Abstract
The integrin αE(CD103)β7 (αEβ7) is expressed by intraepithelial lymphocytes, dendritic cells and regulatory T cells. It plays an important role in the mucosal immune system by retaining lymphocytes within the epithelium and is involved in graft rejection, immunity against tumours and the generation of gut-homing effector cells. In gut and breast, the ligand for αEβ7 is E-cadherin but in human oral mucosa and skin, there is evidence that lymphocytes use an alternative, unknown, ligand. In the present study, the I domain of the human αE subunit, which contains the E-cadherin-binding site, was locked in a highly active, ‘open’ and an inactive, ‘closed’ conformation by the introduction of disulphide bonds and these domains were expressed as IgG Fc fusion proteins. αE fusion proteins recognize E-cadherin, the only known ligand for αEβ7. This interaction was inhibited by an antibody that blocks the αE-binding site on E-cadherin and by the omission of Mn2+, which is essential for integrin function in vitro. The locked ‘open’ conformation of αE adhered to human oral and skin keratinocytes, including the E-cadherin-negative H376 cell line, and this was not inhibited by blocking antibody against the αEβ7-binding site on E-cadherin, providing further evidence for the existence of an alternative ligand for αEβ7 in skin and oral mucosa. The interaction with E-cadherin and the alternative ligand was Mn2+ dependent and mediated by the metal ion-dependent coordination site (MIDAS) of the locked ‘open’αE I domain, independently of the β7 subunit.
Keywords: adhesion, integrin, keratinocytes, mucosal immunology, T cells
Introduction
Integrins are transmembrane adhesion molecules that mediate the attachment of cells to adjacent cells or to the extracellular matrix. They comprise an α and a β subunit: the α subunit is involved in ligand binding and the β subunit is involved in the regulation of ligand binding.1 The integrin αE(CD103)β7 (αEβ7) was first identified on human intraepithelial lymphocytes (IEL) with the monoclonal antibody (mAb) HML-12 and on mouse IEL with the mAb M290.3,4 It is expressed by the majority of T cells present in the gut and lung epithelia and by 40% of the T cells in the lamina propria. αEβ7 is also expressed by IEL at other mucosal surfaces such as the breast, uterus,2,4 oral mucosa5 and skin,6 but only a small proportion of T cells from other compartments are positive for αEβ7.4 In addition to IEL, αEβ7 is also expressed by a subset of CD4+ CD25+ T-regulatory cells, which express the transcription factor FoxP3,7–9 and by dendritic cells. These dendritic cells are important for T-cell activation10,11 and induction of FoxP3 expression by T-regulatory cells.12αEβ7 is clearly a unique integrin that may have many important roles to play in the immune response.
The expression of αEβ7 is up-regulated by transforming growth factor β1 (TGF-β1),4 which is produced by many cell types, including epithelial cells,13 and consequently the up-regulation of αE expression occurs when IEL move into or near the epithelial compartment. αEβ7 has an important role in the gut to retain IEL within the mucosal epithelia through interaction with epithelial cells.14,15αEβ7 is also involved in the retention of IEL within the oral mucosa16 and the epidermis of the skin.17 In addition to cell adhesion, αEβ7 has recently been shown to influence shape and motility of dendritic epidermal T cells in mice in a ligand-dependent manner.18 Therefore it appears that, in addition to the gut, αEβ7 expression in the skin is important not only for retention of lymphocytes but also for their movement and migration within this epithelial compartment.
To date, only one ligand has been identified for αEβ7, namely the calcium-dependent cell-adhesion molecule E-cadherin, expressed exclusively by epithelial cells.19 However, in addition to E-cadherin, there is strong evidence to suggest that another ligand for αEβ7 exists on oral and skin keratinocytes. A previous study has shown that TGF-β1-activated peripheral blood lymphocytes bind to a human E-cadherin-negative oral keratinocyte cell line (H376) via αEβ7 to the same extent as they bind to an E-cadherin-positive cell line (H357). Lymphocyte adhesion to both cell lines was inhibited by a mAb against-αEβ7 but not by an antibody that blocks the αEβ7-binding site on E-cadherin.16 This strongly suggests the presence of a novel alternative ligand present on both the E-cadherin-positive and -negative cell lines.
The αE subunit contains an inserted (I) domain,20 which has previously been shown to be critical for integrin–ligand interactions.21,22 Metal cation coordination is also required for integrin function. Crystallization of the I domain from the integrin subunit, αM, in the presence of Mg2+23 and with Mn2+24 showed that changes in metal coordination are linked to large conformational changes in the integrin protein, which are required for activation. This suggests that integrins are able to exist in two alternative conformational states, namely a high-affinity active state and a low-affinity inactive state, which are dependent on metal cation coordination.24 The metal cation-binding site is located on the surface of the I domain where it is coordinated by six separate groups known as the metal ion-dependent coordination site (MIDAS) motif. It is the MIDAS motif that is directly involved in ligand binding by αE.25
It is possible to lock the MIDAS of integrin I domains in a high- or a low-affinity conformation by introducing amino acid mutations. This has given greater insight into how ligand binding is regulated by conformational change within the I domain. Locked ‘open’ or high-affinity conformations and locked ‘closed’ or low-affinity conformations of the I domains of αM,26,27αL28 and mouse αE29 have been developed. The ‘open’ and ‘closed’ conformations of the mouse αE I domain were modelled on the αM I domain, which shares 38% amino acid identity with αE.25 Locking the I domain of αE ‘open’ increases its sensitivity to Mn2+, the cation required for αE–ligand interactions, and the isolated ‘open’ I domain is inactive without it. The ‘open’αEβ7 heterodimer is also more active than the wild-type αEβ7 heterodimer in the presence of Mn2+, and the locked ‘closed’αE I domain is less effective than both the ‘open’ and wild-type αEβ7 heterodimers in the presence of Mn2+ or Mg2+.29
In the present study, locked ‘open’ and ‘closed’ forms of the human αE I domain were developed, expressed as Fc fusion proteins and used to investigate the interaction between αEβ7 and the ligand on oral and skin keratinocytes. Our results show that binding of the human αE I domain to oral and skin keratinocytes is independent of E-cadherin and provides further evidence for the existence of a second ligand for αEβ7.
Materials and methods
MCF-7 is a breast adenocarcinoma cell line, and H357 and H376 cell lines are derived from oral squamous cell carcinoma and were a gift from Professor S. S. Prime, Bristol Dental School, UK. UP is an immortal human skin keratinocyte cell.30
Primary normal oral keratinocytes (NOK) were obtained from clinically healthy tissue removed during minor oral surgery procedures with ethical committee approval (Sheffield Research Ethics Committee, reference number: 04/Q2305/78) H376, H357, UP and NOK were maintained in keratinocyte growth medium.31
The following antibodies were used: E4.6 mouse anti-human E-cadherin (a gift from Jonathan Higgins, Harvard Medical School, USA), which blocks the αE-binding site on E-cadherin19 and HECD-1 mouse anti-human E-cadherin [Calbiochem (Merck), Beeston, UK], which blocks homotypic binding by E-cadherin and does not block αE binding.32
Production of mutated human αE I domain fusion proteins
The ‘open’ and ‘closed’ conformations of the mouse I domain was originally modelled on the crystal structure of the I domain from αMβ2.24 Amino acids F341 and A348 and V343 and A348C were mutated to cysteine to lock the mouse I domain ‘open’ and ‘closed’ respectively.29 The mouse and human I domains show over 70% homology and therefore amino acids in the human I domain that correspond to those selected in the mouse were chosen for mutation. The I domain DNA sequence of the α E subunit was amplified using the polymerase chain reaction (PCR) and cloned into the pCR®-Blunt-TOPO® Vector (Invitrogen, Paisley, UK) in order to create a ‘sticky-ended’ insert. The I domain was then inserted into a pIg signal vector, to enable it to be expressed as a human IgG Fc fusion protein. The Quikchange mutagenesis kit [Stratagene (Agilent), Stockport, UK] was used to create the amino acid mutations required to lock the I domain ‘open’ or ‘closed’. Mutated and wild-type pIg signal vectors were transfected into COS 7 cells using DEAE dextran. Cell supernatants were harvested after 7 days and the amount of fusion protein present in the supernatants was determined by enzyme-linked immunosorbent assay (ELISA) using a goat anti-human Fc (Sigma, Poole, UK) for capture and a biotinylated goat anti-human Fc [Jackson (Stratech), Newmarket, UK] for detection. Between 1·5 and 0·8 μg/ml of fusion protein was produced per transfection.
Flow cytometry
Expression of E-cadherin was determined by flow cytometry. Cells were stained with E4.6 and HECD-1, then with anti-mouse fluorescein isothiocyanate (FITC)-conjugated secondary antibody (Sigma). After these incubations with antibody, cells were washed twice, resuspended in 500 μl of phosphate-buffered saline (PBS) and analysed using a FACS calibur (Becton Dickinson Bioscience, Oxford, UK) and FlowJo computer software (Tree Star, Ashland, OR).
Reverse transcription PCR
Reverse transcription PCR was used to determine the expression of E-cadherin messenger RNA (mRNA) in H357 and H376 cell lines. The primers used were: forward, TCAGCGTGTGTGACTGTGAA; and reverse, CTCTTCTCCGCCTCCTTCTT. Both primers are intron spanning. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a control. All primers were purchased from Sigma UK.
Cell adhesion assays
A 96-well plate was coated, overnight at 4°, with 100 μl of goat anti-human Fc (Jackson) in PBS. The plate was washed with PBS and then blocked, for 90 min at room temperature, with PBS containing 1% bovine serum albumin (BSA) (Sigma), washed with 100 μl of Hanks’ balanced salt solution (HBSS) (Invitrogen) containing 1 mm Mn2+, then coated with 100 μl of fusion proteins (0·8 μg/ml diluted in HBSS) and incubated for 60 min at 37°. PBS containing 1% BSA was used to determine background cell adhesion in the absence of fusion protein. Epithelial cells were removed from flasks using cell-dissociation buffer (Sigma), and single-cell suspensions, of 40 000 cells/well, were added to the plate. In some experiments, fusion proteins were pretreated with 10 mm dithiothreitol (DTT) for 60 min at room temperature in order to reduce disulphide bonds, before the addition of cells. A standard curve was set up using doubling dilutions, from 40 000 cells down to 300. Adhered cells were supplied with the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] substrate (Promega, Southampton, UK) and the absorbence of the assay plate and of the standard curve was measured at 490 nm.
Statistics
Statistical analysis on data gained from cell adhesion assays was conducted using prism software (GraphPad, Software Inc. La Jolla, CA, USA). All results are expressed as the mean ± standard error of the mean. Statistical tests were performed using one way analysis of variance (anova) and Bonferroni post tests. A value of P< 0·05 was considered statistically significant.
Results
Adhesion of MCF-7 cells to human αE I domain fusion proteins is mediated by E-cadherin
Cell adhesion assays were carried out using the breast epithelial cell line, MCF-7, which expresses the αE ligand E-cadherin (Fig. 1a) in order to determine whether mutated αE I domains, expressed as an Fc fusion protein, retain the specificity of the native αEβ7 molecule. Significantly more MCF-7 cells adhered to the ‘open’ than to the ‘wild-type’ and ‘closed’ fusion proteins, which was not significantly different from background adhesion in the absence of fusion protein. Serial dilution of the ‘open’ fusion protein was associated with a decrease in the percentage of MCF-7 cell binding, whereas serial dilution of the wild-type and ‘closed’ fusion proteins had no effect on cell binding (Fig. 2a). The interaction of MCF-7 cells with the ‘open’ fusion protein was dependent on Mn2+ and could be significantly and specifically inhibited by the addition of the mAb E4.6 against the αEβ7-binding site on E-cadherin but not by the mAb HECD-1 against the homotypic-binding site (Fig. 2b,c). Cell adhesion to the wild-type and ‘closed’ fusion proteins was unaffected by either mAb (data not shown).
Figure 1.
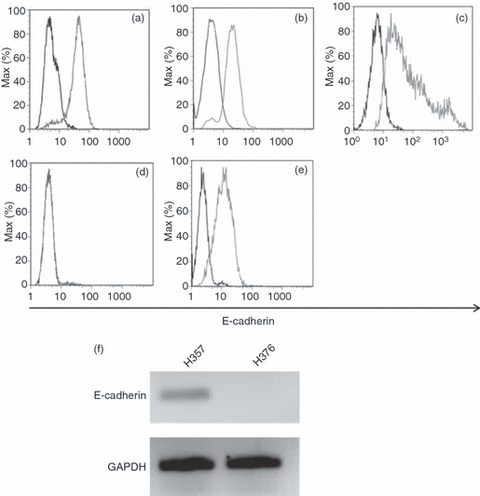
E-cadherin expression by epithelial cells. Cell-surface expression of E-cadherin, as determined by flow cytometry with mAb E4.6 (HECD-1 staining not shown), on (a) MCF-7, (b) H357, (c) normal oral keratinocytes (NOK), (d) H376 and (e) UP cells. In each case the dark histogram shows control cells and the light histogram shows cells stained for E-cadherin. (f) Expression of E-cadherin messenger RNA by H357 and H376 cells. Representative results n = 3. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Figure 2.
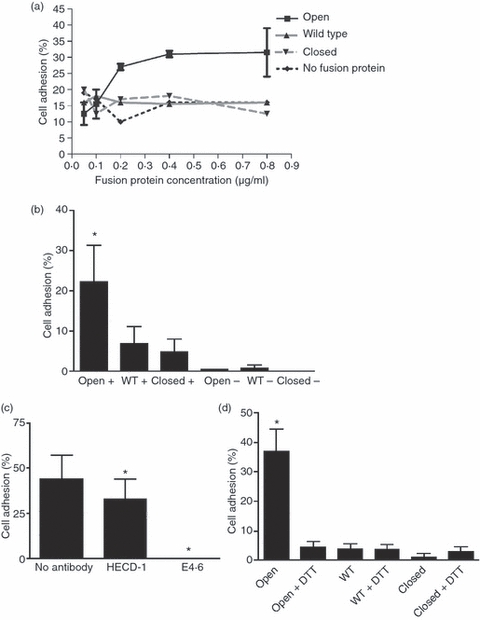
Adhesion of MCF-7 cells to human αE I domain fusion proteins. (a) The percentage binding of MCF-7 cells to serial dilutions of fusion protein in the presence of Mn2+. Bovine serum albumin (BSA) (1%) was used as a negative control. (b) The percentage of MCF-7 cell binding to ‘open’, wild-type and ‘closed’ fusion proteins in the presence (+) and absence (−) of Mn2+. Background adhesion to BSA was subtracted. Combined results from three separate experiments are shown, and error bars represent the standard error of the mean (SEM); *, P < 0·05. (c) The effect of mAbs (HECD-1 and E4.6) to E-cadherin on MCF-7 cell binding to the ‘open’ fusion protein in the presence of Mn2+. Background adhesion to BSA was subtracted. Combined results from three separate experiments are shown, and error bars represent the SEM; *, P < 0·05. (d) Percentage of MCF-7 cell binding to ‘open’, wild-type and ‘closed’ fusion proteins in the presence and absence of dithiothreitol (DTT). Background adhesion to BSA was subtracted. Combined results from three separate experiments are shown, and error bars represent the SEM; *, P < 0·001. WT, wild type.
The increase in activity of the ‘open’αE I domain is caused by the additional disulphide bonds introduced by site-directed mutagenesis
Previous work has shown that reduction of the disulphide bonds introduced to lock ‘open’ the I domains of αM and αL also reduced the ligand-binding capacity to the level of the wild-type I domain.26,33 To confirm whether this is also true for the human ‘open’αE I domain, fusion proteins were treated with the reducing agent DTT before addition to the assay plates and adhesion of MCF-7 cells was determined. Treatment with DTT dramatically reduced cell adhesion to the ‘open’ fusion protein to a level comparable to that observed with wild-type and ‘closed’ fusion proteins (Fig. 2d).
Human αE I domain fusion proteins adhere to oral keratinocytes in an E-cadherin-independent manner
Expression of E-cadherin by the oral keratinocyte cell line H357 and by NOK was confirmed (Fig. 1b,c, respectively) as was loss of expression of E-cadherin protein and mRNA by H376 cells (Fig. 1d,f). Cell adhesion of H357 cells and NOK to the human αE I domain fusion proteins was determined. Significantly more H357 and NOK adhered to the ‘open’ fusion protein than to the ‘closed’ or wild-type proteins and this interaction was dependent on Mn2+ (Fig. 3a). In addition, binding of NOK to the ‘closed’ and wild-type fusion proteins was also inhibited by removal of Mn2+. In contrast to the results with the MCF-7 cell line, addition of the blocking mAb, E4.6, did not have any significant effect on cell adhesion of H357 or NOK to the ‘open’ fusion protein (Fig. 3b). In order to determine whether disruption of the introduced disulphide bonds into the I domain would affect cell adhesion, H357 cells were treated with DTT. This resulted in a significant decrease in cell adhesion to the ‘open’ fusion protein but had no effect on binding to the wild-type or the ‘closed’ fusion proteins (Fig. 3c).
Figure 3.
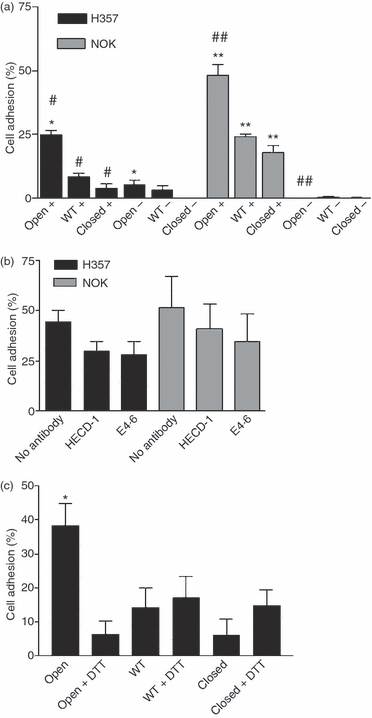
Adhesion of the oral keratinocyte cell line H357 and primary normal oral keratinocytes (NOK) to human αE I domain fusion proteins. (a) The percentage of H357 and NOK cells binding to ‘open’, wild-type and ‘closed’ fusion proteins in the presence (+) and absence (−) of Mn2+. Background adhesion to bovine serum albumin (BSA) was subtracted. *, P < 0·001; #, P < 0·001; **, P < 0·001; and ##, P < 0·001. (b) The effect of mAbs (HECD-1 and E4.6) to E-cadherin on the binding of H357 and NOK cells to ‘open’ fusion protein in the presence of Mn2+. Background adhesion to BSA was subtracted. (c) The percentage of H357 cells bound to ‘open’, wild-type and ‘closed’ fusion proteins in the presence and absence of dithiothreitol (DTT). Background adhesion to BSA was subtracted. All results were combined from three separate experiments. Error bars represent the standard error of the mean (SEM). WT, wild type.
These results suggest that binding of αEβ7 to oral keratinocytes is independent of E-cadherin and that there may be an alternative ligand for αEβ7. In order to investigate this, further adhesion assays were conducted with the E-cadherin-negative oral keratinocyte cell line, H376. H376 cells adhered to the ‘open’ fusion protein, and this interaction was Mn2+ dependent (Fig. 4a). In addition, H376 cells also adhered to the wild-type fusion protein to almost the same extent as the ‘open’ fusion protein and this binding was also Mn2+ dependent. (Fig. 4a). Adhesion of H376 cells to the ‘open’ fusion protein was unaffected by the addition of either the blocking or non-blocking anti-E-cadherin mAbs (Fig. 4b). Treatment of H376 cells with DTT caused a significant reduction in cell adhesion to the ‘open’ fusion protein but did not affect cell binding to the wild-type and ‘closed’ fusion proteins (Fig. 4c).
Figure 4.
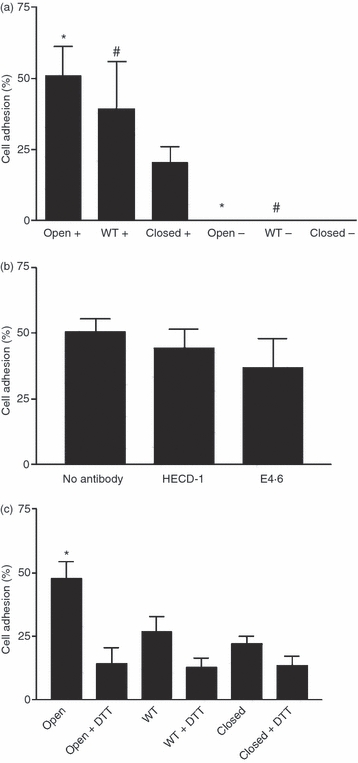
Adhesion of the oral keratinocyte cell line H376 to human αE I domain fusion proteins. (a) The percentage of H376 cells binding to ‘open’, wild-type and ‘closed’ fusion proteins in the presence (+) and absence (−) of Mn2+. Background adhesion to bovine serum albumin (BSA) was subtracted. *, P < 0·01; #, P < 0·05. (b) The effect of mAbs (HECD-1 and E4.6) to E-cadherin on the binding of H376 cells to the ‘open’ fusion protein in the presence of Mn2+. Background adhesion to BSA was subtracted. (c) The percentage of H376 cells binding to ‘open’, wild-type and ‘closed’ fusion proteins in the presence and absence of dithiothreitol (DTT). *, P < 0·01. Background adhesion to BSA was subtracted. All results were combined from three separate experiments. Error bars represent the standard error of the mean (SEM). WT, wild type.
Human αE I domain fusion proteins adhere to skin keratinocytes in an E-cadherin-independent manner
In order to determine whether skin keratinocytes also bind to the fusion proteins in an E-cadherin-independent manner, adhesion of the E-cadherin-positive skin keratinocyte cell line UP (Fig. 1e) was determined. This cell line adhered to the ‘open’ fusion protein in a Mn2+-dependent manner, but showed very little binding to either the wild-type or ‘closed’ fusion proteins (Fig. 5a). Cell adhesion was unaffected by blocking with the mAb E4.6 to E-cadherin (Fig. 5b). These results therefore suggest that in addition to oral keratinocytes, the ‘open’αE I domain may interact with skin keratinocytes in an E-cadherin-independent manner.
Figure 5.
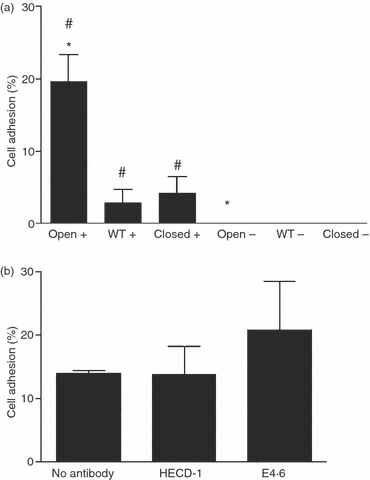
Adhesion of the skin keratinocyte cell line UP to human αE I domain fusion proteins. (a) The percentage of UP cell binding to ‘open’, wild-type and ‘closed’ fusion proteins in the presence (+) and absence (−) of Mn2+. Background adhesion to bovine serum albumin (BSA) was subtracted. *, P < 0·001; #, P < 0·01. (b) The effect of antibodies to E-cadherin (HECD-1 and E4.6) on ‘open’ fusion protein binding to UP cells in the presence of Mn2+. Background adhesion to BSA was subtracted. All results are combined from three separate experiments. Error bars represent the standard error of the mean (SEM). WT, wild type.
Discussion
Mutated integrin I domains provide a useful tool in the study of integrin–ligand interactions as they enable ligand binding to different conformational states to be studied. In this study we have shown that specific mutations of the human αE I domain result in a conformationally highly active ‘open’ form which, when expressed as an Fc fusion protein, binds to E-cadherin-expressing breast epithelial cells in the same way as wild-type αEβ7. Furthermore, we have shown that this fusion protein retains the wild-type binding characteristics for human oral and skin keratinocytes and binds in an E-cadherin-independent manner. These results provide additional evidence for a novel second ligand for αEβ7 in human oral mucosa and skin.
The human I domain fusion proteins were produced by mutating the same amino acids as those used to create the locked ‘open’ and ‘closed’ forms of mouse αE I domain29 and were tested for their binding capacity to the E-cadherin-positive human breast epithelial cell line MCF-7. Our results showed that the binding characteristics of the human αE fusion proteins are similar to those shown previously for the mouse: only the ‘open’ (and not the wild-type or ‘closed’) fusion proteins bind MCF-7 cells. The increase in binding observed with the ‘open’ fusion proteins can be accounted for by the introduction of disulphide bonds as it is lost by pretreatment with DTT. Reduction of disulphide bonds with DTT appears to shift the ‘open’ I domain of αE to the ‘closed’ conformation, which is also favoured by the wild-type I domain because of its lower energy state.
It is known that integrin function is dependent on the coordination of metal cations in vitro,24 and changes in extracellular Mn2+ can regulate the affinity of αEβ7 for E-cadherin.34 Our results also show that binding of the ‘open’ fusion protein is Mn2+ dependent, which suggests that the human locked ‘open’ I domain appears to retain flexibility in the MIDAS despite the introduction of disulphide bonds. This was also observed previously with the mouse ‘open’αE I domain,34 whereas the ‘open’ I domain of αL is insensitive to metal-ion activation as a result of additional rigidity in its MIDAS.28 It has been shown previously that MCF-7 cells use E-cadherin to bind both to αEβ7+ lymphocytes as well as to αEβ7 expressed as an Fc fusion protein. These interactions may be blocked specifically by a mAb (E4.6) against the putative αEβ7-binding site on E-cadherin, but not by antibodies against the homotypic binding site.19,35 In the present study, MCF-7 cell adhesion was abolished in the presence of mAb E4.6 but unaffected in the presence of the non-blocking mAb HECD-1. This suggests that the ‘open’ fusion protein specifically binds MCF-7 cells through interacting with E-cadherin. The activity of the locked ‘open’ I domain appears to be comparable to that of the intact αEβ7 molecule on both lymphocytes and when expressed as an Fc fusion protein. Thus, it seems that MCF-7 cell adhesion can occur in the absence of the β7 subunit. This is supported by the findings of Corps et al.,29 who showed previously that antibodies which block the I domain of the β7 subunit inhibit the function of the wild-type αI domain but not the locked ‘open’ form.
The proportion of cell binding to the ‘open’ fusion protein was variable and ranged from 20 to 50%. This level of adhesion is relatively low compared with adhesion assays which utilize cell-to-cell binding.16 One possible explanation is that homotypic adhesion of E-cadherin might leave fewer cellular ligands free to bind the ‘open’ fusion protein. However, single-cell suspensions were used and the homotypic- and αEβ7+-binding sites on E-cadherin are separate. Furthermore, if homotypic binding was operative in our assay and affected the binding of E-cadherin to the ‘open’ fusion protein, it might be expected that incubation with HECD-1, which blocks homotypic adhesion, would result in increased binding of cells to the ‘open’ fusion protein. This was not seen and thus it is unlikely that homotypic adhesion affects binding to the fusion protein in our assay system. The 25–50% adhesion seen our studies is similar to that seen in other studies29,35 using Fc fusion proteins but the reasons for these relatively low levels are not clear.
A previous study by Brown et al.16 suggested that a second ligand for αEβ7 exists on human oral and skin keratinocytes. They showed that TGF-β1 activated peripheral blood lymphocytes bind to both E-cadherin-positive and -negative oral keratinocyte cell lines and in both cases this was inhibited by antibodies against αEβ7 but not by the mAb E4.6, which binds to the αEβ7-binding site on E-cadherin.16 One potential criticism of this study is that it was not possible to conclusively rule out the involvement of another molecule on the lymphocyte surface in the adhesion to H376 cells because the antibody against αEβ7 may non-specifically inhibit cell binding, although an irrelevant antibody suggested that this was not the case.16 In order to clarify this point we investigated whether the same two oral keratinocyte cell lines (NOK and a skin keratinocyte cell line, UP) interact with the αE I domain fusion proteins in a similar way to the intact αEβ7 molecule.
The oral E-cadherin-negative H357 cell line, the skin UP and NOK showed greater binding to the ‘open’ than to the ‘closed’ or wild-type fusion proteins and only binding of the ‘open’ fusion protein was completely abolished in the absence of Mn2+. These findings suggest that, like the MCF-7 cell line, binding of the ‘open’ fusion protein to oral and skin keratinocytes is mediated solely by the I domain of αE. However, unlike MCF-7 cells, binding was not inhibited by the mAb E4.6 against the αEβ7-binding site on E-cadherin, This is in agreement with Brown et al.,16 who found that the adhesion of αEβ7-positive lymphocytes to H357 cells could not be inhibited by E4.6. Although it could be argued that binding of the ‘open’ fusion protein is mediated by an epitope on E-cadherin not blocked by the mAb E4.6, it is also possible that cell adhesion is E-cadherin independent. Our finding, that the E-cadherin-negative H376 cells also adhered to the ‘open’ fusion protein, strongly suggests that an alternative ligand for αEβ7 exists on oral and skin keratinocytes. Our data show that this E-cadherin-independent interaction is mediated solely by the αE I domain.
Adhesion of the ‘open’αE fusion protein to the ligand on oral keratinocytes was dependent on Mn2+ ions, suggesting that like E-cadherin it also interacts with the MIDAS. Previously, Strauch et al.35 showed that there is potentially another ligand for αEβ7 expressed by E-cadherin-negative human intestinal microvascular endothelial cells (HIMEC), This ligand also interacts with the MIDAS of αEβ7. Whether the alternative ligands on oral keratinocytes and HIMEC are the same remains unclear. However, they may be structurally related to E-cadherin, and must possess amino acid groups that are capable of interacting with the MIDAS of αE.
Unlike the H357 and UP cell lines, H376 cells also interacted with the wild-type and ‘closed’ fusion proteins. They adhered to the wild-type and to the ‘open’ fusion proteins to the same extent, and only slightly less to the ‘closed’ fusion protein. Furthermore, the interaction between H376 and wild-type fusion protein was reduced when Mn2+ ions were omitted, whereas removal of Mn2+ had no effect on the adhesion of the wild-type fusion protein to H357 and UP cells. NOK adhesion resembled that of H376 cells in that binding of ‘closed’ and wild-type fusion proteins was Mn2+ dependent. This suggests that despite being E-cadherin negative, H376 cells may be phenotypically closer to normal keratinocytes than to the H357 cell line. One possible explanation for the binding of wild-type fusion protein is that the ligand on H376 and NOK may have a greater affinity for αEβ7 than E-cadherin. When treated with Mn2+, the wild-type fusion protein may be sufficiently active to interact with this alternative ligand. Evidence that integrins can have different affinities for different ligands has been provided by studies on α4β1 integrin.36,37 E-cadherin may therefore be the low-affinity ligand for αEβ7, and a high-affinity ligand may be expressed by oral and skin keratinocytes.
However, one problem with this explanation is that H357 and UP cells do not interact with the wild-type and ‘closed’ fusion proteins, although our study suggests that they express the alternative ligand for αEβ7. It is possible that the alternative ligand is co-expressed with E-cadherin on H357 cells and only interacts with αEβ7 when E-cadherin is unavailable, for example when it is blocked by antibody. E-cadherin is highly expressed by H357 cells and may have a higher avidity than the alternative ligand, which may facilitate its interaction with αEβ7 in preference to the alternative ligand. A second possibility could be that H376 cells express the alternative ligand more strongly than H357 cells to compensate for loss of E-cadherin expression. Lastly, there may, in fact, be two alternative ligands for αEβ7: a high-affinity ligand expressed by H376 and NOK; and a lower-affinity ligand expressed by H357 and UP cells. However, overall, the reasons for the differences in the interaction of H357 and H376 cells with the αE I domain fusion proteins remains unclear.
In conclusion, this study provides new evidence for the existence of an alternative ligand for αEβ7 integrin on oral and skin keratinocytes. The nature of this ligand is unknown but it may be structurally related to E-cadherin as it appears to use the same mechanism to interact with the I domain of αE. Further work is required to identify this ligand and to understand the role of αEβ7 in the oral mucosa and skin.
Acknowledgments
We are grateful to Dr Jonathon M. G. Higgins, Havard Medical School & Brigham and Women’s Hospital USA for providing human αE cDNA and the E4.6 antibody against the binding site of αEβ7 on E-cadherin. Sarah Jenkinson was supported by The University of Sheffield Internal Scholarship.
Glossary
Abbreviations:
- αEβ7
αE(CD103)β7
- DTT
dithiothreitol
- HIMEC
human intestinal microvascular endothelial cells
- I domain
inserted domain
- IEL
intraepithelial lymphocytes
- mAb
monoclonal antibody
- MIDAS
metal ion-dependent coordination site
- mRNA
messenger RNA
- NOK
normal oral keratinocytes
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- TGF-β
transforming growth factor-β
Disclosures
The authors have no financial conflict of interest to disclose.
References
- 1.Kilshaw PJ, Higgins JMG. Integrin αEβ7: molecular features and functional significance in the immune system. In: Gullberg D, editor. I Domains in Integrins. Austin Texas: Landes Bioscience; 2003. pp. 95–116. [Google Scholar]
- 2.Cerf-Bensussan NN, Jarry J, Browse N, Lisowska-Grospierre B, Guy-Grand D, Griscelli C. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur J Immunol. 1987;17:1279–85. doi: 10.1002/eji.1830170910. [DOI] [PubMed] [Google Scholar]
- 3.Kilshaw PJ, Baker KC. A unique surface antigen on intraepithelial lymphocytes in the mouse. Immunol Lett. 1988;18:149–54. doi: 10.1016/0165-2478(88)90056-9. [DOI] [PubMed] [Google Scholar]
- 4.Kilshaw PJ, Murant SJ. A new surface antigen on intraepithelial lymphocytes in the intestine. Eur J Immunol. 1990;20:2201–7. doi: 10.1002/eji.1830201008. [DOI] [PubMed] [Google Scholar]
- 5.Walton LJ, Thornhill MH, Macey MG, Farthing PM. Cutaneous lymphocyte antigen (CLA) and αEβ7 integrins are expressed by mononuclear cells in skin and oral lichen planus. J Oral Pathol Med. 1997;26:402–7. doi: 10.1111/j.1600-0714.1997.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 6.Simonitsch I, Volc-Platzer B, Mosberger I, Radaszkiewicz T. Expression of monoclonal antibody HML-1-defined αEβ7 integrin in cutaneous T cell lymphoma. Am J Pathol. 1994;145:1148–58. [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Greenwald RJ, Lafuente EM, Tzachanis D, Berezovskaya A, Freeman GJ, Sharp AH, Boussiotis VA. Rap1-GTP is a negative regulator of Th cell function and promotes the generation of CD4+ CD103+ regulatory T cells. J Immunol. 2005;175:3133–9. doi: 10.4049/jimmunol.175.5.3133. [DOI] [PubMed] [Google Scholar]
- 8.Leithäuser F, Meinhardt-Krajina T, Fink K, Wotschke B, Möller P, Reimann J. Foxp3-Expressing CD103+ regulatory T cells accumulate in dendritic cell aggregates of the colonic mucosa in murine transfer colitis. Am J Pathol. 2006;168:1898–909. doi: 10.2353/ajpath.2006.050228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allakhverdi Z, Fitzpatrick D, Boisvert A, Baba N, Bouguermouh S, Sarfati M, Delespesse G. Expression of CD103 identifies human regulatory T-cell subsets. J Allergy Clin Immunol. 2006;118:1342–9. doi: 10.1016/j.jaci.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 10.Annacker O, Coombes JL, Malmstrom V, et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–61. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Förster R, Agace WW. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–73. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coombes JL, Siddiqui KRR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialised population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β- and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnard JA, Beauchamp RD, Coffey RJ, Moss HL. Regulation of intestinal epithelial cell growth by transforming growth factor type β. Proc Natl Acad Sci USA. 1989;86:1578. doi: 10.1073/pnas.86.5.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw SK, Hermanow-Vosatka A, Shibahara T, et al. Migration of intestinal epithelial lymphocytes into a polarized epithelial monolayer. Am J Pathol. 1998;275:584–91. doi: 10.1152/ajpgi.1998.275.3.G584. [DOI] [PubMed] [Google Scholar]
- 15.Cepek KL, Parker CM, Madara JL, Brenner MB. Integrin αEβ7 mediates adhesion of T lymphocytes to epithelial cells. J Immunol. 1993;150:3459–70. [PubMed] [Google Scholar]
- 16.Brown DW, Furness J, Speight PM, Thomas GJ, Li J, Thornhill MH, Farthing PM. Mechanisms of binding of cutaneous lymphocyte antigen positive and αEβ7 positive lymphocytes to oral and skin keratinocytes. Immunol. 1999;98:9–15. doi: 10.1046/j.1365-2567.1999.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pauls K, Schön M, Kubitza RC, et al. Role of integrin αE(CD103)β7 for tissue-specific epidermal localization of CD8+ T lymphocytes. J Invest Dermatol. 2001;117:569–75. doi: 10.1046/j.0022-202x.2001.01481.x. [DOI] [PubMed] [Google Scholar]
- 18.Schlickum S, Sennefelder H, Friedrich M, Harms G, Lohse MJ, Kilshaw PJ, Schön MP. Integrin αE(CD103)β7 influences cellular shape and motility in a ligand-dependent fashion. Blood. 2008;112:619–25. doi: 10.1182/blood-2008-01-134833. [DOI] [PubMed] [Google Scholar]
- 19.Cepek K, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E-Cadherin and the αEβ7 integrin. Nature. 1994;372:190–3. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 20.Shaw SK, Cepek KL, Murphy EA, Russell GJ, Brenner MB, Parker CM. Molecular cloning of the human mucosal lymphocyte integrin αE subunit. J Biol Chem. 1994;269:6016–25. [PubMed] [Google Scholar]
- 21.Landis CR, Bennett RI, Hogg N. A novel LFA-1 activation epitope maps to the I domain. J Cell Biol. 1993;120:1519–27. doi: 10.1083/jcb.120.6.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kern A, Briseswitz R, Bank I, Marcantonio EE. The role of the I domain in ligand binding of the human integrin α1β1. J Biol Chem. 1994;269:22811–6. [PubMed] [Google Scholar]
- 23.Lee J, Rieu R, Arnaout MA, Liddington R. Crystal structure of the A domain from the α subunit of integrin CR3 (CD11b/CD18) Cell. 1995;80:631–8. doi: 10.1016/0092-8674(95)90517-0. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, Bankson LA, Arnaout MA, Liddington RC. Two conformations of the integrin A-domain (I domain): a pathway for activation? Structure. 1995;3:1333–40. doi: 10.1016/s0969-2126(01)00271-4. [DOI] [PubMed] [Google Scholar]
- 25.Higgins MJ, Cernadas M, Tan K, Irie A, Wang J, Takada Y, Brenner MB. The role of the α and β chains in ligand recognition by β7 integrins. J Biol Chem. 2000;275:25652–64. doi: 10.1074/jbc.M001228200. [DOI] [PubMed] [Google Scholar]
- 26.Shimaoki M, Lu C, Salas A, Xiao T, Takagi J, Springer TM. Stabilising the integrin αM inserted domain in alternative conformations with a range of engineered disulphide bonds. Proc Natl Acad Sci USA. 2002;99:16737–41. doi: 10.1073/pnas.252633099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCleverty C, Liddington RC. Engineered allosteric mutants of the integrin αMβ2 I domain: structural and functional studies. J Biochem. 2003;372:121–7. doi: 10.1042/BJ20021273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimaoki M, Lu C, Palframan RT, von Andrian UH, McCormack A, Takagi J, Springer TA. Reversibly locking a protein fold in an active conformation with a disulphide bond: integrin αL I domains with high affinity and antagonist in vivo. Proc Natl Acad Sci USA. 2001;98:6009–14. doi: 10.1073/pnas.101130498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corps EM, Robertson A, Dauncey MJ, Kilshaw PJ. Role of the αI domain in ligand binding by integrin αEβ7. Eur J Immunol. 2003;33:2599–608. doi: 10.1002/eji.200324156. [DOI] [PubMed] [Google Scholar]
- 30.Pei XF, Gorman PA, Watt FM. Two strains of human keratinocytes transfected with HPV16 DNA: comparison with the normal parental cells. Carcinogenesis. 1991;12:277–84. doi: 10.1093/carcin/12.2.277. [DOI] [PubMed] [Google Scholar]
- 31.Dalvi N, Thomas GJ, Marshall JF, Morgan M, Bass R, Ellis V, Speight PM, Whawell SA. Modulation of the urokinase type plasminogen activator receptor (uPAR) by the beta 6 integrin subunit. Biochem Biophys Res Commun. 2004;317:92–9. doi: 10.1016/j.bbrc.2004.02.178. [DOI] [PubMed] [Google Scholar]
- 32.Watabe M, Nagafuchi A, Tsukita S, Takeichi M. Induction of polarised cell-cell association and retardation of growth by activation of the E-cadherin-catenin adhesion system in a dispersed carcinoma line. J Cell Biol. 1994;127:247–56. doi: 10.1083/jcb.127.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu C, Shimaoki M, Ferzly M, Oxvig C, Takagi J, Springer T. An isolated, surface-expressed I domain of the integrin αLβ2 is sufficient for strong adhesive function when locked in an open conformation with a disulphide bond. Proc Natl Acad Sci USA. 2001;98:2387–92. doi: 10.1073/pnas.041606398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins MJ, Mandlebrot DA, Shaw SK, et al. Direct and regulated interaction of integrin αEβ7 with E-Cadherin. J Cell Biol. 1998;140:197–210. doi: 10.1083/jcb.140.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strauch UG, Mueller RC, Li XY, Cernadas M, Higgins JMG, Binion DG, Parker CM. Integrin αE(CD103)β7 mediates adhesion to intestinal microvascular endothelial cell line via an E-cadherin independent interaction. J Immunol. 2001;166:3506–14. doi: 10.4049/jimmunol.166.5.3506. [DOI] [PubMed] [Google Scholar]
- 36.Berlin C, Berg EL, Briskin MJ, et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–95. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 37.Briskin MJ, McEvoy LM, Butcher EC. MAdCAM-1 has homology to immunoglobulin and mucin-like adhesion receptors and to IgA1. Nature. 1993;363:461–4. doi: 10.1038/363461a0. [DOI] [PubMed] [Google Scholar]


