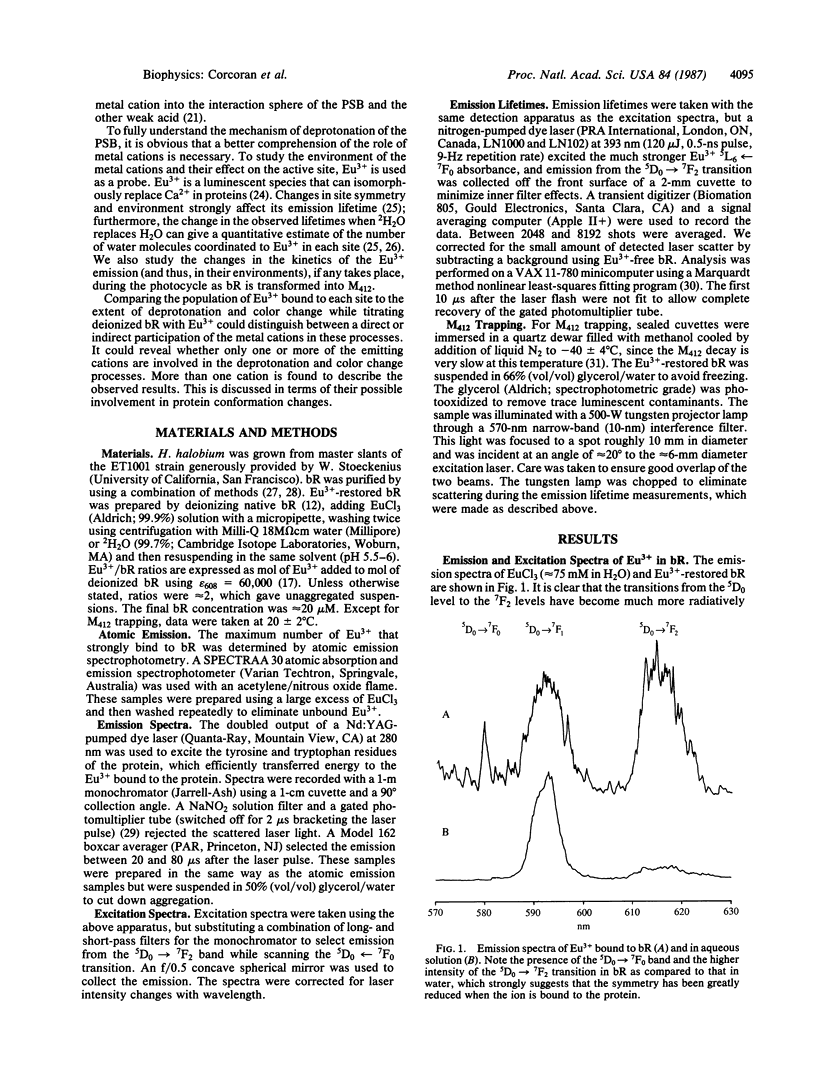
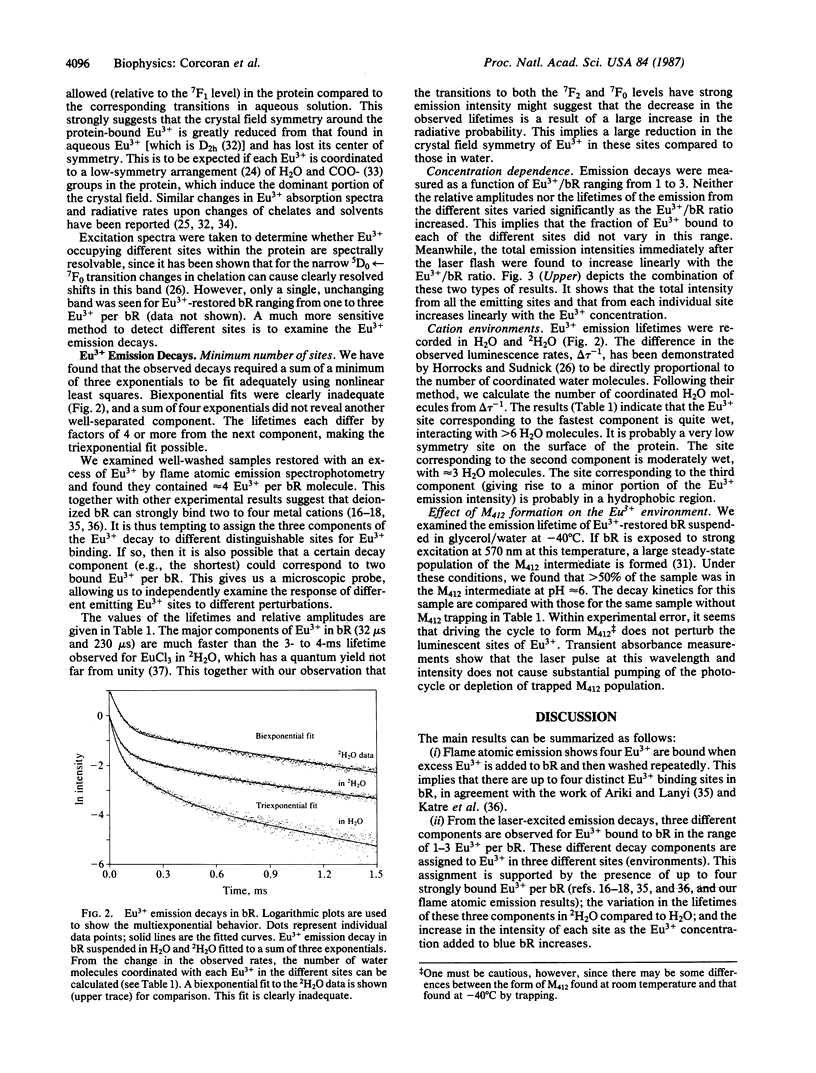
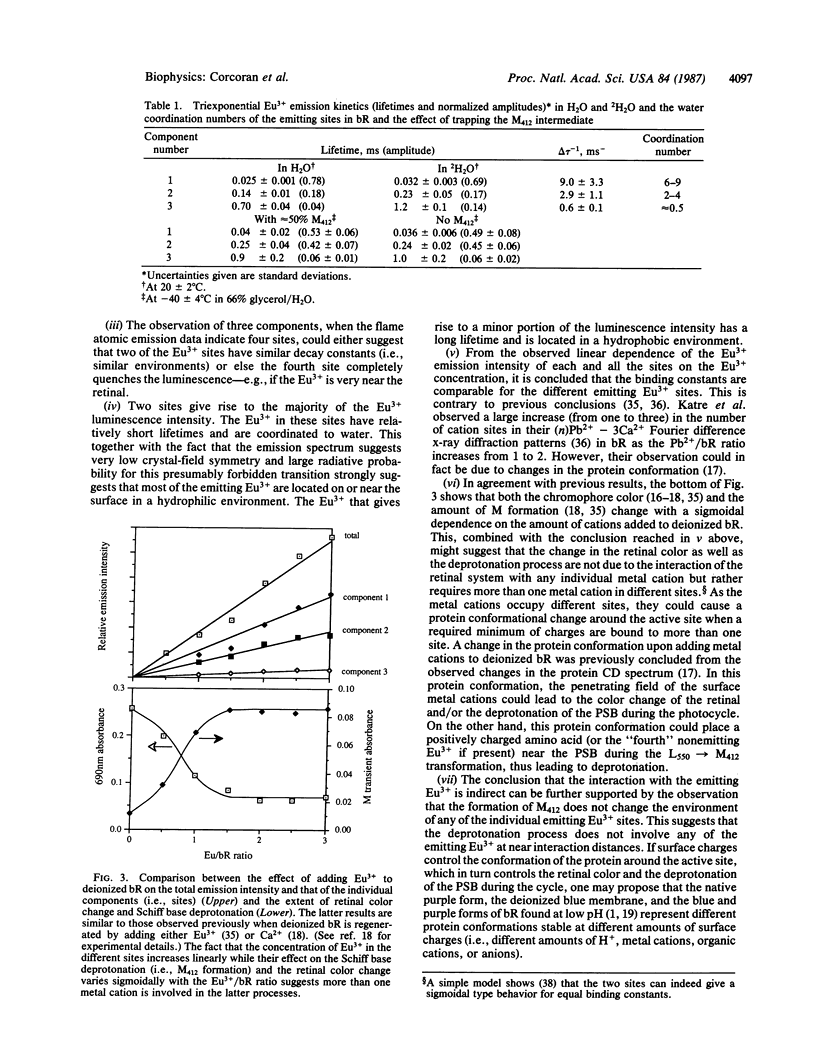
Abstract
The removal of metal cations inhibits the deprotonation process of the protonated Schiff base during the photocycle of bacteriorhodopsin. To understand the nature of the involvement of these cations, a spectroscopic and kinetic study was carried out on bacteriorhodopsin samples in which the native Ca2+ and Mg2+ were replaced by Eu3+, a luminescent cation. The decay of Eu3+ emission in bacteriorhodopsin can be fitted to a minimum of three decay components, which are assigned to Eu3+ emission from three different sites. This is supported by the response of the decay components to the presence of 2H2O and to the changes in the Eu3+/bR molar ratio. The number of water molecules coordinated to Eu3+ in each site is determined from the change in its emission lifetime when 2H2O replaces H2O. Most of the emission originates from two “wet” sites of low crystal-field symmetry—e.g., surface sites. Protonated Schiff base deprotonation has no discernable effect on the emission decay of protein-bound Eu3+, suggesting an indirect involvement of metal cations in the deprotonation process. Adding Eu3+ to deionized bacteriorhodopsin increases the emission intensity of each Eu3+ site linearly, but the extent of the deprotonation (and color) changes sigmoidally. This suggests that if only the emitting Eu3+ ions cause the deprotonation and bacteriorhodopsin color change, ions in more than one site must be involved—e.g., by inducing protein conformation changes. The latter could allow deprotonation by the interaction between the protonated Schiff base and a positive field of cations either on the surface or within the protein.
Keywords: Eu3+ concentration dependence, cation environments, cation participation, effect of photocycle, deprotonation mechanism
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariki M., Lanyi J. K. Characterization of metal ion-binding sites in bacteriorhodopsin. J Biol Chem. 1986 Jun 25;261(18):8167–8174. [PubMed] [Google Scholar]
- Aton B., Doukas A. G., Callender R. H., Becher B., Ebrey T. G. Resonance Raman studies of the purple membrane. Biochemistry. 1977 Jun 28;16(13):2995–2999. doi: 10.1021/bi00632a029. [DOI] [PubMed] [Google Scholar]
- Becher B. M., Cassim J. Y. Improved isolation procedures for the purple membrane of Halobacterium halobium. Prep Biochem. 1975;5(2):161–178. doi: 10.1080/00327487508061568. [DOI] [PubMed] [Google Scholar]
- Bogomolni R. A., Baker R. A., Lozier R. H., Stoeckenius W. Light-driven proton translocations in Halobacterium halobium. Biochim Biophys Acta. 1976 Jul 9;440(1):68–88. doi: 10.1016/0005-2728(76)90114-6. [DOI] [PubMed] [Google Scholar]
- Bridgen J., Walker I. D. Photoreceptor protein from the purple membrane of Halobacterium halobium. Molecular weight and retinal binding site. Biochemistry. 1976 Feb 24;15(4):792–798. doi: 10.1021/bi00649a010. [DOI] [PubMed] [Google Scholar]
- Chance B., Porte M., Hess B., Oesterhelt D. Low temperature kinetics of H+ changes of bacterial rhodopsin. Biophys J. 1975 Sep;15(9):913–917. doi: 10.1016/S0006-3495(75)85865-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. H., Chen J. G., Govindjee R., Ebrey T. Cation binding by bacteriorhodopsin. Proc Natl Acad Sci U S A. 1985 Jan;82(2):396–400. doi: 10.1073/pnas.82.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. H., Jonas R., Melchiore S., Govindjee R., Ebrey T. G. Mechanism and role of divalent cation binding of bacteriorhodopsin. Biophys J. 1986 Mar;49(3):731–739. doi: 10.1016/S0006-3495(86)83699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronister E. L., Corcoran T. C., Song L., El-Sayed M. A. On the molecular mechanisms of the Schiff base deprotonation during the bacteriorhodopsin photocycle. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8580–8584. doi: 10.1073/pnas.83.22.8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachev L. A., Kaulen A. D., Skulachev V. P. Time resolution of the intermediate steps in the bacteriorhodopsin-linked electrogenesis. FEBS Lett. 1978 Mar 1;87(1):161–167. doi: 10.1016/0014-5793(78)80157-4. [DOI] [PubMed] [Google Scholar]
- Dupuis P., Corcoran T. C., El-Sayed M. A. Importance of bound divalent cations to the tyrosine deprotonation during the photocycle of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3662–3664. doi: 10.1073/pnas.82.11.3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhard M., Gerwert K., Hess B., Kreutz W., Siebert F. Light-driven protonation changes of internal aspartic acids of bacteriorhodopsin: an investigation by static and time-resolved infrared difference spectroscopy using [4-13C]aspartic acid labeled purple membrane. Biochemistry. 1985 Jan 15;24(2):400–407. doi: 10.1021/bi00323a024. [DOI] [PubMed] [Google Scholar]
- Hanamoto J. H., Dupuis P., El-Sayed M. A. On the protein (tyrosine)-chromophore (protonated Schiff base) coupling in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7083–7087. doi: 10.1073/pnas.81.22.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess B., Kuschmitz D. Kinetic interaction between aromatic residues and the retinal chromophore of bacteriorhodopsin during the photocycle. FEBS Lett. 1979 Apr 15;100(2):334–340. doi: 10.1016/0014-5793(79)80364-6. [DOI] [PubMed] [Google Scholar]
- Katre N. V., Kimura Y., Stroud R. M. Cation binding sites on the projected structure of bacteriorhodopsin. Biophys J. 1986 Aug;50(2):277–284. doi: 10.1016/S0006-3495(86)83461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y., Ikegami A., Stoeckenius W. Salt and pH-dependent changes of the purple membrane absorption spectrum. Photochem Photobiol. 1984 Nov;40(5):641–646. doi: 10.1111/j.1751-1097.1984.tb05353.x. [DOI] [PubMed] [Google Scholar]
- Lewis A., Spoonhower J., Bogomolni R. A., Lozier R. H., Stoeckenius W. Tunable laser resonance raman spectroscopy of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4462–4466. doi: 10.1073/pnas.71.11.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Govindjee R., Ebrey T. G. A correlation between proton pumping and the bacteriorhodopsin photocycle. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7079–7082. doi: 10.1073/pnas.81.22.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. W., Weaver L. H. Binding of lanthanide ions to thermolysin. Biochemistry. 1974 Apr 9;13(8):1719–1725. doi: 10.1021/bi00705a025. [DOI] [PubMed] [Google Scholar]
- Mowery P. C., Lozier R. H., Chae Q., Tseng Y. W., Taylor M., Stoeckenius W. Effect of acid pH on the absorption spectra and photoreactions of bacteriorhodopsin. Biochemistry. 1979 Sep 18;18(19):4100–4107. doi: 10.1021/bi00586a007. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Scherrer P., Packer L., Seltzer S. Effect of iodination of the purple membrane on the photocycle of bacteriorhodopsin. Arch Biochem Biophys. 1981 Dec;212(2):589–601. doi: 10.1016/0003-9861(81)90402-1. [DOI] [PubMed] [Google Scholar]
- Scherrer P., Stoeckenius W. Selective nitration of tyrosines-26 and -64 in bacteriorhodopsin with tetranitromethane. Biochemistry. 1984 Dec 4;23(25):6195–6202. doi: 10.1021/bi00320a047. [DOI] [PubMed] [Google Scholar]



