Abstract
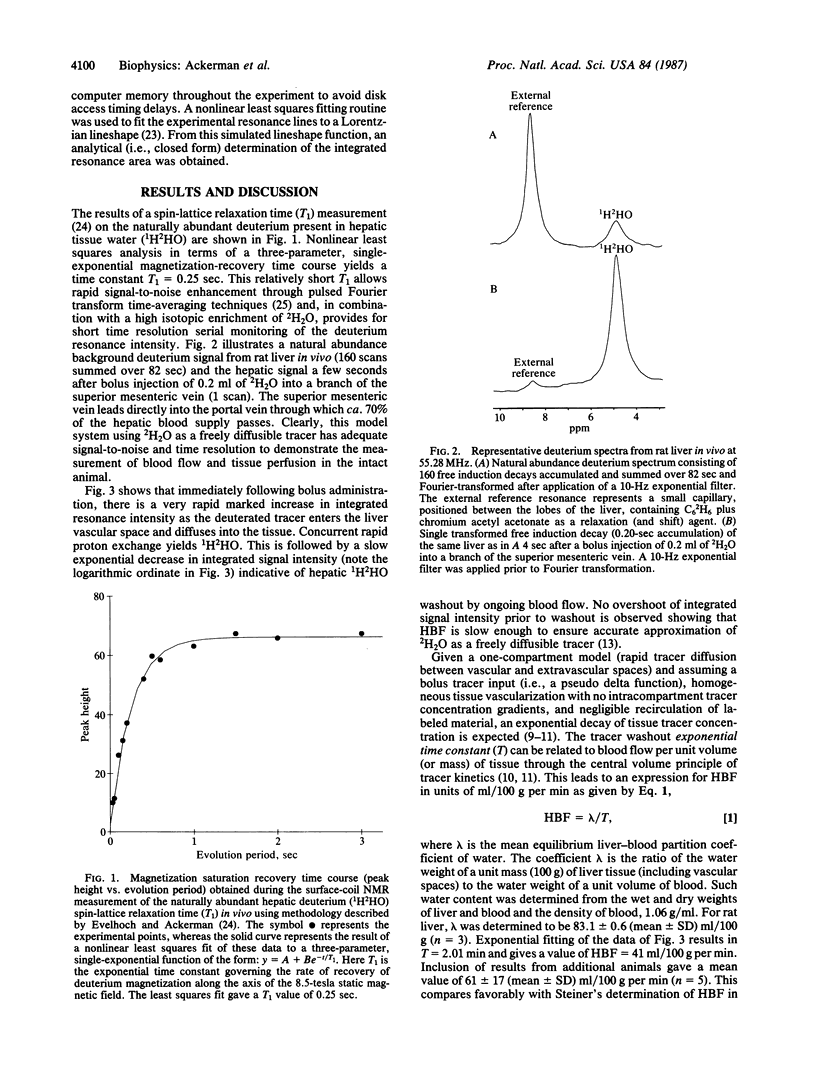
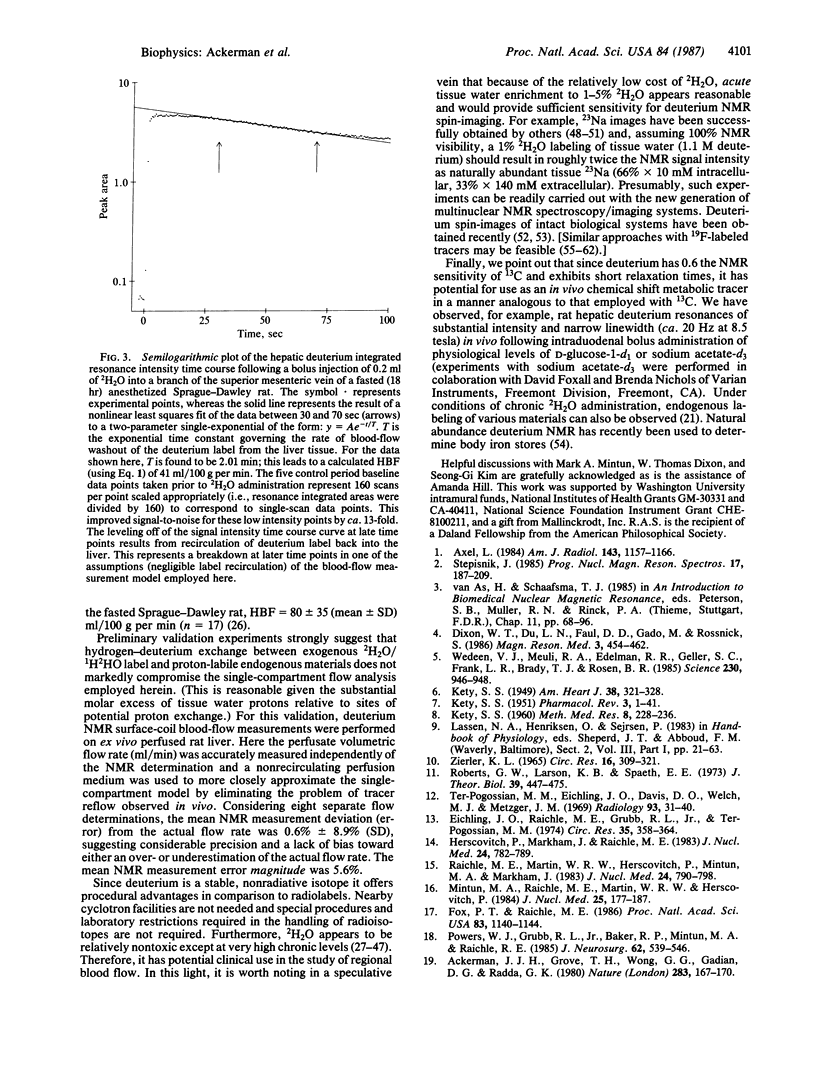
The use of deuterium oxide (2H2O) is proposed as a freely diffusible nuclear magnetic resonance (NMR) blood flow and tissue perfusion tracer of potential clinical utility. Deuterium is a stable, nonradiative isotope commercially available as 2H2O at enrichment levels of essentially 100%--i.e., 110 molar equivalent deuterium. This high concentration, together with the short relaxation time of the spin 1 (quadrupole) deuterium nuclide, provides substantial sensitivity for NMR spectroscopy. As a result, when 2H2O is administered in a bolus fashion to a specific tissue or organ in vivo, the deuterium NMR intensity time course can be analyzed, using mathematical models developed by others for radiolabeled tracers, to measure the rate of blood flow and tissue perfusion. Such an application is demonstrated herein at a static magnetic field of 8.5 tesla. Using single-compartment flow modeling, hepatic blood flow and tissue perfusion in fasted (18 hr) male Sprague-Dawley rats was determined to be 61 +/- 17 (mean +/- SD) ml/100 g per min (n = 5).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMAROSE A. P., CZAJKA D. M. Cytopathic effects of deuterium oxide on the male gonads of the mouse and dog. Exp Cell Res. 1962 Feb;26:43–61. doi: 10.1016/0014-4827(62)90201-x. [DOI] [PubMed] [Google Scholar]
- Ackerman J. J., Grove T. H., Wong G. G., Gadian D. G., Radda G. K. Mapping of metabolites in whole animals by 31P NMR using surface coils. Nature. 1980 Jan 10;283(5743):167–170. doi: 10.1038/283167a0. [DOI] [PubMed] [Google Scholar]
- Axel L. Blood flow effects in magnetic resonance imaging. AJR Am J Roentgenol. 1984 Dec;143(6):1157–1166. doi: 10.2214/ajr.143.6.1157. [DOI] [PubMed] [Google Scholar]
- BACHNER P., MCKAY D. G., RITTENBERG D. THE PATHOLOGIC ANATOMY OF DEUTERIUM INTOXICATION. Proc Natl Acad Sci U S A. 1964 Mar;51:464–471. doi: 10.1073/pnas.51.3.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brereton I. M., Irving M. G., Field J., Doddrell D. M. Preliminary studies on the potential of in vivo deuterium NMR spectroscopy. Biochem Biophys Res Commun. 1986 May 29;137(1):579–584. doi: 10.1016/0006-291x(86)91250-7. [DOI] [PubMed] [Google Scholar]
- CZAJKA D. M., FINKEL A. J. Effect of deuterium oxide on the reproductive potential of mice. Ann N Y Acad Sci. 1960 Nov 25;84:770–779. doi: 10.1111/j.1749-6632.1960.tb39109.x. [DOI] [PubMed] [Google Scholar]
- CZAJKA D. M., FINKEL A. J., FISCHER C. S., KATZ J. J. Physiological effects of deuterium on dogs. Am J Physiol. 1961 Aug;201:357–362. doi: 10.1152/ajplegacy.1961.201.2.357. [DOI] [PubMed] [Google Scholar]
- DeLayre J. L., Ingwall J. S., Malloy C., Fossel E. T. Gated sodium-23 nuclear magnetic resonance images of an isolated perfused working rat heart. Science. 1981 May 22;212(4497):935–936. doi: 10.1126/science.7233188. [DOI] [PubMed] [Google Scholar]
- Dixon W. T., Du L. N., Faul D. D., Gado M., Rossnick S. Projection angiograms of blood labeled by adiabatic fast passage. Magn Reson Med. 1986 Jun;3(3):454–462. doi: 10.1002/mrm.1910030311. [DOI] [PubMed] [Google Scholar]
- Eichling J. O., Raichle M. E., Grubb R. L., Jr, Ter-Pogossian M. M. Evidence of the limitations of water as a freely diffusible tracer in brain of the rhesus monkey. Circ Res. 1974 Sep;35(3):358–364. doi: 10.1161/01.res.35.3.358. [DOI] [PubMed] [Google Scholar]
- Feinberg D. A., Crooks L. A., Kaufman L., Brant-Zawadzki M., Posin J. P., Arakawa M., Watts J. C., Hoenninger J. Magnetic resonance imaging performance: a comparison of sodium and hydrogen. Radiology. 1985 Jul;156(1):133–138. doi: 10.1148/radiology.156.1.4001399. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard J. K., Kichura G. M., Ackerman J. J., Eisenberg J. D., Billadello J. J., Sobel B. E., Gross R. W. Quantitative 31P nuclear magnetic resonance analysis of metabolite concentrations in Langendorff-perfused rabbit hearts. Biophys J. 1985 Nov;48(5):803–813. doi: 10.1016/S0006-3495(85)83839-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES A. M., BENNETT E. L., CALVIN M. Further studies on sterility produced in male mice by deuterium oxide. Ann N Y Acad Sci. 1960 Nov 25;84:763–769. doi: 10.1111/j.1749-6632.1960.tb39108.x. [DOI] [PubMed] [Google Scholar]
- HUGHES A. M., CALVIN M. Production of sterility in mice by deuterium oxide. Science. 1958 Jun 20;127(3312):1445–1446. doi: 10.1126/science.127.3312.1445. [DOI] [PubMed] [Google Scholar]
- Hayes C. J., Palmer J. D. The suppression of mouse spontaneous locomotor activity by the ingestion of deuterium oxide. Experientia. 1976 Apr 15;32(4):469–470. doi: 10.1007/BF01920801. [DOI] [PubMed] [Google Scholar]
- Herscovitch P., Markham J., Raichle M. E. Brain blood flow measured with intravenous H2(15)O. I. Theory and error analysis. J Nucl Med. 1983 Sep;24(9):782–789. [PubMed] [Google Scholar]
- Hilal S. K., Maudsley A. A., Ra J. B., Simon H. E., Roschmann P., Wittekoek S., Cho Z. H., Mun S. K. In vivo NMR imaging of sodium-23 in the human head. J Comput Assist Tomogr. 1985 Jan-Feb;9(1):1–7. doi: 10.1097/00004728-198501000-00001. [DOI] [PubMed] [Google Scholar]
- Hughes A. M., Bennett E. L., Calvin M. PRODUCTION OF STERILITY IN MICE BY DEUTERIUM OXIDE. Proc Natl Acad Sci U S A. 1959 Apr;45(4):581–586. doi: 10.1073/pnas.45.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving M. G., Brereton I. M., Field J., Doddrell D. M. In vivo determination of body iron stores by natural-abundance deuterium magnetic resonance spectroscopy. Magn Reson Med. 1987 Jan;4(1):88–92. doi: 10.1002/mrm.1910040111. [DOI] [PubMed] [Google Scholar]
- Joseph P. M., Fishman J. E., Mukherji B., Sloviter H. A. In vivo 19F NMR imaging of the cardiovascular system. J Comput Assist Tomogr. 1985 Nov-Dec;9(6):1012–1019. doi: 10.1097/00004728-198511000-00003. [DOI] [PubMed] [Google Scholar]
- KATZ J. J., CRESPI H. L., CZAJKA D. M., FINKEL A. J. Course of deuteriation and some physiological effects of deuterium in mice. Am J Physiol. 1962 Nov;203:907–913. doi: 10.1152/ajplegacy.1962.203.5.907. [DOI] [PubMed] [Google Scholar]
- KATZ J. J., CRESPI H. L., HASTERLIK R. J., THOMSON J. F., FINKEL A. J. Some observations on biological effects of deuterium, with special reference to effects on neoplastic processes. J Natl Cancer Inst. 1957 May;18(5):641–659. [PubMed] [Google Scholar]
- KETY S. S. The theory and applications of the exchange of inert gas at the lungs and tissues. Pharmacol Rev. 1951 Mar;3(1):1–41. [PubMed] [Google Scholar]
- Kanwar K. C., Verma R. Biologic effects of orally administered deuterium oxide on rat liver. Exp Pathol (Jena) 1977;13(4-5):255–261. doi: 10.1016/s0014-4908(77)80010-0. [DOI] [PubMed] [Google Scholar]
- Kanwar K. C., Verma R. Oral D2O administration and enzymatic changes in rat testis. Acta Biol Med Ger. 1976;35(5):577–581. [PubMed] [Google Scholar]
- Longmaid H. E., 3rd, Adams D. F., Neirinckx R. D., Harrison C. G., Brunner P., Seltzer S. E., Davis M. A., Neuringer L., Geyer R. P. In vivo 19F NMR imaging of liver, tumor, and abscess in rats. Preliminary results. Invest Radiol. 1985 Mar-Apr;20(2):141–145. doi: 10.1097/00004424-198503000-00009. [DOI] [PubMed] [Google Scholar]
- McFarland E., Koutcher J. A., Rosen B. R., Teicher B., Brady T. J. In vivo 19F NMR imaging. J Comput Assist Tomogr. 1985 Jan-Feb;9(1):8–15. doi: 10.1097/00004728-198501000-00002. [DOI] [PubMed] [Google Scholar]
- Med M. Prenatal development of thoracic intervertebral articulation. Folia Morphol (Praha) 1977;25(2):175–177. [PubMed] [Google Scholar]
- Money K. E., Myles W. S. Heavy water nystagmus and effects of alcohol. Nature. 1974 Feb 8;247(5440):404–405. doi: 10.1038/247404a0. [DOI] [PubMed] [Google Scholar]
- Nelson T. R., Newman F. D., Schiffer L. M., Reith J. D., Cameron S. L. Fluorine nuclear magnetic resonance: calibration and system optimization. Magn Reson Imaging. 1985;3(3):267–273. doi: 10.1016/0730-725x(85)90356-x. [DOI] [PubMed] [Google Scholar]
- Nunnally R. L., Babcock E. E., Horner S. D., Peshock R. M. Fluorine-19 NMR spectroscopy and imaging investigations of myocardial perfusion and cardiac function. Magn Reson Imaging. 1985;3(4):399–405. doi: 10.1016/0730-725x(85)90404-7. [DOI] [PubMed] [Google Scholar]
- Peng S. K., Ho K. J., Taylor C. B. Biologic effects of prolonged exposure to deuterium oxide. A behavioral, metabolic, and morphologic study. Arch Pathol. 1972 Jul;94(1):81–89. [PubMed] [Google Scholar]
- Powers W. J., Grubb R. L., Jr, Baker R. P., Mintun M. A., Raichle M. E. Regional cerebral blood flow and metabolism in reversible ischemia due to vasospasm. Determination by positron emission tomography. J Neurosurg. 1985 Apr;62(4):539–546. doi: 10.3171/jns.1985.62.4.0539. [DOI] [PubMed] [Google Scholar]
- RABINOWITZ J. L., DEFENDI V., LANGAN J., KRITCHEVSKY D. Hepatic lipogenesis in D2O-fed mice. Ann N Y Acad Sci. 1960 Nov 25;84:727–735. doi: 10.1111/j.1749-6632.1960.tb39104.x. [DOI] [PubMed] [Google Scholar]
- Rebouche C. J., Pearson G. A., Serfass R. E., Roth C. W., Finley J. W. Evaluation of nuclear magnetic resonance spectroscopy for determination of deuterium abundance in body fluids: application to measurement of total-body water in human infants. Am J Clin Nutr. 1987 Feb;45(2):373–380. doi: 10.1093/ajcn/45.2.373. [DOI] [PubMed] [Google Scholar]
- Roberts G. W., Larson K. B., Spaeth E. E. The interpretation of mean transit time measurements for multiphase tissue systems. J Theor Biol. 1973 May;39(2):447–475. doi: 10.1016/0022-5193(73)90111-2. [DOI] [PubMed] [Google Scholar]
- THOMSON J. F., KLIPFEL F. J. Changes in renal function in rats drinking heavy water. Proc Soc Exp Biol Med. 1958 Apr;97(4):758–759. doi: 10.3181/00379727-97-23871. [DOI] [PubMed] [Google Scholar]
- Thomas S. R., Clark L. C., Jr, Ackerman J. L., Pratt R. G., Hoffmann R. E., Busse L. J., Kinsey R. A., Samaratunga R. C. MR imaging of the lung using liquid perfluorocarbons. J Comput Assist Tomogr. 1986 Jan-Feb;10(1):1–9. doi: 10.1097/00004728-198601000-00001. [DOI] [PubMed] [Google Scholar]
- Wedeen V. J., Meuli R. A., Edelman R. R., Geller S. C., Frank L. R., Brady T. J., Rosen B. R. Projective imaging of pulsatile flow with magnetic resonance. Science. 1985 Nov 22;230(4728):946–948. doi: 10.1126/science.4059917. [DOI] [PubMed] [Google Scholar]
- ZIERLER K. L. EQUATIONS FOR MEASURING BLOOD FLOW BY EXTERNAL MONITORING OF RADIOISOTOPES. Circ Res. 1965 Apr;16:309–321. doi: 10.1161/01.res.16.4.309. [DOI] [PubMed] [Google Scholar]



