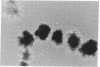
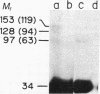
Abstract
Erythropoietin (Epo) is a glycoprotein factor that specifically regulates the proliferation and differentiation of erythroid progenitor cells. Here we describe the isolation of Epo-responsive mouse erythroleukemia cell line SKT6, the characterization of the specific binding of biologically active 125I-labeled human Epo (125I-Epo) to its membrane receptor, and, finally, report information concerning the molecular structure of the receptor. About 75% of erythroid colony-forming precursor cell-like colonies derived from SKT6 cells were hemoglobin-positive after 3- to 4-day exposure to Epo in methylcellulose culture. Radioiodinated Epo bound specifically to SKT6 cells, and Scatchard analysis of the data showed a high affinity for 125I-Epo (Kd = 0.15 nM) but displayed only a small number of specific receptors (approximately equal to 470 per cell). Membrane components that specifically interact with 125I-Epo were identified by covalent crosslinking with disuccinimidyl suberate, and three receptor species with apparent Mr 63,000, 94,000, and 119,000 were found in membrane from SKT6 cells, suggesting the complex structure of the receptor molecules. Specific bindings were also detected in all of the Epo-unresponsive Friend erythroleukemia cells examined, and cross-linking study revealed the presence of only the 63,000 species as a binding site.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn C. D., Jarvis J. H., Greenman J. M. A quantitative bioassay for erythropoietin using mouse fetal liver cells. Exp Hematol. 1975 Jan;3(1):65–78. [PubMed] [Google Scholar]
- Fieldsteel A. H., Kurahara C. Moloney leukaemia virus as a helper in retrieving Friend virus from a non-infectious reticulum cell sarcoma. Nature. 1969 Sep 20;223(5212):1274–1274. doi: 10.1038/2231274a0. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J. F., Ebbe S. N., Hollander L., Cutting H. O., Miller M. E., Cronkite E. P. Radioimmunoassay of erythropoietin: circulating levels in normal and polycythemic human beings. J Lab Clin Med. 1982 May;99(5):624–635. [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa Y., Aida M., Inoue Y. Isolation and characterization of high and low differentiation-inducible Friend leukemia lines. Gan. 1976 Oct;67(5):767–770. [PubMed] [Google Scholar]
- Jacobs K., Shoemaker C., Rudersdorf R., Neill S. D., Kaufman R. J., Mufson A., Seehra J., Jones S. S., Hewick R., Fritsch E. F. Isolation and characterization of genomic and cDNA clones of human erythropoietin. 1985 Feb 28-Mar 6Nature. 313(6005):806–810. doi: 10.1038/313806a0. [DOI] [PubMed] [Google Scholar]
- Kasuga M., van Obberghen E., Yamada K. M., Harrison L. C. Autoantibodies against the insulin receptor recognize the insulin binding subunits of an oligomeric receptor. Diabetes. 1981 Apr;30(4):354–357. doi: 10.2337/diab.30.4.354. [DOI] [PubMed] [Google Scholar]
- Koyasu S., Nakauchi H., Kitamura K., Yonehara S., Okumura K., Tada T., Yahara I. Production of interleukin 3 and gamma-interferon by an antigen-specific mouse suppressor T cell clone. J Immunol. 1985 May;134(5):3130–3136. [PubMed] [Google Scholar]
- Krantz S. B., Goldwasser E. Specific binding of erythropoietin to spleen cells infected with the anemia strain of Friend virus. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7574–7578. doi: 10.1073/pnas.81.23.7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin F. K., Suggs S., Lin C. H., Browne J. K., Smalling R., Egrie J. C., Chen K. K., Fox G. M., Martin F., Stabinsky Z. Cloning and expression of the human erythropoietin gene. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7580–7584. doi: 10.1073/pnas.82.22.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- McDonald J. D., Lin F. K., Goldwasser E. Cloning, sequencing, and evolutionary analysis of the mouse erythropoietin gene. Mol Cell Biol. 1986 Mar;6(3):842–848. doi: 10.1128/mcb.6.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod D. L., Shreeve M. M., Axelrad A. A. Improved plasma culture system for production of erythrocytic colonies in vitro: quantitative assay method for CFU-E. Blood. 1974 Oct;44(4):517–534. [PubMed] [Google Scholar]
- Mishina Y., Obinata M. Induction of commitment of murine erythroleukemia cells (TSA8) to CFU-E with DMSO. Exp Cell Res. 1986 Feb;162(2):319–325. doi: 10.1016/0014-4827(86)90337-x. [DOI] [PubMed] [Google Scholar]
- Miyake T., Kung C. K., Goldwasser E. Purification of human erythropoietin. J Biol Chem. 1977 Aug 10;252(15):5558–5564. [PubMed] [Google Scholar]
- Ramsay R. G., Ikeda K., Rifkind R. A., Marks P. A. Changes in gene expression associated with induced differentiation of erythroleukemia: protooncogenes, globin genes, and cell division. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6849–6853. doi: 10.1073/pnas.83.18.6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennick D. M., Lee F. D., Yokota T., Arai K. I., Cantor H., Nabel G. J. A cloned MCGF cDNA encodes a multilineage hematopoietic growth factor: multiple activities of interleukin 3. J Immunol. 1985 Feb;134(2):910–914. [PubMed] [Google Scholar]
- Shibuya T., Mak T. W. Isolation and induction of erythroleukemic cell lines with properties of erythroid progenitor burst-forming cell (BFU-E) and erythroid precursor cell (CFU-E). Proc Natl Acad Sci U S A. 1983 Jun;80(12):3721–3725. doi: 10.1073/pnas.80.12.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejedor F., Ballesta J. P. Iodination of biological samples without loss of functional activity. Anal Biochem. 1982 Nov 15;127(1):143–149. doi: 10.1016/0003-2697(82)90156-7. [DOI] [PubMed] [Google Scholar]
- Todokoro K., Ikawa Y. Sequential expression of proto-oncogenes during a mouse erythroleukemia cell differentiation. Biochem Biophys Res Commun. 1986 Mar 28;135(3):1112–1118. doi: 10.1016/0006-291x(86)91043-0. [DOI] [PubMed] [Google Scholar]
- Yokota T., Lee F., Rennick D., Hall C., Arai N., Mosmann T., Nabel G., Cantor H., Arai K. Isolation and characterization of a mouse cDNA clone that expresses mast-cell growth-factor activity in monkey cells. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1070–1074. doi: 10.1073/pnas.81.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]