Abstract
In the crystal structure of the title compound, C22H19N3O3, intermolecular C—H⋯O hydrogen bonds link the molecules into a zigzag chain parallel to the face diagonal of the ac plane. The methoxy phenyl rings make a dihdral angle of 32.38 (7)° and form dihedral angles of 0.66 (8) and 24.17 (7)° with the fused benzooxazine ring system.
Related literature
For the Baeyer–Villiger oxidation of 1-alkyl-3-arylimino-2-indolinone with m-chloroperbenzoic acid to afford 1-alkyl-4-(arylimino)-1H benzo[d][1,3]oxazin-2(4H)-one, see: Mehrdad et al. (2011 ▶); Azizian et al. (2000 ▶); Jadidi et al. (2008 ▶). For a related structure, see: Asgari et al. (2011 ▶).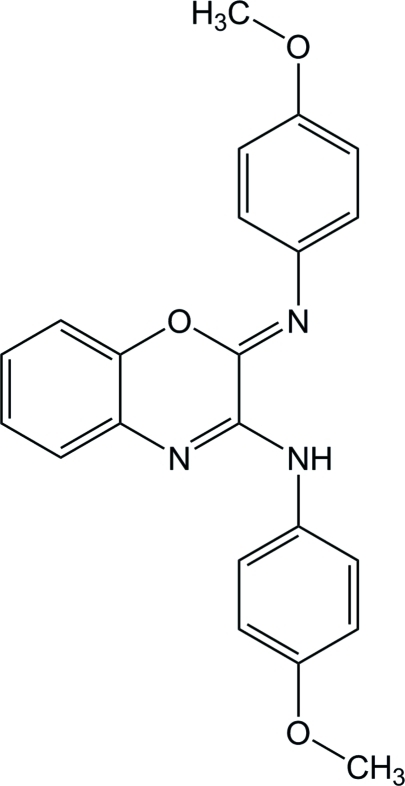
Experimental
Crystal data
C22H19N3O3
M r = 373.40
Monoclinic,
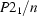
a = 14.4225 (14) Å
b = 8.0836 (5) Å
c = 16.2749 (14) Å
β = 107.263 (7)°
V = 1811.9 (3) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 298 K
0.60 × 0.13 × 0.04 mm
Data collection
Stoe IPDS II diffractometer
21467 measured reflections
4893 independent reflections
3190 reflections with I > 2σ(I)
R int = 0.111
Refinement
R[F 2 > 2σ(F 2)] = 0.083
wR(F 2) = 0.195
S = 1.15
4893 reflections
253 parameters
H-atom parameters constrained
Δρmax = 0.24 e Å−3
Δρmin = −0.28 e Å−3
Data collection: X-AREA (Stoe & Cie, 2005 ▶); cell refinement: X-AREA; data reduction: X-AREA; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810050294/bt5425sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810050294/bt5425Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C7—H7⋯O3i | 0.93 | 2.59 | 3.423 (3) | 149 |
Symmetry code: (i) 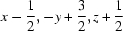 .
.
Acknowledgments
The authors thank the Vice President of Research Affairs at Shahid Beheshti University, General Campus, for financial support.
supplementary crystallographic information
Comment
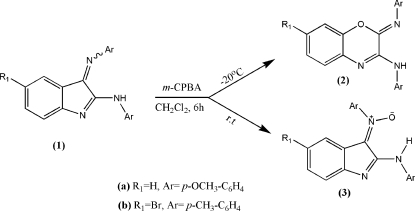
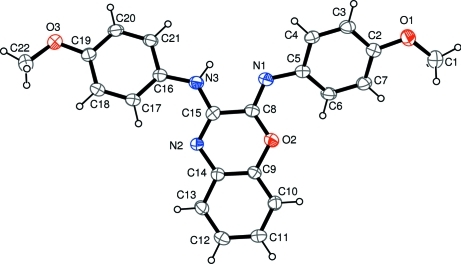
Recently, we reported a Baeyer–Villiger oxidation of 1-alkyl-3-arylimino-2-indolinone with m-chloroperbenzoic acid to afford 1-alkyl-4-(arylimino)-1H benzo[d][1,3]oxazin-2(4H)-one (Azizian et al., 2000; Jadidi et al., 2008). As a continuation of this work, 2-arylimino-N-aryl-2H-benzo[b][1,4]oxazin-3-amines (2) or N-aryl-N-(2-arylamino-3H-indol-3-ylidene)amine N-oxides (3) were obtained in two different temperatures by Baeyer-Villiger oxidation reaction (Fig. 1) of N-aryl-3-(arylimino)-3H-indol-2-amines (1) (Mehrdad et al., 2011). In this paper, we report the structure of (2Z)-2-(4-methoxyphenylimino)-N-(4-methoxyphenyl)- 2H-benzo[b][1,4]oxazin-3-amine (2a). The molecular structure of the title compound is shown in Fig. 2.
The methoxy phenyl rings, A (C2—C7) and B (C16—C21) and benzooxazine ring C (C9—C14/C8/O2/N2/C15) enclose the dihedral angles: A/B = 32.38 (7)°, A/C = 10.66 (8)° and B/C = 24.17 (7)°. Intermolecular C—H···O interactions (Table 1) stabilize the crystal structure.
Experimental
The solution of N-Aryl-3-(Arylimino)-3H-indol-2-amine (1a) (1.0 mmol) in 25 ml CH2Cl2 was cooled to 253K. Then, m-CPBA (1.5 mmol) dissolved in 25 ml CH2Cl2 was added dropwise to the stirred solution of (1a). After stirring for 6 h at 253K, product (2a) was formed (monitoring by TLC). The crude product was poured into water and extracted with CH2Cl2 (60 ml). The organic layer was dried over Na2SO4, and evaporation of the solvent afforded the crude product (2a), which was purified on silica gel by column chromatography using 90:10 n-hexane:ethyl acetate as eluent to afford (2a) as a light yellow solid (90%); m.p. = 169–171°C (Mehrdad et al., 2011).
Refinement
All H atoms were positioned geometrically, with N—H=0.86 Å, Cmethyl—H=0.96Å and Caromatic—H=0.93Å and constrained to ride on their parent atoms, with Uiso(H)=1.2Ueq(C,N).
Figures
Fig. 1.
Reaction scheme.
Fig. 2.
The molecular structure of the title molecule, with the atom-numbering scheme. Displacement ellipsoids are drawn at the 30% probability level.
Fig. 3.
Unit-cell packing diagram for (I).
Crystal data
| C22H19N3O3 | F(000) = 784 |
| Mr = 373.40 | Dx = 1.369 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2yn | Cell parameters from 21467 reflections |
| a = 14.4225 (14) Å | θ = 1.7–29.3° |
| b = 8.0836 (5) Å | µ = 0.09 mm−1 |
| c = 16.2749 (14) Å | T = 298 K |
| β = 107.263 (7)° | Needle, yellow |
| V = 1811.9 (3) Å3 | 0.60 × 0.13 × 0.04 mm |
| Z = 4 |
Data collection
| Stoe IPDS II diffractometer | 3190 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.111 |
| graphite | θmax = 29.3°, θmin = 1.7° |
| Detector resolution: 0.15 mm pixels mm-1 | h = −18→19 |
| rotation method scans | k = −10→11 |
| 21467 measured reflections | l = −22→22 |
| 4893 independent reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.083 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.195 | H-atom parameters constrained |
| S = 1.15 | w = 1/[σ2(Fo2) + (0.0578P)2 + 0.8502P] where P = (Fo2 + 2Fc2)/3 |
| 4893 reflections | (Δ/σ)max = 0.002 |
| 253 parameters | Δρmax = 0.24 e Å−3 |
| 0 restraints | Δρmin = −0.28 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | −0.0070 (3) | 0.2134 (5) | 0.0453 (2) | 0.0719 (9) | |
| H1A | −0.0208 | 0.3142 | 0.0707 | 0.086* | |
| H1B | 0.0435 | 0.1542 | 0.0866 | 0.086* | |
| H1C | −0.0645 | 0.1464 | 0.0281 | 0.086* | |
| C2 | 0.1045 (2) | 0.3483 (3) | −0.01638 (17) | 0.0497 (6) | |
| C3 | 0.1271 (2) | 0.3897 (3) | −0.09054 (17) | 0.0521 (7) | |
| H3 | 0.0870 | 0.3551 | −0.1437 | 0.063* | |
| C4 | 0.2088 (2) | 0.4820 (3) | −0.08625 (16) | 0.0476 (6) | |
| H4 | 0.2224 | 0.5109 | −0.1368 | 0.057* | |
| C5 | 0.27136 (19) | 0.5328 (3) | −0.00716 (16) | 0.0459 (6) | |
| C6 | 0.2465 (2) | 0.4957 (4) | 0.06647 (17) | 0.0557 (7) | |
| H6 | 0.2861 | 0.5319 | 0.1196 | 0.067* | |
| C7 | 0.1629 (2) | 0.4047 (4) | 0.06239 (18) | 0.0570 (7) | |
| H7 | 0.1465 | 0.3822 | 0.1124 | 0.068* | |
| C8 | 0.43609 (19) | 0.6338 (3) | 0.05119 (16) | 0.0446 (6) | |
| C9 | 0.54000 (18) | 0.5791 (3) | 0.18936 (16) | 0.0436 (6) | |
| C10 | 0.5543 (2) | 0.5004 (4) | 0.26776 (18) | 0.0518 (6) | |
| H10 | 0.5058 | 0.4342 | 0.2773 | 0.062* | |
| C11 | 0.6412 (2) | 0.5210 (4) | 0.33167 (17) | 0.0536 (7) | |
| H11 | 0.6514 | 0.4685 | 0.3844 | 0.064* | |
| C12 | 0.7131 (2) | 0.6199 (4) | 0.31721 (18) | 0.0554 (7) | |
| H12 | 0.7713 | 0.6340 | 0.3606 | 0.066* | |
| C13 | 0.6990 (2) | 0.6979 (4) | 0.23896 (17) | 0.0525 (7) | |
| H13 | 0.7476 | 0.7644 | 0.2298 | 0.063* | |
| C14 | 0.61137 (18) | 0.6767 (3) | 0.17343 (15) | 0.0428 (6) | |
| C15 | 0.5157 (2) | 0.7295 (3) | 0.03396 (16) | 0.0447 (6) | |
| C16 | 0.55436 (18) | 0.8817 (3) | −0.08379 (15) | 0.0435 (6) | |
| C17 | 0.6539 (2) | 0.9088 (4) | −0.04862 (17) | 0.0536 (7) | |
| H17 | 0.6862 | 0.8660 | 0.0053 | 0.064* | |
| C18 | 0.7048 (2) | 0.9990 (4) | −0.09332 (18) | 0.0578 (7) | |
| H18 | 0.7713 | 1.0150 | −0.0693 | 0.069* | |
| C19 | 0.6583 (2) | 1.0656 (3) | −0.17326 (17) | 0.0487 (6) | |
| C20 | 0.5592 (2) | 1.0388 (4) | −0.20832 (17) | 0.0548 (7) | |
| H20 | 0.5268 | 1.0827 | −0.2620 | 0.066* | |
| C21 | 0.5087 (2) | 0.9484 (4) | −0.16457 (16) | 0.0505 (6) | |
| H21 | 0.4425 | 0.9312 | −0.1894 | 0.061* | |
| C22 | 0.8046 (2) | 1.1618 (5) | −0.1983 (2) | 0.0777 (10) | |
| H22A | 0.8289 | 1.0517 | −0.1997 | 0.093* | |
| H22B | 0.8296 | 1.2060 | −0.1412 | 0.093* | |
| H22C | 0.8250 | 1.2305 | −0.2379 | 0.093* | |
| N1 | 0.35461 (16) | 0.6221 (3) | −0.01063 (14) | 0.0499 (5) | |
| N2 | 0.59911 (15) | 0.7521 (3) | 0.09392 (12) | 0.0413 (5) | |
| N3 | 0.49602 (16) | 0.7909 (3) | −0.04487 (13) | 0.0494 (5) | |
| H3A | 0.4381 | 0.7720 | −0.0774 | 0.059* | |
| O1 | 0.02353 (17) | 0.2511 (3) | −0.02758 (14) | 0.0700 (6) | |
| O2 | 0.45078 (13) | 0.5596 (3) | 0.12731 (12) | 0.0556 (5) | |
| O3 | 0.70194 (16) | 1.1580 (3) | −0.22196 (13) | 0.0655 (6) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.066 (2) | 0.077 (2) | 0.076 (2) | −0.0097 (17) | 0.0262 (17) | 0.0031 (18) |
| C2 | 0.0490 (15) | 0.0481 (15) | 0.0498 (14) | 0.0023 (12) | 0.0114 (12) | −0.0024 (12) |
| C3 | 0.0606 (17) | 0.0492 (15) | 0.0416 (13) | −0.0021 (13) | 0.0074 (12) | −0.0070 (12) |
| C4 | 0.0567 (16) | 0.0476 (15) | 0.0367 (12) | 0.0030 (12) | 0.0111 (11) | −0.0008 (11) |
| C5 | 0.0479 (14) | 0.0464 (14) | 0.0405 (12) | 0.0063 (11) | 0.0088 (11) | −0.0015 (11) |
| C6 | 0.0488 (15) | 0.075 (2) | 0.0393 (13) | −0.0014 (14) | 0.0072 (11) | −0.0043 (13) |
| C7 | 0.0549 (17) | 0.075 (2) | 0.0414 (13) | −0.0001 (15) | 0.0143 (12) | 0.0018 (13) |
| C8 | 0.0438 (13) | 0.0448 (14) | 0.0420 (13) | 0.0052 (11) | 0.0077 (11) | −0.0031 (11) |
| C9 | 0.0378 (13) | 0.0457 (14) | 0.0445 (13) | 0.0027 (10) | 0.0079 (10) | 0.0008 (11) |
| C10 | 0.0464 (14) | 0.0535 (16) | 0.0554 (15) | 0.0022 (12) | 0.0147 (12) | 0.0093 (13) |
| C11 | 0.0533 (16) | 0.0587 (17) | 0.0434 (13) | 0.0080 (13) | 0.0061 (12) | 0.0054 (13) |
| C12 | 0.0479 (15) | 0.0627 (18) | 0.0486 (14) | −0.0022 (13) | 0.0035 (12) | −0.0079 (13) |
| C13 | 0.0467 (15) | 0.0584 (17) | 0.0499 (15) | −0.0085 (12) | 0.0104 (12) | −0.0057 (13) |
| C14 | 0.0438 (13) | 0.0431 (13) | 0.0414 (12) | 0.0015 (11) | 0.0125 (10) | −0.0020 (10) |
| C15 | 0.0531 (15) | 0.0393 (13) | 0.0443 (13) | 0.0065 (11) | 0.0186 (11) | −0.0017 (10) |
| C16 | 0.0440 (13) | 0.0461 (14) | 0.0400 (12) | 0.0054 (11) | 0.0119 (11) | −0.0026 (10) |
| C17 | 0.0440 (14) | 0.0676 (18) | 0.0433 (14) | 0.0022 (13) | 0.0036 (12) | 0.0082 (13) |
| C18 | 0.0421 (14) | 0.075 (2) | 0.0497 (14) | −0.0037 (14) | 0.0041 (12) | 0.0031 (15) |
| C19 | 0.0533 (15) | 0.0501 (15) | 0.0423 (13) | 0.0014 (12) | 0.0136 (12) | −0.0018 (11) |
| C20 | 0.0530 (16) | 0.0699 (19) | 0.0372 (12) | 0.0059 (14) | 0.0068 (12) | 0.0054 (13) |
| C21 | 0.0422 (13) | 0.0640 (18) | 0.0408 (13) | 0.0028 (12) | 0.0052 (11) | −0.0016 (12) |
| C22 | 0.0560 (19) | 0.095 (3) | 0.084 (2) | −0.0101 (18) | 0.0233 (18) | 0.012 (2) |
| N1 | 0.0459 (12) | 0.0551 (14) | 0.0447 (11) | −0.0003 (10) | 0.0071 (10) | −0.0023 (10) |
| N2 | 0.0417 (11) | 0.0446 (12) | 0.0361 (10) | −0.0024 (9) | 0.0095 (8) | −0.0002 (9) |
| N3 | 0.0446 (12) | 0.0576 (14) | 0.0431 (11) | 0.0033 (10) | 0.0087 (9) | 0.0008 (10) |
| O1 | 0.0668 (14) | 0.0815 (15) | 0.0617 (13) | −0.0229 (12) | 0.0193 (11) | −0.0067 (11) |
| O2 | 0.0446 (10) | 0.0604 (12) | 0.0558 (11) | −0.0025 (9) | 0.0058 (9) | 0.0078 (9) |
| O3 | 0.0595 (13) | 0.0817 (15) | 0.0544 (11) | −0.0078 (11) | 0.0157 (10) | 0.0101 (11) |
Geometric parameters (Å, °)
| C1—O1 | 1.416 (4) | C11—H11 | 0.9300 |
| C1—H1A | 0.9600 | C12—C13 | 1.381 (4) |
| C1—H1B | 0.9600 | C12—H12 | 0.9300 |
| C1—H1C | 0.9600 | C13—C14 | 1.402 (4) |
| C2—O1 | 1.374 (3) | C13—H13 | 0.9300 |
| C2—C3 | 1.382 (4) | C14—N2 | 1.393 (3) |
| C2—C7 | 1.386 (4) | C15—N2 | 1.318 (3) |
| C3—C4 | 1.378 (4) | C15—N3 | 1.325 (3) |
| C3—H3 | 0.9300 | C16—C21 | 1.393 (4) |
| C4—C5 | 1.397 (3) | C16—C17 | 1.396 (4) |
| C4—H4 | 0.9300 | C16—N3 | 1.401 (3) |
| C5—C6 | 1.382 (4) | C17—C18 | 1.385 (4) |
| C5—N1 | 1.417 (4) | C17—H17 | 0.9300 |
| C6—C7 | 1.397 (4) | C18—C19 | 1.384 (4) |
| C6—H6 | 0.9300 | C18—H18 | 0.9300 |
| C7—H7 | 0.9300 | C19—O3 | 1.370 (3) |
| C8—N1 | 1.304 (3) | C19—C20 | 1.390 (4) |
| C8—O2 | 1.336 (3) | C20—C21 | 1.371 (4) |
| C8—C15 | 1.479 (4) | C20—H20 | 0.9300 |
| C9—C14 | 1.381 (4) | C21—H21 | 0.9300 |
| C9—C10 | 1.385 (4) | C22—O3 | 1.415 (4) |
| C9—O2 | 1.389 (3) | C22—H22A | 0.9600 |
| C10—C11 | 1.381 (4) | C22—H22B | 0.9600 |
| C10—H10 | 0.9300 | C22—H22C | 0.9600 |
| C11—C12 | 1.384 (4) | N3—H3A | 0.8600 |
| O1—C1—H1A | 109.5 | C12—C13—H13 | 120.1 |
| O1—C1—H1B | 109.5 | C14—C13—H13 | 120.1 |
| H1A—C1—H1B | 109.5 | C9—C14—N2 | 121.9 (2) |
| O1—C1—H1C | 109.5 | C9—C14—C13 | 118.8 (2) |
| H1A—C1—H1C | 109.5 | N2—C14—C13 | 119.4 (2) |
| H1B—C1—H1C | 109.5 | N2—C15—N3 | 123.5 (2) |
| O1—C2—C3 | 115.8 (2) | N2—C15—C8 | 121.4 (2) |
| O1—C2—C7 | 124.8 (3) | N3—C15—C8 | 115.1 (2) |
| C3—C2—C7 | 119.5 (3) | C21—C16—C17 | 117.8 (2) |
| C4—C3—C2 | 120.5 (2) | C21—C16—N3 | 116.8 (2) |
| C4—C3—H3 | 119.8 | C17—C16—N3 | 125.4 (2) |
| C2—C3—H3 | 119.8 | C18—C17—C16 | 120.6 (2) |
| C3—C4—C5 | 121.0 (2) | C18—C17—H17 | 119.7 |
| C3—C4—H4 | 119.5 | C16—C17—H17 | 119.7 |
| C5—C4—H4 | 119.5 | C19—C18—C17 | 120.9 (3) |
| C6—C5—C4 | 118.2 (3) | C19—C18—H18 | 119.5 |
| C6—C5—N1 | 125.9 (2) | C17—C18—H18 | 119.5 |
| C4—C5—N1 | 115.9 (2) | O3—C19—C18 | 125.3 (3) |
| C5—C6—C7 | 121.0 (3) | O3—C19—C20 | 116.1 (2) |
| C5—C6—H6 | 119.5 | C18—C19—C20 | 118.5 (3) |
| C7—C6—H6 | 119.5 | C21—C20—C19 | 120.7 (2) |
| C2—C7—C6 | 119.8 (3) | C21—C20—H20 | 119.7 |
| C2—C7—H7 | 120.1 | C19—C20—H20 | 119.7 |
| C6—C7—H7 | 120.1 | C20—C21—C16 | 121.4 (2) |
| N1—C8—O2 | 122.8 (2) | C20—C21—H21 | 119.3 |
| N1—C8—C15 | 117.7 (2) | C16—C21—H21 | 119.3 |
| O2—C8—C15 | 119.5 (2) | O3—C22—H22A | 109.5 |
| C14—C9—C10 | 121.3 (2) | O3—C22—H22B | 109.5 |
| C14—C9—O2 | 120.6 (2) | H22A—C22—H22B | 109.5 |
| C10—C9—O2 | 118.1 (2) | O3—C22—H22C | 109.5 |
| C11—C10—C9 | 119.5 (3) | H22A—C22—H22C | 109.5 |
| C11—C10—H10 | 120.3 | H22B—C22—H22C | 109.5 |
| C9—C10—H10 | 120.3 | C8—N1—C5 | 125.9 (2) |
| C10—C11—C12 | 120.0 (3) | C15—N2—C14 | 117.6 (2) |
| C10—C11—H11 | 120.0 | C15—N3—C16 | 130.4 (2) |
| C12—C11—H11 | 120.0 | C15—N3—H3A | 114.8 |
| C13—C12—C11 | 120.6 (3) | C16—N3—H3A | 114.8 |
| C13—C12—H12 | 119.7 | C2—O1—C1 | 118.4 (2) |
| C11—C12—H12 | 119.7 | C8—O2—C9 | 118.8 (2) |
| C12—C13—C14 | 119.9 (3) | C19—O3—C22 | 118.5 (2) |
| O1—C2—C3—C4 | −177.7 (3) | C17—C18—C19—O3 | 179.2 (3) |
| C7—C2—C3—C4 | 2.0 (4) | C17—C18—C19—C20 | −0.6 (5) |
| C2—C3—C4—C5 | 1.3 (4) | O3—C19—C20—C21 | −179.8 (3) |
| C3—C4—C5—C6 | −3.4 (4) | C18—C19—C20—C21 | 0.0 (4) |
| C3—C4—C5—N1 | 177.9 (2) | C19—C20—C21—C16 | 0.5 (5) |
| C4—C5—C6—C7 | 2.3 (4) | C17—C16—C21—C20 | −0.4 (4) |
| N1—C5—C6—C7 | −179.3 (3) | N3—C16—C21—C20 | −179.8 (3) |
| O1—C2—C7—C6 | 176.6 (3) | O2—C8—N1—C5 | 0.2 (4) |
| C3—C2—C7—C6 | −3.2 (4) | C15—C8—N1—C5 | 178.3 (2) |
| C5—C6—C7—C2 | 1.0 (5) | C6—C5—N1—C8 | 25.8 (4) |
| C14—C9—C10—C11 | 0.6 (4) | C4—C5—N1—C8 | −155.7 (3) |
| O2—C9—C10—C11 | −178.2 (3) | N3—C15—N2—C14 | −178.2 (2) |
| C9—C10—C11—C12 | 0.1 (4) | C8—C15—N2—C14 | 2.6 (3) |
| C10—C11—C12—C13 | −0.4 (5) | C9—C14—N2—C15 | 1.1 (4) |
| C11—C12—C13—C14 | −0.1 (4) | C13—C14—N2—C15 | −179.9 (2) |
| C10—C9—C14—N2 | 177.9 (2) | N2—C15—N3—C16 | 3.1 (4) |
| O2—C9—C14—N2 | −3.4 (4) | C8—C15—N3—C16 | −177.7 (2) |
| C10—C9—C14—C13 | −1.1 (4) | C21—C16—N3—C15 | −171.6 (3) |
| O2—C9—C14—C13 | 177.7 (2) | C17—C16—N3—C15 | 9.0 (5) |
| C12—C13—C14—C9 | 0.9 (4) | C3—C2—O1—C1 | −176.0 (3) |
| C12—C13—C14—N2 | −178.1 (3) | C7—C2—O1—C1 | 4.3 (5) |
| N1—C8—C15—N2 | 177.6 (2) | N1—C8—O2—C9 | −180.0 (2) |
| O2—C8—C15—N2 | −4.3 (4) | C15—C8—O2—C9 | 2.0 (3) |
| N1—C8—C15—N3 | −1.7 (3) | C14—C9—O2—C8 | 1.6 (4) |
| O2—C8—C15—N3 | 176.5 (2) | C10—C9—O2—C8 | −179.6 (2) |
| C21—C16—C17—C18 | −0.2 (4) | C18—C19—O3—C22 | 12.6 (5) |
| N3—C16—C17—C18 | 179.2 (3) | C20—C19—O3—C22 | −167.6 (3) |
| C16—C17—C18—C19 | 0.7 (5) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C7—H7···O3i | 0.93 | 2.59 | 3.423 (3) | 149 |
Symmetry codes: (i) x−1/2, −y+3/2, z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BT5425).
References
- Asgari, D., Mehrdad, M., Ghanbari, M., Jadidi, K., Behzad, S. K. & Khavasi, H. R. (2011). Acta Cryst. E67 Submitted [BT5429]
- Azizian, J., Mehrdad, M., Jadidi, K. & Sarrafi, Y. (2000). Tetrahedron Lett. 41, 5265–5268.
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst. 32, 837–838.
- Jadidi, K., Ghahremanzadeh, R., Mehrdad, M., Ghanbari, M. & Arvin-Nezhad, H. (2008). Monatsh. Chem. 139, 277–280.
- Mehrdad, M., Ghanbari, M., Jadidi, K., Asgari, D. & Khavasi, H. R. (2011). In preparation.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Stoe & Cie (2005). X-AREA Stoe & Cie, Darmstadt, Germany.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810050294/bt5425sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810050294/bt5425Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report