Abstract
Dendritic cells (DCs) are initiators of innate immunity and acquired immunity as cells linking these two bio-defence systems through the production of cytokines such as interferon-α (IFN-α) and interleukin-12 (IL-12). Nucleic acids such as DNA from damaged cells or pathogens are important activators not only for anti-microbial innate immune responses but also in the pathogenesis of IFN-related autoimmune diseases. Plasmacytoid DCs are regarded as the main effectors for the DNA-mediated innate immunity by possessing DNA-sensing toll-like receptor 9 (TLR9). We here found that double-stranded DNA (dsDNA) complexed with lipotransfectants triggered activation of human monocyte-derived DCs (moDCs), leading to the preferential production of IFN-α but not IL-12. This indicates that myeloid DCs also function as supportive effectors against the invasion of pathogenic microbes through the DNA-mediated activation in innate immunity. The dsDNA with lipotransfectants can be taken up by moDCs without co-localization of endosomal LAMP1 staining, and the dsDNA-mediated IFN-α production was not impaired by chloroquine. These findings indicate that moDC activation by dsDNA does not involve the endosomal TLR pathway. In contrast, single-stranded RNA (ssRNA) stimulated moDCs to secrete IL-12 but not IFN-α. This process was inhibited by chloroquine, suggesting an involvement of the TLR pathway in ssRNA-mediated moDC activation. As might be inferred from our findings, myeloid DCs may function as a traffic control between innate immunity via IFN-α production and acquired immunity via IL-12 production, depending on the type of nucleic acids. Our results provide a new insight into the biological action of myeloid DCs underlying the DNA-mediated activation of protective or pathogenic immunity.
Keywords: dendritic cells, double-stranded DNA, interferon-α, single-stranded RNA
Introduction
It has been widely accepted that immature dendritic cells (DCs) serve as sentinels for invading antigens at the frontline at the periphery, and then act as global initiators of innate immunity by producing inflammatory cytokines such as type I interferons (IFNs), which function as activators of many types of innate immune effectors and directly influence invading pathogens.1 Furthermore, DCs sequentially initiate acquired immunity by priming naive CD4+ T cells through producing interleukin-12 (IL-12), which is indispensable for triggering the T helper type 1 response. The DCs are important in the initiation of both innate and acquired immunity as cells linking these two bio-defence systems.2,3
Upon microbial invasion, various types of inflammatory responses occur in the periphery, and nucleic acids from regional damaged cells or pathogens can appear at the sites of inflammation, to be recognized by DCs as danger signals, and to activate DCs to initiate innate immunity. Endosomal toll-like receptors (TLRs), such as TLR3, TLR7, TLR8 and TLR9, which are sensors for these nucleic acids and mediate the activation signal cascades leading to the production of cytokines,4,5 are expressed in DC subsets.6,7 In general, myeloid DCs, which are regarded as conventional DCs, have the ability to produce large amounts of IL-12 upon TLR-mediated microbial recognition,1,8 indicating a critical contribution to T helper type 1-mediated acquired immunity. Only in response to double-stranded (ds) RNA such as poly(I:C), can myeloid DCs produce IFN-α.8,9 Of the DC subsets, plasmacytoid DCs (pDCs) are regarded as the major producers of type I IFNs by the selective expression of TLR7 and TLR9, which recognize single-stranded (ss) RNA and ssDNA, respectively. Indeed, human pDCs are able to produce robust amounts of type I IFNs, but not IL-12, in response to DNA such as microbial DNA with unmethylated CpG motifs.10 Recent studies have revealed a central role of pDCs for self-DNA in the pathogenesis of autoimmune diseases. In patients with psoriasis or systemic lupus erythematosus (SLE), anti-microbial peptide LL37 or nuclear protein high-mobility group box 1 protein (HMGB1) released from tissue injury helps to promote stabilization and delivery of self-DNA into early endosomes in pDCs,11,12 leading to the induction of aberrant type I IFN production and driving autoimmunity. Hence, DNA is a potent activator of immune responses during infection or tissue damage if optimal host-derived transporter agents are present, and pDCs are thought to be the key effectors that initiate the DNA-mediated immune responses.
However, it has been reported that non-pDCs as well as pDCs produce IFN-α in response to the DNA virus, herpes simplex virus type-1, via both TLR9-independent and TLR9-dependent pathways.13 Although there is abundant evidence that human myeloid DCs lack TLR96,7 and the mechanism underlying the sensing of DNA in myeloid DCs remains elusive, the type I IFN production is dependent on viral entry, but not on TLR9, and the entry-dependent IFN induction requires the presence of viral genomic DNA in myeloid DCs.14 These findings suggest a contribution of myeloid DCs to the DNA-mediated innate immune response by TLR-independent DNA recognition machinery. Extensive analysis during the last few years has identified three different cytosolic DNA sensors – DAI (DLM-1/ZBP1),15 AIM216 and LRRFIP117 – that activate the innate immune system. Therefore, it is plausible that, in the inflammatory sites in the periphery, DNA from damaged cells or pathogens could be incorporated into immature myeloid DC subsets, as well as pDCs, and activate them if it could form a complex with host-derived transporter agents. Along with this speculation, we here examined an in vitro model in which dsDNA was incorporated into human monocyte-derived DCs (moDCs) as a complex form with various transfectants for enhancing DNA transport, and we offer new insights into the biological action of myeloid DCs underlying the DNA-mediated activation of antimicrobial immunity or autoimmunity.
Materials and methods
Media and reagents
RPMI-1640 supplemented with 2 mm l-glutamine, 100 U/ml penicillin, 100 ng/ml streptomycin and heat-inactivated 10% fetal bovine serum (Biosource International, Camarillo, CA) was used for cell cultures. For cell stimulation, we used 25 μg/ml dsRNA poly(I:C) (InvivoGen, San Diego, CA), 1 μg/ml R848 (InvivoGen), unconjugated or FITC-conjugated dsDNA poly(dA-dT)/poly(dA-dT) (Sigma-Aldrich, St Louis, MO), and ssRNA poly(U) (Sigma-Aldrich). Reagents used for facilitating the transfection of nucleic acid were as follows: lipofectamine 2000, which is a mixture of DOSPA and DOPE at a ratio of 3 : 1, where DOSPA is 2,3-dioleyloxy-N-[2(sperminecarbox-amido)ethyl]-N, N dimethyl-1-propanaminium trifluoroacetate and DOPE is the neutral lipid dioleoyl phosphatidylethanolamine (Invitrogen, Carlsbad, CA); LyoVec®, which is a mixture of DMPTA and DOPE, where DMPTA is dimyristoleyl phos[homethyl trimethyl ammonium] (InvivoGen); DOTAP (N-[1-(2, 3-dioleoyloxy) propyl]-N, N, N trimethyl ammonium methyl sulphate) (Roche Diagnostics, Indianapolis, IN); and Protamine (Mochida Pharmaceutical Co. Ltd, Tokyo, Japan).
Generation of monocyte-derived human DCs
The moDCs were generated as previously described.8 Briefly, human peripheral blood mononuclear cells were separated from the heparinized peripheral blood of healthy donors by standard gradient centrifugation with Ficoll–Hypaque (Amersham Pharmacia Biotech, Uppsala, Sweden). The low-density peripheral blood mononuclear cells were harvested, and CD14+ cells were positively selected using CD14-microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) to reach > 95% purity according to restaining with anti-CD14. To induce DC differentiation, 106 cells/ml CD14+ monocytes were cultured in complete medium (RPMI-1640 with 10% FBS), 800 IU/ml human recombinant granulocyte–macrophage colony-stimulating factor (rGM-CSF; R&D Systems, Minneapolis, MN), and 200 IU/ml human rIL-4 (R&D Systems) at 37° in 5% CO2. On day 3, medium containing GM-CSF and IL-4 was added. On day 5, non-adherent DCs were harvested by gentle pipetting.
Isolation of human blood DC subsets
Human peripheral blood DC subsets (myeloid BDCA1+ DCs and pDCs) were isolated from PBMCs from healthy adult donors, as described previously.8 CD11c+ BDCA3− lineage− CD4+ cells (as myeloid BDCA1+ DCs) and CD11c− BDCA4+ lineage− CD4+ cells (as pDCs) were sorted by FACS Aria® (BD Biosciences, San Diego, CA) to reach > 99% purity according to restaining with anti-BDCA1 or anti-BDCA2.
Cultures of DCs with transfectants
Either dsDNA or poly(U) was mixed with lipofectamine 2000 [1 μg dsDNA or poly(U) per 1 μl of lipofectamine 2000], LyoVec [0·5–1·5 μg dsDNA or poly(U) per 50 μl LyoVec], DOTAP [1–2 μg dsDNA or poly(U) per 5–10 μg DOTAP], or protamine [1 μg dsDNA per 2 μg protamine, or 1 μg poly(U) per 1 μg protamine] for 30 min at 37° to make complexes [dsDNA/transfectants or poly(U)/transfectants], then moDCs (5 × 104 cells/200 μl/well in a 96-well flat-bottom culture plate) were cultured in the presence of these mixtures [final concentration 25 μg/ml of dsDNA or poly(U)]. The moDCs cultured with transfectants alone or medium alone, served as controls. In some experiments, chloroquine (Sigma-Aldrich) dissolved in PBS was added to moDCs with transfectants. The PBS was diluted in parallel to serve as a vehicle control.
Detection of intracellular dsDNA
The cells cultured with FITC-conjugated dsDNA or FITC-conjugated poly(U) for 4 hr were cytocentrifuged, and the specimen was stained with phycoerythrin (PE) -labelled anti-lysosome-associated membrane protein (LAMP1; BD Biosciences) followed by 4’,6-diamidino-2-phenylindole (DAPI; Nacalai Tesque, Inc., Kyoto, Japan). The stained specimens were observed using a confocal microscope (LSM510-META; Carl Zeiss, Zentralbereich Forschung, Germany). Furthermore, intracellular dsDNA was detected using a FACSCalibur® equipped with CellQuest software (BD Biosciences).
Analysis of cell surface markers
To analyse the expression of co-stimulatory molecules, the DCs after culture for 24 hr were stained with FITC-labelled anti-CD86 (BD Biosciences) and then analysed using a FACSCalibur®.
Measurement of cytokine production
The production of cytokines (IFN-α and IL-12p40) in the culture supernatants was determined by ELISA after 24 hr culture (ELISA kits from R&D Systems). Also dsDNA/LyoVec Complexes® and ssPolyU/LyoVec Complexes® (InvivoGen) were used for both the intracellular cytokine staining and ELISA. Intracellular cytokine staining in DCs was performed after 8 hr of culture. Brefeldin A (10 μg/ml; Sigma-Aldrich) was added for the last 2 hr. After stimulation, cells were fixed and permeabilized using the FIX and PERM kit (CALTAG, Burlingame, CA) and then stained with FITC-labelled anti-IFN-α2 monoclonal antibody (Chromaprobe, Maryland Heights, MO) and PE-labelled anti-IL-12 p40+ p70 monoclonal antibody (BioLegend, San Diego, CA). Dead cells were excluded on the basis of side- and forward-scatter characteristics.
Results
The dsDNA is incorporated intracellularly in DCs by transfectants
We first examined whether dsDNA is actually incorporated by moDCs. Lipofectamine, LyoVec and DOTAP are usually used in the method known as a liposome transfection (lipotransfection), and cationic liposomes formed by these reagents show advanced DNA and mRNA transfection.18 Protamine, used as an anti-heparin treatment, is a natural cationic protein in the testis and spontaneously associates with nucleic acids. Hence, we used these as nucleic acid transfection reagents. FITC-labelled dsDNA was added to the culture of moDCs for 4 hr. As visualized in Fig. 1, the labelled dsDNA was readily detected as associating with moDCs by flow cytometry when given in complex with any transfectants. No dsDNA was detected at all in moDCs when added without transfectants. We found that LyoVec, lipofectamine and DOTAP markedly enhanced the incorporation of dsDNA into moDCs but protamine did so only modestly, which might enable it to access the endosomal or cytosolic compartment of moDCs (Fig. 1a). The dsDNA was most efficiently delivered into moDCs when DOTAP or lipofectamine was used as a transfectant. Further analysis with confocal microscopy at the single cell level for the intracellular distribution revealed that, whereas dsDNA transfected by LyoVec was internalized intracellularly (but distinct from the organelles, as shown by the staining of LAMP1, an endosomal/lysosomal marker), ssRNA poly(U) transfected by LyoVec was co-localized with LAMP1 (Fig. 1b).
Figure 1.
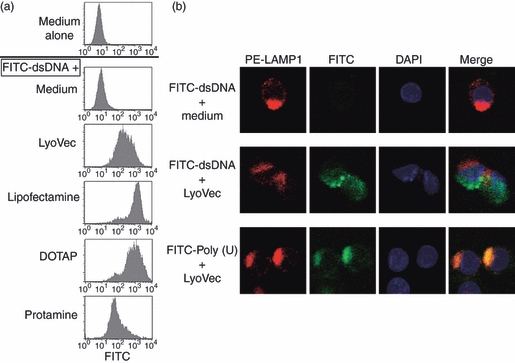
Detection of incorporated double-stranded (ds) DNA in monocyte-derived dendritic cells (moDCs). FITC-labelled dsDNA poly(dA-dT)/poly(dA-dT) was mixed with the indicated transfectants, and these complexed forms of dsDNA were added to the culture with moDCs for 4 hr. The cells were flow cytometrically analysed (a). Furthermore, the incorporated FITC-labelled dsDNA or FITC-labelled single-stranded (ss) RNA poly(U) in the cells after culture with LyoVec were visualized (green) by immunofluorescence microscopy together with LAMP1 antibody (red) and nuclei staining with DAPI (blue) (b). Similar results were observed in at least three independent donors.
Incorporated dsDNA induces DC maturation
We next examined whether dsDNA can activate moDCs in the presence of transfectants. Dendritic cells were cultured with dsDNA and transfectants for 24 hr, and the expression of the co-stimulatory molecule CD86 was analysed by flow cytometry (Fig. 2). We used also ssRNA poly(U), which is sensed by TLR8 in human myeloid DCs.19 Neither dsDNA nor poly(U) up-regulated CD86 expression on moDCs in the absence of these transfectants. We here found that, although LyoVec or lipofectamine itself could induce the CD86 up-regulation to some extent, the addition of dsDNA further increased the CD86 expression when compared with the expression induced by transfectants alone (Fig. 2a,b). The dsDNA in combination with DOTAP also significantly augmented the CD86 expression on moDCs (Fig. 2b). Like dsDNA, poly(U) could increase CD86 expression in the presence of lipofectamine, LyoVec, or DOTAP. Notably, in combination with protamine, poly(U) but not dsDNA was a potent inducer of CD86 expression on moDCs (Fig. 2a,b).
Figure 2.
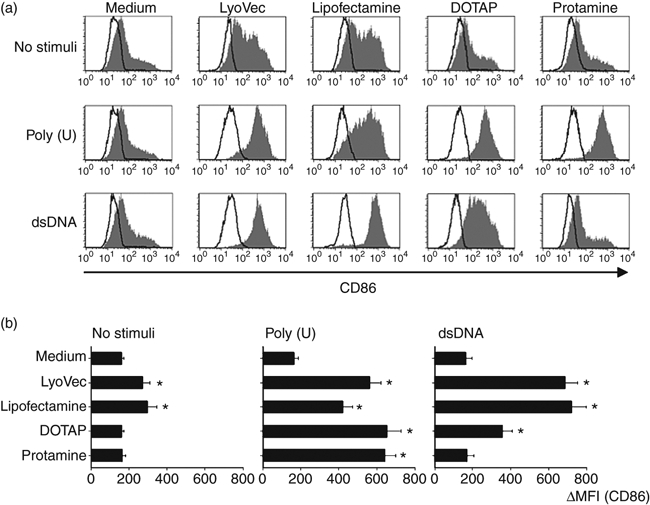
Analysis of CD86 expression on monocyte-derived dendritic cells (moDCs) after incorporation of double-stranded (ds) DNA complexed with transfectants. CD86 expression on moDCs stimulated with dsDNA/transfectants or poly(U)/transfectants for 24 hr were analysed by flow cytometry. (a) The staining profiles of CD86 and an isotype-matched control are indicated by shaded and open areas, respectively. (b) Mean fluorescence intensity (MFI), which is calculated by the subtraction of MFI with the control from that with anti-CD86, is shown. Data are shown as means ± SEM of three independent donors. Statistical significance compared with medium control was determined using paired Student’s t-test (*P< 0·01).
dsDNA and ssRNA induce different cytokine secretion from moDCs
Cytokine secretion is one of the hallmarks of the activation mode of DCs. As Figs 1 and 2 show, dsDNA complexed with lipotransfectants (lipofectamine, LyoVec or DOTAP) was well incorporated in moDCs to up-regulate CD86 expression, and we here found that moDCs produced IFN-α when stimulated with dsDNA in the presence of these lipotransfectants (lipofectamine, LyoVec and DOTAP), but not protamine (Fig. 3), indicating that dsDNA somehow stimulates the cascades of moDCs leading to the type I IFN synthesis. However, moDCs produced less IL-12 in response to the dsDNA in the presence of these lipotransfectants. In contrast to dsDNA, ssRNA poly(U) stimulated moDCs to preferentially secrete IL-12 but not IFN-α when cultured with all the transfectants, including protamine (Fig. 3). Similar to the response against poly(U), synthetic small compound R848, which also triggers TLR8 in human myeloid DCs,20,21 strongly induced IL-12 but not IFN-α, even in the absence of the transfectants (Fig. 3). This indicates that the activation of TLR8-mediated signalling pathways is not involved in IFN synthesis, unlike DNA-mediated activation. In our experimental setting, we used synthetic dsRNA poly(I:C) and found that poly(I:C) induced both IL-12 and IFN-α from moDCs even in the absence of the transfectants (Fig. 3), being consistent with the previous investigations.6,8 Poly(I:C) seems to be efficiently internalized by some transfectants, as indicated by further amplification of cytokine responses.
Figure 3.
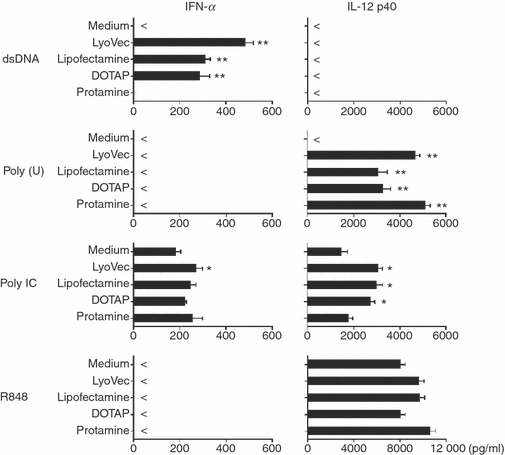
Interferon-α (IFN-α) and interleukin-12 (IL-12) production from monocyte-derived dendritic cells (moDCs) after incorporation of dsDNA. The moDCs were stimulated with dsDNA/transfectants or poly(U)/transfectants for 24 hr. The concentrations of IFN-α and IL-12 p40 in the culture supernatants were measured by ELISA. Data are shown as mean ± SEM of five independent donors. Statistical significance compared with medium control was determined using paired Student’s t-test (*P< 0·05 and **P< 0·01).
The contrary cytokine responsiveness after the culture of dsDNA versus ssRNA was determined in a dose-dependent fashion (except the 25 μg/ml dsDNA) when moDCs were stimulated with dsDNA complexed with LyoVec or poly(U) complexed with LyoVec (Fig. 4). We further examined the cytokine secretion by two-colour staining of intracellular anti-IFN-α and anti-IL-12 at the single-cell level. Eight hours after activation with dsDNA complexed with LyoVec, 5·2% of the DCs produced IFN-α but few cells produced IL-12 (Fig. 5). In response to poly(U) complexed with LyoVec, 11% of the DCs produced IL-12 but not IFN-α. When stimulated with both dsDNA and poly(U) at the same time, there was a small but notable fraction of cells producing both IFN-α and IL-12 (2·8%), in addition to single IFN-α-producing cells (3·6%) and IL-12-producing cells (9·4%) (Fig. 5), suggesting a possibility that the same DCs respond to both dsDNA and poly(U).
Figure 4.
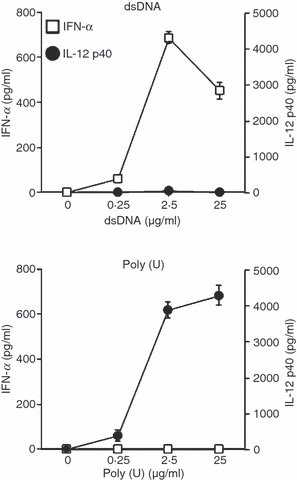
Contrary cytokine response in monocyte-derived dendritic cells (moDCs) in response to double-stranded (ds)DNA versus single-stranded (ss)RNA. The moDCs were stimulated with dsDNA/LyoVec® Complexes or ssPolyU/LyoVec® Complexes. After 24 hr of culture with different doses of dsDNA/LyoVec® Complexes and ssPolyU/LyoVec® Complexes, the concentrations of IFN-α and IL-12 p40 in the culture supernatants were measured by ELISA. Data are shown as mean ± SEM of three independent donors.
Figure 5.
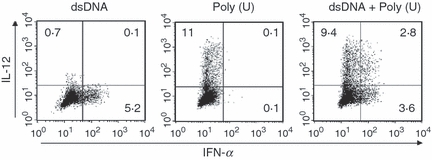
Intracellular cytokine expression in monocyte-derived dendritic cells (moDCs) in response to double-stranded (dsDNA) versus single-stranded (ss) RNA. The moDCs were stimulated with dsDNA/LyoVec® Complexes and/or ssPolyU/LyoVec® Complexes. After 8 hr of stimulation, intracellular cytokine (IFN-α and IL-12 p40+70) production in moDCs was analysed by flow cytometry. Percentages of the cytokine-producing DCs are indicated in each dot-blot profile. Similar results were observed in three independent donors and the results of a representative experiment are shown.
Purified blood myeloid DC subset also shows contrary cytokine responsiveness after stimulation with dsDNA and ssRNA
The moDCs are widely used in experimental and clinical studies of the functional basis of human DCs. In this study, we used moDCs for in vitro culture (with GM-CSF and IL-4) of magnetic bead-sorted monocytes of > 95%, but not 99%, purity. Therefore, the generated moDCs may show heterogeneity during culture or may contain a small fraction of other cell types such as pDCs. To exclude the possibility that different fractions of the generated moDCs respond to dsDNA or ssRNA, we performed an experiment using a purified blood myeloid BDCA1+ DC subset devoid of pDCs by multi-colour cell sorting (> 99% purity) (Fig. 6a) to examine their ability to produce cytokines. The isolated blood myeloid BDCA1+ DC subset as well as the generated moDCs preferentially produced IFN-α rather than IL-12 in response to dsDNA complexed with LyoVec whereas they produced IL-12 but not IFN-α in response to poly(U) complexed with LyoVec (Fig. 6b). This pattern is similar to the cytokine secretion of moDCs (Figs 3 and 4) and supports the functional attribute of differential cytokine responsiveness on human myeloid DCs in response to dsDNA and ssRNA.
Figure 6.
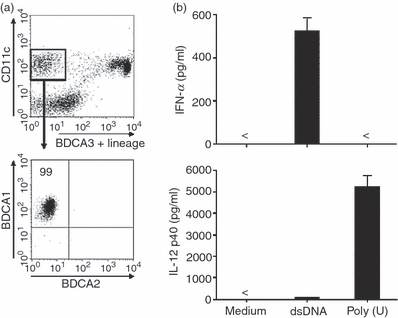
Cytokine response of purified blood myeloid BDCA1+ dendritic cell (DC) subset after stimulation with double-stranded (ds) DNA and single-stranded (ss) RNA. (a) A DC-enriched population in human peripheral blood mononuclear cells (after being negatively selected using magnetic beads conjugated with anti-CD3, anti-CD14, anti-CD19 and anti-CD56) were flow cytometrically analysed by the staining of anti-CD11c, anti-CD4 and anti-BDCA3+ anti-lineage marker (anti-CD3, -CD14, -CD15, -CD16, -CD19, -CD235a) to detect CD11c+ BDCA1+ myeloid DCs (CD11c+ BDCA1+ BDCA3− BDCA2− cells) (upper blot) to reach > 99% purity according to restaining with anti-BDCA1 or anti-BDCA2 (lower blot). The upper dot blot in this figure was shown after gating of the CD4-positive fraction. (b) The sorted blood myeloid BDCA1+ DC subset was stimulated with dsDNA/LyoVec® Complexes or ssPolyU/LyoVec® Complexes. After 24 hr of culture, the concentrations of interferon-α and interleukin-12 p40 in the culture supernatants were measured by ELISA. Data are shown as mean ± SEM of three independent donors.
dsDNA-mediated IFN-α production is independent of chloroquine
It is generally accepted that human myeloid cell subsets, including blood myeloid DCs, monocytes and moDCs lack TLR9.6,7 Paradoxically, there is an investigation showing that human moDCs weakly but definitely express TLR9, which allows induction of IFN-α by responding to CpG-DNA.22 Therefore, we next examined whether dsDNA-induced IFN-α in moDCs involves the TLR9-dependent pathway. Plasmacytoid-derived IFN-α production in response to CpG2216 was completely inhibited by chloroquine, which inhibits endosomal acidification and TLR activation (Fig. 7), consistent with several other reports.23,24 The moDC-derived IFN-α production in response to dsDNA complexed with LyoVec was only slightly but not significantly impaired by chloroquine. Meanwhile, poly(U)-mediated IL-12 production from moDCs was partially but significantly inhibited in a dose-dependent manner by chloroquine (Fig. 7). These findings suggest that moDC activation by ssRNA involves the TLR-mediated pathway, and that dsDNA induces IFN-α through a TLR-independent pathway in moDCs.
Figure 7.
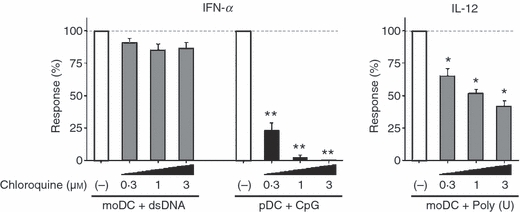
Effects of chloroquine on interferon-α (IFN-α) and interleukin-12 (IL-12) production from monocyte-derived moDCs in response to dsDNA or ssRNA. The moDCs were stimulated with dsDNA/LyoVec® Complexes or ssPolyU/LyoVec® Complexes for 24 hr in the absence (with vehicle) or presence of different doses of chloroquine (0–3 μm). The concentrations of IFN-α and IL-12 p40 in the culture supernatants were measured by ELISA and the percentage of the cytokine production compared with the vehicle control (absence of chloroquine) was calculated. Data are shown as mean ± SEM of three independent donors. Statistical significance compared with vehicle control was determined using paired Student’s t-test (*P< 0·05 and **P< 0·01).
Discussion
Environmental nucleic acids from invading viruses or bacteria commonly induce immune responses.25,26 Meanwhile, recognition of endogenous nucleic acids from cell death has been implicated in the pathogenesis of IFN-driven autoimmune diseases.27 Hence, nucleic acid-mediated innate immunity has two sides; protection against the invasion of pathogenic microbes and induction of undesirable immune responses. Therefore, it seems important to elucidate the activation process after the incorporation of nucleic acids into DCs that is functioning as a trigger of innate immunity through cytokine secretion.
Among nucleic acids, DNA is a potent stimulator of type I IFN production, which not only plays a central role in anti-microbial infection but also triggers and amplifies a pathogenic autoimmune process in systemic lupus erythematosus28 or psoriasis.29 The pDCs, through expressing TLR9, are considered to be major producers of type I IFNs in response to DNA such as microbial DNA or endogenous DNA,11 and to be key effectors of DNA-mediated immunity. However, less is known about the DNA-mediated molecular events in myeloid DCs that induce the type I IFN response. There are some reports showing that dsDNA is a potent inducer of type I IFNs in other cell types, such as glomerular endothelial cells, fibroblasts and macrophages,15,17,30 but no direct evidence in experiments using human myeloid DCs. The present study identifies dsDNA as a potent trigger of IFN response in human myeloid DCs and suggests that they have a role in the DNA-mediated activation of protective or pathogenic innate immunity. In support of our data, the dsDNA virus herpes simplex virus type 1 stimulates moDCs to express type I IFNs.31 Although human myeloid DCs have the capability to produce IL-12 in response to the ligands for TLRs that are expressed in myeloid DCs (TLR2, -3, -4, -5 and -8),8 our finding that dsDNA could preferentially induce IFN-α rather than IL-12 from myeloid DCs suggests a unique functional attribute as danger signal in the context of immunopathology. Anti-tumour activity of IFN-α has already been clarified.32,33 In this context, myeloid DCs residing in the inflammatory area of tumour-bearing conditions could have an anti-tumour effect by producing IFN-α if self-DNA from damaged cells or tumours is well incorporated into the DCs. In addition, our results suggest the feasibility of moDCs, usually used as a cell source of ‘DC therapy’, also being effective in the direct attack against tumours through IFN-α production. Consistent with our data showing that protamine favours ssRNA rather than dsDNA to function as a transfectant, it has been reported that protamine-condensed mRNA is protected from RNase-mediated degradation and this protamine-stabilized mRNA can directly activate DCs through the TLR-MyD88-dependent pathway.34 This indicates that the stabilized form of nucleic acid can be used for effective vaccination. Hence, exploitation of efficient DNA-based vaccines requires a well-matched transfectant for moDCs. Our present data would provide useful insights for the development of efficient DNA-based immune therapies.
Our observations showing both intracellular distribution of dsDNA without co-localization of endosomal marker LAMP1 and chloroquine-independent IFN-α production induced by dsDNA indicate that endosomal TLRs do not mediate moDC recognition of dsDNA upon liposomal transfection. As such, the dsDNA virus adenovirus stimulates type I IFN production through a TLR-independent sensing mechanism35 and dsDNA is thought to be recognized by cytosolic DNA sensors.15,16 As a result of recent intense research, LRRFIP1 has been proposed as a potent cytosolic sensor of dsDNA that enhances the induction of type I IFN genes via a β-catenin-dependent pathway,17 but little is known about whether human myeloid DCs functionally express this sensor to provoke an IFN response. This is an important question to be clarified in future studies.
In contrast to the DNA-sensing machinery, the RNA-sensing system has been well identified. Plasmacytoid DCs possess TLR7 recognizing ssRNA,19,36 whereas human myeloid DCs have TLR8, a homologue of TLR7.21 Both TLR7 and TLR8 in each human DC subset contribute to the recognition of self-RNA in the auto-inflammatory response.37 However, TLR7 in pDCs and TLR8 in myeloid DCs have specialized functions in producing type 1 IFNs versus IL-12, respectively. This is elucidated by a study showing that pDCs preferentially produced IFN-α, whereas myeloid DCs produced IL-12 in response to imidazoquinoline R848, which triggers TLR7 in pDCs and TLR8 in myeloid DCs.8,19,20 Similarly, it has been observed that ssRNA induces human moDCs to produce tumour necrosis factor-α and IL-6 without IFN-α but induces pDCs to produce IFN-α.37 Myeloid DCs have also endosomal TLR3, which recognizes long dsRNA, to induce type I IFNs.9 In addition to these TLRs, cytosolic RNA helicases retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs) act as dsRNA sensors and are expressed in a wide variety of cells. It has recently been demonstrated that, in response to RNA, neither RIG-I nor melanoma differentiation-associated gene 5 (MDA5) played a significant role in pDCs in producing IFN-α, whereas these RLRs mainly functioned on the IFN-α production in myeloid DCs or monocytes.38,39 In support of these findings, we here showed that, although ssRNA poly(U) as well as R848, which are ligands for TLR8, was incapable of inducing IFN-α production in myeloid DCs, dsRNA poly(I:C), a ligand for TLR3 and MDA5, led to IFN-α production.
Considering our results showing the differential responsiveness of IFN-α and IL-12 production after the stimulation with dsDNA and ssRNA, it appears that myeloid DCs function as a traffic control that turns the immune system toward the innate immunity through the production of IFN-α or toward the acquired immunity via IL-12-driven T helper type 1 differentiation, depending on the type of nucleic acids. This may contribute to the regulatory mechanism to provoke an optimal innate and acquired immune response in the physiological environment.
Collectively, we have clarified that dsDNA complexed with lipotransfectants allows the activation of human myeloid DCs in a TLR-independent way, leading to the preferential production of IFN-α. This finding indicates that myeloid DCs might organize a supportive or fail-safe system against the invasion of pathogenic microbes through the DNA-mediated activation of innate immune responses and highlights additional cellular targets of IFN-related diseases.
Acknowledgments
The authors thank Ms Mihoko Inoue and Ms Mitsuko Ueda for manuscript preparation and Dr Tsuneyasu Kaisho for scientific advice. This work was supported by Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; Grant Number: 21591289, Grant-in-Aid from The Japan Medical Association, and Takeda Science Foundation.
Glossary
Abbreviations:
- DC
dendritic cell
- dsDNA
double-stranded DNA
- IFN
interferon
- IL
interleukin
- moDC
monocyte-derived dendritic cell
- pDC
plasmacytoid dendritic cell
- ssRNA
single-stranded RNA
- TLR
toll-like receptor
Disclosures
The authors have no conflicting financial interests.
References
- 1.Bogdan C. The function of type I interferons in antimicrobial immunity. Curr Opin Immunol. 2000;12:419–24. doi: 10.1016/s0952-7915(00)00111-4. [DOI] [PubMed] [Google Scholar]
- 2.Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J Exp Med. 2000;192:219–26. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–56. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 4.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 5.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 6.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–9. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–93. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 8.Ito T, Kanzler H, Duramad O, Cao W, Liu YJ. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood. 2006;15:2423–31. doi: 10.1182/blood-2005-07-2709. [DOI] [PubMed] [Google Scholar]
- 9.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 10.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 11.Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–9. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 12.Finke D, Eloranta ML, Rönnblom L. Endogenous type I interferon inducers in autoimmune diseases. Autoimmunity. 2009;42:349–52. doi: 10.1080/08916930902831829. [DOI] [PubMed] [Google Scholar]
- 13.Hochrein H, Schlatter B, O’Keeffe M, et al. Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and -independent pathways. Proc Natl Acad Sci USA. 2004;101:11416–21. doi: 10.1073/pnas.0403555101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasumussen SB, Sørensen LN, Malmgaard L, Ank N, Baines JD, Chen ZJ, Paludan SR. Type I interferon production during herpes simplex virus infection is controlled by cell-type-specific viral recognition through Toll-like receptor 9, the mitochondrial antiviral signaling protein pathway, and novel recognition systems. J Virol. 2007;81:13315–24. doi: 10.1128/JVI.01167-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takaoka A, Wang Z, Choi MK, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:423–4. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 16.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;26:514–8. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang P, An H, Liu X, Wen M, Zheng Y, Rui Y, Cao X. The cytosolic nucleic acid sensor LRRFIP1 mediates the production of type I interferon via a beta-catenin-dependent pathway. Nat Immunol. 2010;11:487–94. doi: 10.1038/ni.1876. [DOI] [PubMed] [Google Scholar]
- 18.Felgner JH, Kumar R, Sridhar CN, et al. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J Biol Chem. 1994;269:2550–61. [PubMed] [Google Scholar]
- 19.Heil F, Hemmi H, Hochrein H, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 20.Ito T, Amakawa R, Kaisho T, et al. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J Exp Med. 2002;195:1507–12. doi: 10.1084/jem.20020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, Lipford G, Bauer S. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 22.Hoene V, Peiser M, Wanner R. Human monocyte-derived dendritic cells express TLR9 and react directly to the CpG-A oligonucleotide D19. J Leukoc Biol. 2006;80:1328–36. doi: 10.1189/jlb.0106011. [DOI] [PubMed] [Google Scholar]
- 23.Osawa Y, Iho S, Takauji R, et al. Collaborative action of NF-kappaB and p38 MAPK is involved in CpG DNA-induced IFN-alpha and chemokine production in human plasmacytoid dendritic cells. J Immunol. 2006;177:4841–52. doi: 10.4049/jimmunol.177.7.4841. [DOI] [PubMed] [Google Scholar]
- 24.Hornung V, Schlender J, Guenthner-Biller M, Rothenfusser S, Endres S, Conzelmann KK, Hartmann G. Replication-dependent potent IFN-alpha induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J Immunol. 2004;173:5935–43. doi: 10.4049/jimmunol.173.10.5935. [DOI] [PubMed] [Google Scholar]
- 25.Ishii KJ, Akira S. Innate immune recognition of, and regulation by, DNA. Trends Immunol. 2006;27:523–32. doi: 10.1016/j.it.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Koyama S, Ishii KJ, Coban C, Akira S. Innate immune responses to viral infection. Cytokine. 2008;43:336–41. doi: 10.1016/j.cyto.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 28.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;16:1540–3. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 29.Nestle FO, Conrad C, Tun-Kyi A, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202:135–43. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hägele H, Allam R, Pawar RD, Reichel CA, Krombach F, Anders HJ. Double-stranded DNA activates glomerular endothelial cells and enhances albumin permeability via a toll-like receptor-independent cytosolic DNA recognition pathway. Am J Pathol. 2009;175:1896–904. doi: 10.2353/ajpath.2009.090182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Izaguirre A, Barnes BJ, Amrute S, et al. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J Leukoc Biol. 2003;74:1125–38. doi: 10.1189/jlb.0603255. [DOI] [PubMed] [Google Scholar]
- 32.Miki A, Yano Y, Kato H, Seo Y, Kuriyama M, Azuma T, Hayashi Y. Anti-tumor effect of pegylated interferon in the rat hepatocarcinogenesis model. Int J Oncol. 2008;32:603–8. [PubMed] [Google Scholar]
- 33.Moriya F, Ogasawara S, Basaki Y, et al. Growth inhibitory effects of pegylated IFN-alpha2b and 5-fluorouracil in combination on renal cell carcinoma cell lines in vitro and in vivo. Int J Oncol. 2008;33:647–55. doi: 10.1016/j.ijrobp.2008.06.320. [DOI] [PubMed] [Google Scholar]
- 34.Scheel B, Teufel R, Probst J, et al. Toll-like receptor-dependent activation of several human blood cell types by protamine-condensed mRNA. Eur J Immunol. 2005;35:1557–66. doi: 10.1002/eji.200425656. [DOI] [PubMed] [Google Scholar]
- 35.Nociari M, Ocheretina O, Schoggins JW, Falck-Pederson E. Sensing infection by adenovirus: toll-like receptor-independent viral DNA recognition signals activation of the interferon regulatory factor 3 master regulator. J Virol. 2007;81:4145–57. doi: 10.1128/JVI.02685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hornung V, Guenthner-Biller M, Bourquin C, et al. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005;11:263–70. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- 37.Ganguly D, Chamilos G, Lande R, et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med. 2009;206:1983–94. doi: 10.1084/jem.20090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ablasser A, Poeck H, Anz D, et al. Selection of molecular structure and delivery of RNA oligonucleotides to activate TLR7 versus TLR8 and to induce high amounts of IL-12p70 in primary human monocytes. J Immunol. 2009;182:6824–33. doi: 10.4049/jimmunol.0803001. [DOI] [PubMed] [Google Scholar]
- 39.Kato H, Sato S, Yoneyama M, et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]


