Abstract
Langerhans’ cells (LCs) represent a specific subset of dendritic cells (DCs) which are important for detecting and processing pathogens that penetrate the skin and epithelial barriers. The aim of our study was to explain what makes their in vitro counterparts – monocyte-derived Langerhans’-like cells (MoLCs) – unique compared with monocyte-derived dendritic cells (MoDCs). Immature MoDCs were generated by incubating peripheral blood monocytes with granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4. The addition of transforming growth factor-β (TGF-β) to this cytokine cocktail resulted in the generation of MoLCs. MoLCs showed a lower expression of CD83, CD86, HLA-DR and CCR7 compared with MoDCs, regardless of their maturational status. Both immature and mature MoLCs secreted higher quantities of IL-23 compared with MoDCs and this finding correlated with a higher secretion of IL-17 in co-culture of MoLCs with allogeneic CD4+ T cells. Mature MoLCs, which produced higher levels of IL-12 and lower levels of IL-10 compared with mature MoDCs, were more potent at inducing interferon-γ (IFN-γ) production by CD4+ T cells in the co-culture system. In conclusion, the finding that mature MoLCs stimulate stronger T-helper 1 and T-helper 17 immune responses than mature MoDCs, makes them better candidates for use in the preparation of anti-tumour DC vaccines.
Keywords: cytokine production, monocyte-derived dendritic cells, monocyte-derived Langerhans-like cells, phenotypization, T-helper cell polarization
Introduction
Dendritic cells (DCs) are members of a leucocyte population that is highly specialized in all phases of processing an antigen, including its uptake, transportation and presentation to target cells. DCs can be separated into different subpopulations, depending on their tissue locations, pathways of migration and mechanisms of dealing with the antigen challenge.1 Generally, DCs can be migratory or resident, and localized in lymphoid (thymus, spleen, lymph nodes and mucosa-associated lymphoid tissues) and non-lymphoid (in sterile organs such as the pancreas, heart, liver and kidney or in those connected with the environment, i.e. the skin, gut, lung or urinary tract) tissues.2 Regardless of where they are located, the principal functions of DCs are to maintain self-tolerance or to initiate specific immune responses to particular antigens.3
Langerhans’ cells (LCs) are a specific subset of DCs that are considered important for detecting and processing pathogens that penetrate epithelial barriers. While in an immature state, they are resident in the epidermal layer of the skin or in the other stratified epithelia that line the cavities of the respiratory, gastrointestinal and urogenital systems.4 Immature LCs express high levels of: (i) Langerin (CD207), a type II lectin receptor with a pattern-recognition role, (ii) Birbeck granules, unorthodox organelles that might have a role in the alternative antigen-presenting pathway, (iii) CD1a, a family of molecules that are able to present microbial lipid antigens to T cells and (iv) E-cadherin, an adhesion molecule.2,4,5 When stimulated by danger signals, LCs start to undergo a functional maturation and migrate through the afferent lymphatic vessels towards the nearby lymph nodes, which are capable of priming T cells.5–7 These migratory LCs up-regulate major histocompatibility complex (MHC) class II molecules, costimulatory molecules (i.e. CD40 and CD86) and CC-chemokine receptor 7 (CCR-7), while at the same time down-regulate Langerin and E-cadherin.4,6,7 Recently, it was shown that the maturation and migration of LCs could also be independently initiated,8,9 and thus the third stage in their developmental cycle was introduced. LCs that are phenotypically semimature serve to promote peripheral tolerance in the lymph node either by inducing anergy and apoptosis of specific CD4+ T-cell clones, or by promoting differentiation of naïve CD4+ T cells into T-regulatory cells.10,11
In the dermal layer of the skin, as well as in the connective tissues located beneath stratified epithelia, there is another, phenotypically heterogenous population of DCs, identified as interstitial/dermal DCs (IDDCs). This population consists of three different subsets, categorized according to their expression of CD1a and CD14 molecules: (i) CD14+ CD1a−, (ii) CD14− CD1a− and (iii) CD14− CD1a+.12 It is believed that the CD14+ subset represents a population of dermal macrophages or a group of precursors for other antigen-presenting cells (APCs) in the skin,13 while the CD1a+ subset represents a dermal DC population, distinct from LCs.14 CD1a+ cells showed the capacity to migrate and prime T cells in the lymph node, while CD14+ cells did not have such ability.15 IDDCs express a C-type lectin receptor called DC-SIGN (CD209), which is different from that expressed by LCs.16 Only a small percentage of dermal CD14+ cells, which could serve as precursors for LCs, are Langerin-positive.13 Although LCs and IDDCs have already been phenotypically well distinguished, their function, as well as their cross-talk have not been completely clarified.17
The exact precursors of different DC populations have not yet been confirmed. It has been established that they mostly originate from bone marrow but differentiate in peripheral locations under specific conditions.18 The experiments performed by Qu et al.,19 which improved the initial concept of Randolph et al.,20 have demonstrated the differentiation of skin DCs from mouse Gr-1+ monocytes in vivo. Geissmann et al.21 found a functional correlation between the Gr-1+ subpopulation and human CD14+ monocytes. Therefore, it was presumed that human DCs generated from peripheral blood monocytes (MoDCs) in vitro, in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4,5 resemble those produced in vivo. This system can also be used to generate Langerhans’-like DCs (MoLCs), in the presence of transforming growth factor-β (TGF-β).22
The maturation and migration of DCs can be induced by various inflammatory stimuli. Tumour necrosis factor-α (TNF-α), IL-1β and IL-6 are capable of promoting the maturation of MoDCs that is not dependent on Toll-like receptor (TLR) stimulation,23 while the addition of prostaglandin E2 (PGE2) further enhances their migratory and functional characteristics.24 MoDCs cultured in the presence of TNF-α, IL-1β, IL-6 and PGE2 induced T helper 1 (Th1) polarization of CD4+ T cells.24 The effects of this well-known combination of proinflammatory cytokines on MoLCs have not yet been explored. Taking into account that data analyzing the effect of MoLCs on CD4+ T-helper polarizing capabilities are also scarce, the aim of our study was to explain what makes MoLCs unique compared with MoDCs, before and after treatment with the cocktail of proinflammatory cytokines and mediators.
Materials and methods
Medium and reagents
The culture medium was RPMI-1640 (ICN, Costa Mesa, CA) supplemented with 2 mm l-glutamine, 20 μg/ml of gentamicin, 50 μm 2-mercaptoethanol (2-ME) and 10% heat inactivated fetal calf serum (FCS). Recombinant human IL-4 was purchased from Roche Diagnostics GmbH (Mannheim, Germany). Recombinant human GM-CSF (Leucomax, specific activity 4·44 × 106 UI) was obtained from Schering-Plough (Basel, Switzerland). Recombinant human TGF-β1 (Chinese hamster ovary-cell derived) was from R&D Systems (Minneapolis, MN).
Cell preparation and MoDC/MoLC cultures
MoDCs and MoLCs were generated from peripheral blood mononuclear cells (PBMCs). Briefly, PBMCs from buffy coats of healthy volunteers were isolated by density centrifugation on Lymphoprep (Nycomed, Oslo, Norway), resuspended in 5 ml of RPMI-1640 containing 10% FCS and 50 μM of 2-ME and allowed to adhere to plastic in flasks. After incubation for 1·5 hr at 37°, non-adherent cells were removed. To obtain MoDCs, adherent cells were cultured in 5 ml of control medium (RPMI-1640) containing GM-CSF (100 ng/ml) and IL-4 (20 ng/ml). The addition of TGF-β1 (5 ng/ml) to this cytokine cocktail resulted in the generation of MoLCs.22 After 6 days, MoDCs were replated in medium (RPMI-1640) containing GMCSF + IL-4 or GM-CSF + IL-4 + cocktail of proinflammatory cytokines, and MoLCs were replated in medium containing GM-CSF + IL- 4 + TGF-β1 or GM-CSF + IL-4 + TGF-b1 + cocktail of proinflammatory cytokines; 1 × 106 MoDCs or 1 × 106 MoLCs were added to each ml of the respective medium, and incubation was carried out for a further 2 days. The cocktail of proinflammatory cytokines consisted of TNF-α, IL-1β, IL-6 (10 ng/ml of each; R&D Systems) and PGE2 (1 μg/ml; Sigma, Münich, Germany). After 8 days, cell-free supernatants were collected and stored at −20° for the subsequent determination of cytokine levels.
Immunophenotyping of MoDCs/MoLCs
Control and treated MoDCs and MoLCs (1 × 105 cells/sample tube) were washed in phosphate-buffered saline (PBS) supplemented with 2% FCS and 0·1% sodium azide (NaN3), and incubated for 45 min at 4° with one of the following monoclonal antibodies (mAbs) which was conjugated to either phycoerythrin (PE) or fluorescein isothiocyanate (FITC): HLA-DR–PE, CD1a–PE, CD14–FITC, CD83–FITC, CD86–PE, (Serotec, Oxford, UK), CD54–PE (Serotec) and CCR7–FITC (R&D Systems). Controls consisted of samples with irrelevant (Ir) mouse mAbs, conjugated to PE or FITC (Serotec), and which were reactive with rat antigens and non-reactive with human antigens. Cell fluorescence was analyzed using an EPICS XL-MCL flow cytometer (Coulter, Krefeld, Germany). At least 5000 cells per sample were analyzed.
Immunocytochemistry of MoDCs/MoLCs
Immature cells of both DC subsets were adhered to glass slides covered with poly-l-lysine (2 × 104 cells/slide) using a Shandon Cytospin Centrifuge (Thermo Scientific, Breda, the Netherlands). Cytospin preparations were fixed with 2% pararosaniline in PBS and incubated for 30 min with anti-(human Langerin/CD207) (2 μg/ml, goat IgG; R&D Systems). This was followed by a 15-min incubation with a biotinylated link antibody in the presence of 5% normal human serum and then by a 15-min incubation with alkaline phosphatase-labelled streptavidin (LSAB+ System; AP; DakoCytomation, Glostrup, Denmark). After each step of the staining procedure, the slides were thoroughly washed with Tris-buffered saline (TBS). Staining was complete after a 10-min incubation with the substrate-chromogen solution, and the cytospin preparations were analyzed using light microscopy. Slides in which the primary antibody was omitted served as the negative control. At least 500 cells were analyzed on each cytospin preparation and the percentage of positive cells was determined.
Allogeneic T-cell activation
The ability of T cells to proliferate was tested in an allogeneic mixed leucocyte reaction (MLR). CD4+ T cells were used as responders in the MLR, after their isolation from PBMCs using immunomagnetic sorting with a CD4+ isolation kit (MACS technology; Miltenyi Biotec, Bergisch Gladbach, Germany) following the manufacturer’s instructions. After loading the cell suspension onto a column placed in the magnetic field of a MACS Separator, unlabelled cells run through and this cell fraction consists mainly of CD4+ T cells. The purity of T cells recovered in the negative fraction was 90·8%, as verified by staining with anti-CD3–PE and anti-CD4–FITC followed by flow cytometry analysis. Purified CD4+ T cells (1 × 105 cells/well) were cultured with different numbers of allogeneic MoDCs in complete RPMI-1640 containing 10% FCS, in 96-well round-bottomed cell-culture plates. Different ratios of DC cells : T cells were used. After 5 days of culture, cell proliferation was assessed by pulsing the cells with [3H]thymidine (1 μCi/well; Amersham, Bucks., UK) for the last 18 hr of culture. Labelled cells were harvested onto glass fibre filters and the incorporation of the radionuclide into the DNA was measured using β-scintillation counting (LKB-1219 Rackbeta; Wallac, Turku, Finland). The results were expressed as counts per minute (c.p.m.) ± standard deviation (SD) of triplicate samples.
Cytokine assays
Cells were stimulated, after 8 days of culture, with phorbol 12-myristate 13-acetate (PMA) (20 ng/ml) and ionomycin (500 ng/ml) for 16 hr to stimulate the production of the synthesized cytokines. Cells were harvested and centrifuged, and the cell-free supernatants were collected and stored at −20 for the subsequent determination of cytokine levels. The levels of IL-12, IL-23, IL-27, IL-6 and TGF-β1 were measured using sandwich enzyme-linked immunosorbent assays (ELISAs) from R&D Systems, following the manufacturer’s instructions, in the cell-free supernatants of control or treated MoDCs. TNF-α was determined using an ELISA kit purchased from Bender MedSystems (Vienna, Austria). The levels of T-helper cytokines were evaluated using a FlowCytomix Human Th1/Th2 11plex kit from Bender MedSystems.
Statistical analysis
The significance between the means of experimental data was determined using the Student’s unpaired t-test. Their differences were considered statistically significant if the P value was below 0·05.
Results
Phenotypic characteristics of MoDCs and MoLCs
MoDCs were generated from peripheral blood monocytes after incubation of the cells with GM-CSF and IL-4. The addition of TGF-β to this cytokine cocktail resulted in the generation of MoLCs. The phenotypic characteristics of these DC subsets were determined after 6 days of culture.
Both MoDCs and MoLCs displayed phenotypic characteristics of immature DCs, as judged by the low expression of CD86, CCR7 (Fig. 1) and CD83 (data not shown). While the expression of CD14 was almost completely down-regulated in both subsets, almost all MoLCs, but only about half of the MoDCs, expressed CD1a. Immature (i)MoLCs differed from iMoDCs by having higher forward scatter (FS) parameters and significantly lower expression of HLA-DR, CD-54 and CCR7 (Fig. 1). In addition, the percentage of Langerin+ cells within iMoLCs (70%) was significantly higher than the percentage of Langerin+ cells within iMoDCs (5%) (Fig. 2).
Figure 1.
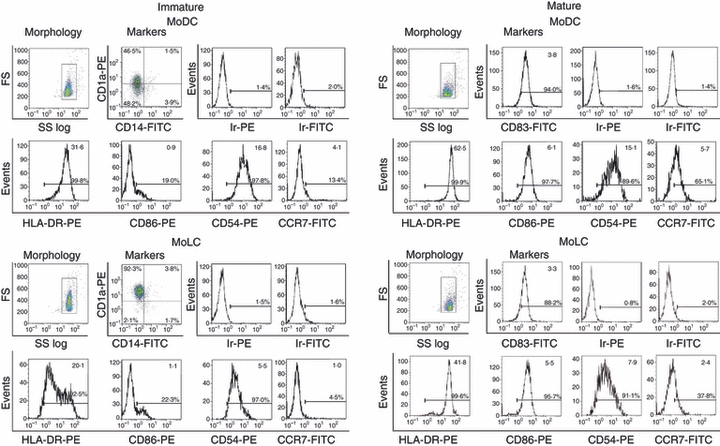
Phenotypic characterization of monocyte-derived dendritic cells (MoDCs) and monocyte-derived Langerhans’ like cells (MoLCs), as determined by flow cytometry. MoDCs were generated from peripheral blood monocytes after incubation with granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). MoLCs were generated under the same conditions but with the addition of transforming growth factor- β (TGF-β) to the cytokine cocktail. Maturation of these DC subsets was induced with a cocktail of proinflammatory mediators, as described in the Materials and methods. The numbers in the upper right corners of single histograms represent mean values of fluorescence intensity within the gated populations [marked on forward scatter (FS) : side scatter (SS) log profiles]. The results are representative of one donor, out of five different experiments. Ir-PE and Ir-FITC represent non-specific (background) fluorescence using irrelevant (Ir) mouse monoclonal antibodies (mAbs) conjugated to the corresponding fluorochrome [i.e. phycoerythrin (PE) and fluorescein isothiocyanate (FITC)], which do not react with human cells.
Figure 2.
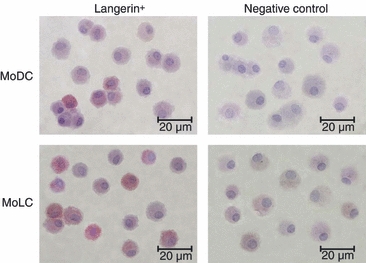
Phenotypic characterization of monocyte-derived dendritic cells (MoDCs) and monocyte-derived Langerhans’ like cells (MoLCs), as determined by immunocytochemistry. Cytospin preparations of immature dendritic cell (iDC) samples were stained with anti-human Langerin IgG, as described in the Materials and methods. At least 500 cells were analyzed on each cytospin using a light microscope and the percentage of positive cells was determined. Original magnification, × 600; the bar represents 20 μm. The slides with the omitted primary antibody served as negative controls.
The iDCs of both lineages were induced to mature by incubation with a standard cocktail of proinflammatory mediators. This resulted in the up-regulation of HLA-DR, CD83, CD86 and CCR7 and a slight decrease in the FS profiles (Fig. 1). Mature (m)MoLCs showed lower expression of HLA-DR and CCR7 compared with mMoDCs, whereas no significant differences in the expression of CD83 and CD86 were seen. The levels of non-specific (background) fluorescence for both immature and mature MoDCs and MoLCs, determined using irrelevant mAbs conjugated to PE and FITC, did not exceed 2%.
Production of cytokines by MoDCs and MoLCs in culture
The levels of cytokines were determined in the cell-free supernatants of both iDCs and mDCs (Fig. 3).
Figure 3.
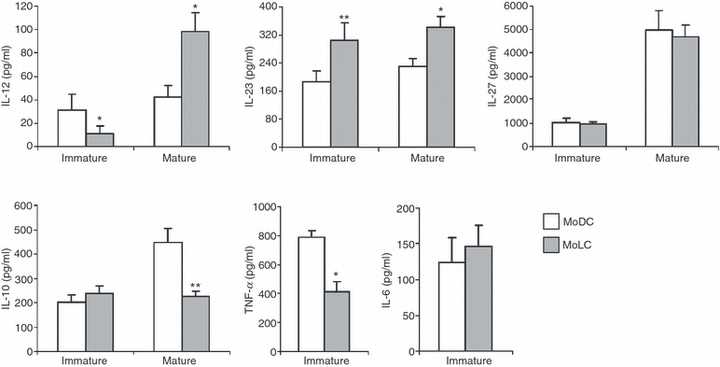
Production of cytokines in monocyte-derived dendritic cells (MoDCs) and monocyte-derived Langerhans’ like cells (MoLCs) culture supernatants. The supernatants of both immature and mature MoDCs and MoLCs were collected after 6 days of culture, and the concentrations of cytokines (in pg/ml) were determined using enzyme-linked immunosorbent assays (ELISAs). Values are given as mean ± standard deviation (SD) of three different experiments. *P < 0·05, **P< 0·01 compared with MoDCs. IL, interleukin; TNF-α, tumour necrosis factor-α.
iMoLCs produced significantly lower levels of IL-12 and TNF-α, and significantly higher levels of IL-23, than iMoDCs. The differences in secretion of IL-6, IL-10 and IL-27 between these DC subsets were not statistically significant.
mMoLCs produced significantly higher quantities of IL-12 and IL-23 and lower quantities of IL-10 compared with mMoDCs, whereas the secretion of IL-27 was not statistically different between the two cell types.
Alloreactive stimulatory capability of MoDCs and MoLCs
The alloreactive stimulatory capability of MoDCs and MoLCs was tested using MLRs where allogeneic CD4+ T cells were responders. CD4+ T cells stimulated with iMoLCs showed a higher proliferation capability than CD4+ T cells stimulated with iMoDCs, at all DC/CD4+ T-cell ratios. All differences were statistically significant except at the highest (1:10) DC/CD4+ T-cell ratio (Fig. 4).
Figure 4.
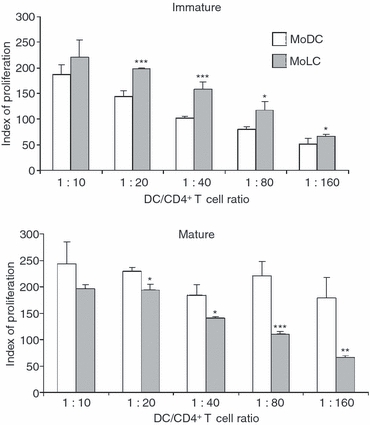
Allostimulatory activity of monocyte-derived dendritic cells (MoDCs) and monocyte-derived Langerhans’ like cells (MoLCs). Immature and mature MoDCs and MoLCs were co-cultured with allogeneic CD4+ T cells at different ratios. After 5 days, the cultures were pulsed with [3H]thymidine for the last 18 hr and the radioactivity was measured as described in the Materials and methods. Values are given as mean ± standard deviation (SD) of triplicate samples from one representative experiment out of three different experiments showing similar results. The basal counts per minute (c.p.m.) in CD4+ T-cell cultures, alone, was 402 ± 53 c.p.m. The basal c.p.m. in DC cultures alone, independently of number and type of DCs, was between 62 and 102 c.p.m. (similar to the background radioactivity). The proliferation index (PI) was calculated as follows: PI = c.p.m. (DC/CD4+ T-cell co-culture)/[c.p.m. (CD4+ T-cell culture alone) + c.p.m. (DC culture alone)]. *P < 0·05, **P < 0·01, ***P < 0·005 compared with MoDCs.
After maturation, the alloreactive stimulatory capability changed in favour of MoDCs. In contrast to iMoLCs, the alloreactive stimulatory capability of mMoLCs was lower than that of mMoDCs and the differences were statistically significant except at the highest (1:10) DC/CD4+ T-cell ratio (Fig. 4).
T-helper polarization capability of MoDCs and MoLCs
The ability of MoDCs and MoLCs to polarize T-helper immune responses was assayed on the basis of the levels of interferon-γ (IFN-γ), IL-17, IL-4, IL-13 and IL-10 in the supernatants of DC/CD4+ T-cell co-cultures (Fig. 5).
Figure 5.
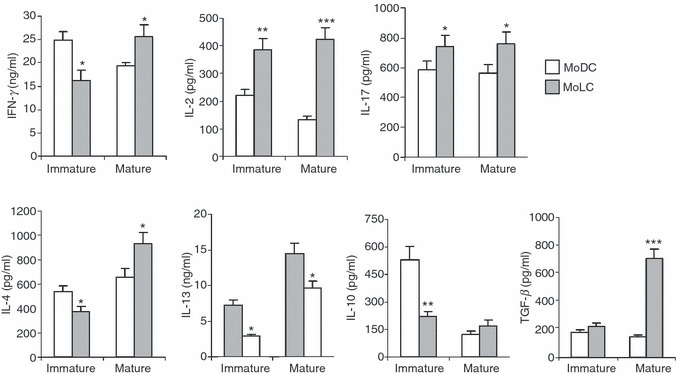
Production of cytokines by allogeneic CD4+ T cells in co-culture with immature or mature monocyte-derived dendritic cells (MoDCs) or monocyte-derived Langerhans’ like cells (MoLCs). The amounts of cytokines in both co-cultures (MoDCs/CD4+ T cells and MoLCs/CD4+ T cells) were measured using enzyme-linked immunosorbent assays (ELISAs) or FlowCytomix Human Th1/Th2 11plex, as described in the Materials and methods. Values are given as mean ± standard deviation (SD) of three independent experiments. *P < 0·05, **P < 0·01, ***P < 0·005 compared with MoDCs. IFN-γ, interferon-γ; IL, interleukin; TNF-α, tumour necrosis factor-α.
CD4+ T cells in co-culture with iMoLCs produced significantly lower amounts of IFN-γ, IL-4, IL-13 and IL-10, but higher amounts of IL-17 and IL-2, compared to these cytokines in co-culture with iMoDCs.
In contrast, mMoLCs significantly up-regulated the production of IFN-γ, IL-2 and IL-17 by CD4+ T cells compared with the levels produced by mMoDCs. It is interesting that the production of T-helper 2 (Th2) cytokines was different. The level of IL-4 in the mMoLC/CD4+ T-cell co-culture was higher than in the corresponding mMoDC/CD4+ T-cell co-culture. The opposite results were obtained for IL-13. In addition, higher quantities of TGF-β were detected in mMoLC/CD4+ T-cell co-cultures compared with mMoDC/CD4+ T-cell co-cultures.
Discussion
Blood monocytes may differentiate into various DC subsets, depending on the cytokine milieu. In our study, iMoDCs were generated by the treatment of monocytes with GM-CSF and IL-4, a well known DC differentiation cocktail.25 The addition of TGF-β to this cocktail resulted in the generation of iMoLCs.22 As the differentiation potential and function of these cells are significantly influenced by the culture conditions and individual genetic variations, we generated these DC subsets from the same blood donors. It is believed that these DC subsets may represent in vitro counterparts of LCs and IDDCs, respectively, whose functions are not well understood. Therefore, the main goal of our study was to define differences in the T-helper polarizing capability of these DC subsets before and after treatment with a DC differentiation cocktail of proinflammatory cytokines and mediators. The components of this cocktail, namely TNF-α, IL-1β, IL-6 and PGE2, which are mostly secreted by local DCs and macrophages in vivo after activation of these cells with various danger signals, are the best-defined DC maturation stimuli.24
According to the phenotypic analysis, iMoLCs were larger and more heterogenous in size, and expressed significantly higher levels of CD1a and Langerin, than iMoDCs. Such characteristics mirrored the differences between ex vivo isolated LCs and IDDCs.15 Lower expression of HLA-DR, CD54 and CCR7 on iMoLCs suggested that these cells were more immature then iMoDCs. The difference in the expression of HLA-DR corresponded well with data obtained from other in vitro studies,26,27 in contrast to those obtained for CD54 and CCR7, which have not yet been published. Although there was no difference in the expression of CD86 between iMoLCs and iMoDCs, its low expression on both DC types corresponded with their immature phenotype.27 Upon maturation, both DC subsets up-regulated most cell-surface markers, but the expression of these markers was lower on mMoLCs than on mMoDCs. These results are in accordance with the fact that TGF-β down-regulates HLA-DR, CD83 and CD86 on DCs.28
The main finding of this study was that iMoLCs induced weaker T-helper 1 (Th1) and Th2, but stronger T-helper 17 (Th17), polarization of allogeneic CD4+ T cells than iMoDCs. In contrast, mMoLCs were able to induce stronger Th1, Th2 and Th17 immune responses than mMoDCs. Several ex vivo studies have shown that both LCs and IDDCs induced Th1 and Th2 polarization of alloreactive CD4+ T cells,29,30 while only LCs were able to induce Th17 polarization of CD4+ T cells.31 Stronger induction of the Th17 response by MoLCs compared with MoDCs is in accordance with the specific request for IL-17 production in epithelia where LCs act as a first line of defense against various pathogens.32–35 Additionally, the Th17 response is also supported by the fact that tissues populated with LCs are enriched with TGF-β.36 Therefore, our results on the in vitro counterparts of LCs and IDDCs shed new light on these important biological phenomena.
Lower production of IFN-γ and higher production of IL-17 by allogeneic CD4+ T cells in co-culture with iMoLCs compared with co-culture of CD4+ T cells and iMoDCs, are in agreement with the lower production of IL-12 and TNF-α and higher production of IL-23, respectively, by iMoLCs compared with iMoDCs. A similar, strong association between the levels of IL-23 and the production of IL-17 in co-culture of CD4+ T cells and mMoLCs was confirmed in this study. Higher levels of IL-12 and lower levels of IL-10 in mMoLC cultures might explain why mMoLCs are more potent inducers of Th1 polarization than mMoDCs. An interesting finding was the lower ability of iMoLCs to induce a Th2 response compared with iMoDCs, as judged by lower levels of both IL-4 and IL-13 in co-culture supernatants. When assessing the production of IL-13, the same finding was confirmed by mMoLCs. However, the opposite was observed for the production of IL-4. One explanation could be differences in the dynamics of secretion of Th2 cytokines in co-culture. Namely, it has been shown that up- and down-regulation of IL-13 production by CD4+ CD45RO+ cells predominantly, is faster than the production of IL-4 in cultures with DCs.37 However, the biological significance of this finding remains to be tested, bearing also in mind that IL-13 is a more potent down-modulator of IFN-γ production than IL-4.38
The proliferation capability of CD4+ T cells stimulated by iMoLCs was higher than that of CD4+ T cells primed with iMoDCs, and the phenomenon correlated with the production of a higher level of IL-2. The reason for this finding is not clear because the expression of a costimulatory ligand (CD86) was similar and the expression of HLA-DR and CD54 was even lower than on iMoDCs. Therefore, it remains to be studied how the expression of this molecule changes as a result of contact with CD4+ T cells, in which CD40 ligand (CD4+ T cells) and CD40 (DCs) is of significant importance.39 Peiser et al.40 showed that stimulation of MoLCs with CD40 ligand was followed by the up-regulation of CD83 and CD86 expression and the production of IL-12p70 and IL-10. In addition, CD40 triggering increased the potency of MoLCs to stimulate CD4+ T-cell proliferation. It is also not clear whether increased production of IL-2 is a cause or a consequence of cellular activation. In this context, lower production of IL-10 in a co-culture of iMoLCs with CD4+ T cells might be important, because IL-10 inhibits the production of IL-2 at the mRNA level without changing the expression or function of the IL-2 receptor (IL-2R).41 Therefore, in our culture system lower levels of IL-10 could have counteracted the production of IL-2, which is a key T-cell growth factor.42
However, maturational stimuli significantly lowered the proliferation capability of CD4+ T cells triggered by mMoLCs, compared with mMoDCs, in spite of higher production of IL-2. This result could be explained by a lower expression of most cell-surface markers on mMoLCs compared with mMoDCs, and a different IL-2/TGF-β ratio. Lower expression of HLA-DR and CD54 on mMoLCs is in line with previously published data which show that mature LCs from skin explants, with a low ability for antigen presentation and reduced affinity to contact alloreactive CD4+ T cells, abrogated their proliferation.29 In contrast, higher production of TGF-β in co-culture of CD4+ T cells and mMoLCs is in accordance with the effect of TGF-β on the modulation of intracellular effects of IL-2. Namely, it has been shown that TGF-β inhibits expression, as well as signalling through IL-2R, mediated by the Jak–Stat pathway.43–45
Anti-tumour vaccine protocols in the last few years have been mainly focused on mMoDCs, which are able to promote the Th1 polarization of CD4+ T cells.46 However, experiments in mice have recently shown that CD4+ Th17 cells provided better protection against malignant melanoma than CD4+ Th1 cells because of their unique ability to promote CD8+ anti-tumour T cells in vivo.47 Th17 cells maintained anti-tumour activity in vivo by producing IFN-γ but not IL-17,48 as a result of the Th17/Th1 phenotypic switch.49 As the mMoLCs in our study were stronger inducers of both Th1 and Th17 responses than mMoDCs, and CD4+ Th17 cells have been found in various human tumours,48,50–52 we hypothesized that mMoLCs might be useful for future vaccine protocols aimed against at least skin and epithelial tumours. However, it should be emphasized that mMoLCs were also stronger inducers of the Th2 immune response than mMoDCs, which could potentially decrease the intensity of the Th17/Th1 response.53 As MoLCs achieved this characteristic after maturation, it would be reasonable to test additional maturation protocols in future experiments in order to determine their potentially useful characteristics for optimal stimulation of anti-tumour immunity. This assumption is supported by earlier studies showing that a well-established maturational cocktail,24 which we used in our study, had the ability to expand a regulatory T-cell population,54 whereas PGE2 could additionally stimulate the production of IL-10, inhibit the secretion of IL-12 and induce Th2 polarization by DCs.55,56
In conclusion, our results demonstrated that both iMoLCs and mMoLCs are better inducers of the Th17 immune response than MoDCs. This function, and the ability of MoLCs to stimulate a stronger Th1 immune response than mMoDCs, make mMoLCs a better candidate for preparation of anti-tumour DC vaccines.
Acknowledgments
This study was supported by a grant (VMA/06-10/A.5) from the Military Medical Academy, Belgrade, Serbia. We would like to express our gratitude to Ms Dejana Dimitrijevic for assistance in English correction.
Disclosure
The authors deny any potential conflict of interest, including all relevant financial interests in any company or institution that might benefit from this publication.
References
- 1.Riedl E, Stockl J, Majdic O, Scheinecker C, Knapp W, Strobl H. Ligation of E-cadherin on in vitro-generated immature Langerhans-type dendritic cells inhibits their maturation. Blood. 2000;96:4276–84. [PubMed] [Google Scholar]
- 2.Merad M, Manz MG. Dendritic cell homeostasis. Blood. 2009;113:3418–27. doi: 10.1182/blood-2008-12-180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 4.Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 2007;204:3119–31. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geissmann F, Dieu-Nosjean MC, Dezutter C, et al. Accumulation of immature Langerhans cells in human lymph nodes draining chronically inflamed skin. J Exp Med. 2002;196:417–30. doi: 10.1084/jem.20020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginhoux F, Collin MP, Bogunovic M, et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204:3133–46. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–47. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 8.Randolph GJ. Is maturation required for Langerhans cell migration? J Exp Med. 2002;196:413–6. doi: 10.1084/jem.20021240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villablanca EJ, Mora JR. A two-step model for Langerhans cell migration to skin-draining LN. Eur J Immunol. 2008;38:2975–80. doi: 10.1002/eji.200838919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grunig G, Banz A, de Waal Malefyt R. Molecular regulation of Th2 immunity by dendritic cells. Pharmacol Ther. 2005;106:75–96. doi: 10.1016/j.pharmthera.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Kuwana M. Induction of anergic and regulatory T cells by plasmacytoid dendritic cells and other dendritic cell subsets. Hum Immunol. 2002;63:1156–63. doi: 10.1016/s0198-8859(02)00754-1. [DOI] [PubMed] [Google Scholar]
- 12.Nestle FO, Zheng XG, Thompson CB, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol. 1993;151:6535–45. [PubMed] [Google Scholar]
- 13.Larregina AT, Morelli AE, Spencer LA, et al. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat Immunol. 2001;2:1151–8. doi: 10.1038/ni731. [DOI] [PubMed] [Google Scholar]
- 14.Angel CE, George E, Brooks AE, Ostrovsky LL, Brown TL, Dunbar PR. Cutting edge: CD1a+ antigen-presenting cells in human dermis respond rapidly to CCR7 ligands. J Immunol. 2006;176:5730–4. doi: 10.4049/jimmunol.176.10.5730. [DOI] [PubMed] [Google Scholar]
- 15.Angel CE, Lala A, Chen CJ, Edgar SG, Ostrovsky LL, Dunbar PR. CD14+ antigen-presenting cells in human dermis are less mature than their CD1a+ counterparts. Int Immunol. 2007;19:1271–9. doi: 10.1093/intimm/dxm096. [DOI] [PubMed] [Google Scholar]
- 16.Ebner S, Ehammer Z, Holzmann S, et al. Expression of C-type lectin receptors by subsets of dendritic cells in human skin. Int Immunol. 2004;16:877–87. doi: 10.1093/intimm/dxh088. [DOI] [PubMed] [Google Scholar]
- 17.Bechetoille N, Andre V, Valladeau J, Perrier E, Dezutter-Dambuyant C. Mixed Langerhans cell and interstitial/dermal dendritic cell subsets emanating from monocytes in Th2-mediated inflammatory conditions respond differently to proinflammatory stimuli. J Leukoc Biol. 2006;80:45–58. doi: 10.1189/jlb.0205109. [DOI] [PubMed] [Google Scholar]
- 18.Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells. Semin Immunol. 2005;17:313–8. doi: 10.1016/j.smim.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Qu C, Edwards EW, Tacke F, et al. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J Exp Med. 2004;200:1231–41. doi: 10.1084/jem.20032152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–61. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 21.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 22.Geissmann F, Prost C, Monnet JP, Dy M, Brousse N, Hermine O. Transforming growth factor beta1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J Exp Med. 1998;187:961–6. doi: 10.1084/jem.187.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sporri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat Immunol. 2005;6:163–70. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- 24.Jonuleit H, Kuhn U, Muller G, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–42. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 25.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeuchi J, Watari E, Shinya E, et al. Down-regulation of Toll-like receptor expression in monocyte-derived Langerhans cell-like cells: implications of low-responsiveness to bacterial components in the epidermal Langerhans cells. Biochem Biophys Res Commun. 2003;306:674–9. doi: 10.1016/s0006-291x(03)01022-2. [DOI] [PubMed] [Google Scholar]
- 27.van der Aar AM, Sylva-Steenland RM, Bos JD, Kapsenberg ML, de Jong EC, Teunissen MB. Loss of TLR2, TLR4, and TLR5 on Langerhans cells abolishes bacterial recognition. J Immunol. 2007;178:1986–90. doi: 10.4049/jimmunol.178.4.1986. [DOI] [PubMed] [Google Scholar]
- 28.Geissmann F, Revy P, Regnault A, et al. TGF-beta 1 prevents the noncognate maturation of human dendritic Langerhans cells. J Immunol. 1999;162:4567–75. [PubMed] [Google Scholar]
- 29.Morelli AE, Rubin JP, Erdos G, et al. CD4+ T cell responses elicited by different subsets of human skin migratory dendritic cells. J Immunol. 2005;175:7905–15. doi: 10.4049/jimmunol.175.12.7905. [DOI] [PubMed] [Google Scholar]
- 30.Klechevsky E, Morita R, Liu M, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathers AR, Janelsins BM, Rubin JP, et al. Differential capability of human cutaneous dendritic cell subsets to initiate Th17 responses. J Immunol. 2009;182:921–33. doi: 10.4049/jimmunol.182.2.921. [DOI] [PubMed] [Google Scholar]
- 32.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165:6107–15. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 33.Kim B, Sarangi PP, Azkur AK, Kaistha SD, Rouse BT. Enhanced viral immunoinflammatory lesions in mice lacking IL-23 responses. Microbes Infect. 2008;10:302–12. doi: 10.1016/j.micinf.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–31. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 35.Kelly MN, Kolls JK, Happel K, et al. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect Immun. 2005;73:617–21. doi: 10.1128/IAI.73.1.617-621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hashimoto K. Regulation of keratinocyte function by growth factors. J Dermatol Sci. 2000;24(Suppl 1):S46–50. doi: 10.1016/s0923-1811(00)00141-9. [DOI] [PubMed] [Google Scholar]
- 37.Webb DC, Cai Y, Matthaei KI, Foster PS. Comparative roles of IL-4, IL-13, and IL-4Ralpha in dendritic cell maturation and CD4+ Th2 cell function. J Immunol. 2007;178:219–27. doi: 10.4049/jimmunol.178.1.219. [DOI] [PubMed] [Google Scholar]
- 38.de Vries JE. The role of IL-13 and its receptor in allergy and inflammatory responses. J Allergy Clin Immunol. 1998;102:165–9. doi: 10.1016/s0091-6749(98)70080-6. [DOI] [PubMed] [Google Scholar]
- 39.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 40.Peiser M, Wanner R, Kolde G. Human epidermal Langerhans cells differ from monocyte-derived Langerhans cells in CD80 expression and in secretion of IL-12 after CD40 cross-linking. J Leukoc Biol. 2004;76:616–22. doi: 10.1189/jlb.0703327. [DOI] [PubMed] [Google Scholar]
- 41.de Waal Malefyt R, Yssel H, de Vries JE. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol. 1993;150:4754–65. [PubMed] [Google Scholar]
- 42.Nelson BH, Willerford DM. Biology of the interleukin-2 receptor. Adv Immunol. 1998;70:1–81. doi: 10.1016/s0065-2776(08)60386-7. [DOI] [PubMed] [Google Scholar]
- 43.Grundstrom S, Dohlsten M, Sundstedt A. IL-2 unresponsiveness in anergic CD4+ T cells is due to defective signaling through the common gamma-chain of the IL-2 receptor. J Immunol. 2000;164:1175–84. doi: 10.4049/jimmunol.164.3.1175. [DOI] [PubMed] [Google Scholar]
- 44.Campbell JD, Cook G, Robertson SE, et al. Suppression of IL-2-induced T cell proliferation and phosphorylation of STAT3 and STAT5 by tumor-derived TGF beta is reversed by IL-15. J Immunol. 2001;167:553–61. doi: 10.4049/jimmunol.167.1.553. [DOI] [PubMed] [Google Scholar]
- 45.Bright JJ, Kerr LD, Sriram S. TGF-beta inhibits IL-2-induced tyrosine phosphorylation and activation of Jak-1 and Stat 5 in T lymphocytes. J Immunol. 1997;159:175–83. [PubMed] [Google Scholar]
- 46.Mailliard RB, Wankowicz-Kalinska A, Cai Q, et al. Alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–7. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- 47.Martin-Orozco N, Muranski P, Chung Y, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–98. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muranski P, Boni A, Antony PA, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–73. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee YK, Turner H, Maynard CL, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang B, Rong G, Wei H, et al. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun. 2008;374:533–7. doi: 10.1016/j.bbrc.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 51.Sfanos KS, Bruno TC, Maris CH, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–61. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang RF. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci U S A. 2008;105:15505–10. doi: 10.1073/pnas.0710686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Jong EC, Smits HH, Kapsenberg ML. Dendritic cell-mediated T cell polarization. Springer Semin Immunopathol. 2005;26:289–307. doi: 10.1007/s00281-004-0167-1. [DOI] [PubMed] [Google Scholar]
- 54.Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar KM. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006;108:2655–61. doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luft T, Jefford M, Luetjens P, et al. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: prostaglandin E(2) regulates the migratory capacity of specific DC subsets. Blood. 2002;100:1362–72. doi: 10.1182/blood-2001-12-0360. [DOI] [PubMed] [Google Scholar]
- 56.Morelli AE, Thomson AW. Dendritic cells under the spell of prostaglandins. Trends Immunol. 2003;24:108–11. doi: 10.1016/s1471-4906(03)00023-1. [DOI] [PubMed] [Google Scholar]


