Abstract
In mice, the plasma cell (PC) niche in the bone marrow is close to the haematopoietic stem cell (HSC) niche. We investigated whether PCs can be mobilized into the peripheral blood (PB) in healthy donors receiving granulocyte colony-stimulating factor (G-CSF) for the induction of HSC mobilization into the PB. G-CSF increased the count of circulating PCs 6-fold, that of circulating B lymphocytes 4-fold and that of circulating HSCs 44-fold. Mobilized circulating PCs comprised CD138− (62·2%) and CD138+ (37·8%) PCs, the latter being more mature based on increased CD27, CD38 and cytoplasmic immunoglobulin expression. Mobilized PCs had a phenotype close to that of steady-state PB PCs or in vitro generated PCs, but they expressed L-selectin only weakly. Finally, a median value of 0·4 × 106/kg donor PCs – one-thirtieth of the overall PC count in a healthy adult – was grafted into patients, which could contribute to immune memory recovery.
Keywords: granulocyte colony-stimulating factor, immunology, mobilization/homing, plasma cells, transplantation
Introduction
After they have been generated in the lymph nodes, plasmablasts exit into the lymphatic system. They flow out into the peripheral blood (PB) via the thoracic duct and have to find a niche in the bone marrow (BM), spleen, mucosa-associated lymphoid tissues (MALTs) or lymph nodes.1 In these niches, plasmablasts further differentiate into mature plasma cells (PCs) and may survive for decades.2 Long-term surviving PCs are responsible for the long-term humoral immune memory. Consistent with this, treatment with anti-CD20 monoclonal antibodies (mAbs), which completely delete B cells, did not affect the levels of circulating immunoglobulins.3 The rarity of the niche supporting the long-term survival of PCs is a key factor of the regulation of humoral responses. In fact, newly generated plasmablasts have to compete with already established long-lived PCs to gain access to these rare niches.4 In mice, the PC niche has been shown to be similar to the haematopoietic stem cell (HSC) and pre-pro B-cell niche. Insertion of the green fluorescent protein (GFP) gene into the stromal cell-derived factor-1 [SDF-1 or chemokine (C-X-C motif) ligand 12 (CXCL 12)] gene made it possible to show that all murine BM PCs as well as HSCs and pre-pro B cells adhere to SDF-1+ vascular cell adhesion molecule (VCAM1)+ cells, which represent 1% of BM cells.5 There are no data regarding the PC niche in the human BM. Many studies have documented the mechanisms of homing of HSCs into the BM and recirculation of these BM HSCs into the blood. CXCR4+ HSCs are attracted to the BM by the SDF-1 chemokine produced by BM stromal cells. Binding of SDF1 to CXCR4 activates the very-late activation antigen type 4 (VLA-4) integrin of HSCs which can adhere to endothelial VCAM1+ cells.6 HSCs are recruited to SDF-1+ stromal cells which are adjacent to endothelial cells. Upon injury, HSCs migrate to the closest osteoblasts which produce various growth factors, such as granulocyte colony-stimulating factor (G-CSF) and interleukin-6 (IL-6).7 More recently, BM stromal cells have been shown to express β3 adrenergic receptors.8 Norepinephrine production by the sympathetic nervous system controls expression of homing molecules by stromal cells. It is noteworthy that a circadian fluctuation of norepinephrine production results in circadian release of a minor population of HSCs into the PB. In mice, these circulating HSCs have been shown to play an important role in innate immune surveillance.9 Accordingly, circulating HSCs home to tissues where they may reside for 36 hr before returning to the PB through the lymphatic system. In the case of infection, Toll-like receptor-mediated activation of HSCs results in down-regulation of the sphingosine phosphate receptor and in situ differentiation of HSCs into innate immune cells: tissue-resident myeloid cells, preferentially dendritic cells.10 This tightly controlled homing of HSCs into the BM and recirculation into the PB may explain why human CD34+ HSCs injected into the PB can rapidly home to and engraft the BM and vice versa. At the same time, it may also explain why HSCs can be mobilized into the PB after CXCR4 antagonist or G-CSF injection.11 The effect of G-CSF is mainly attributable to activation of BM myeloid cells to produce proteases that cleave SDF-1 and adhesion molecules.8
Given the similarity of the PC and HSC BM niches in mice, it is tempting to postulate that similar mechanisms exist for the homing of PCs into the BM and eventually for their recirculation from the BM to the PB. Regarding PC homing, it has been shown that deletion of CXCR4 abrogates homing of murine PCs into the murine BM, similarly to HSCs.12 Regarding the exit of BM PCs into the PB, 2 CD19+CD20− CD38++ PCs/mm3 have been reported in human adults in steady-state conditions.13,14 The origin of circulating PCs remains undetermined but they may be either newly generated PCs in the lymph node or long-lived tissue PCs. After vaccination with tetanus toxin (TT), there is a 4–5-fold increase in the number of circulating PCs, a significant fraction of which do not secrete anti-TT Abs.15 This suggests that newly generated PCs can displace old PCs from their niche and induce them to recirculate.4
In the present study, we investigated the counts and detailed phenotype of circulating PCs in adult healthy donors receiving G-CSF to induce HSC mobilization into the PB. Our results show that a 5-day treatment of healthy individuals with G-CSF increases the count of circulating PCs by 6-fold, that of circulating B lymphocytes by 4-fold and that of circulating HSCs by 44-fold. Circulating PCs comprised both CD19+CD20− CD38++ CD138− plasmablasts and CD19+CD20−CD38++CD138+ PCs.
Materials and methods
Cell samples
PB and leukapheresis samples were obtained from 26 healthy donors (age range 22–66 years) treated with G-CSF (10 μg/kg per day) for 5 days in order to collect HSCs for allograft. In concordance with French ethical law, cells that were not used for the patient’s treatment could be used for research with the donor’s written agreement. Leukapheresis was performed using a continuous flow blood cell separator (COBE Spectra version 4; CaridianBCT, Lakewood, CO). For each donor, a PB sample was obtained at the time at which the leukapheresis procedure was performed and both PB and leukapheresis samples were analysed. PB mononuclear cells (PBMCs) were obtained by density centrifugation using Lymphocyte Separation Medium (Lonza, Walkersville, MD) and analysed. PB from 11 healthy donors (in the absence of acute or chronic infection or recent vaccination) was purchased from the French Blood Centre (Toulouse, France).
Antibodies
Abs conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), energy-coupled dye, peridinin chlorophyll protein (PerCP)-Cy5·5, PE-Cy7, Pacific Blue, allophycocyanin (APC) and APC-H7, specific for human CD19 (clone SJ25C1), CD27 (clone L128), CD29 [β1-integrin (ITGβ1), clone MAR4], CD38 (clone HIT2 or HB7), CD43 (clone 1G10), CD45 (clones 2D1 and HI30), CD49d (ITGα4, clone 9F10), CD49e (ITGα5, clone SAM1), CD56 (N-CAM, clone B159), CD62L (clone DREG-56), CD70 (clone Ki-24), CD106 (VCAM-1, clone 51-10C9), CD117 (clone 104D2), CD184 (CXCR4, clone 12G5), CCR2 (CD192, clone 48607), human leucocyte antigen (HLA)-DR, DP, DQ (clone Tu39), ITGβ7 (clone FIB504), anti-immunoglobulin light chain lambda (IgLCλ, clone JDC-12), anti-immunoglobulin light chain kappa (IgLCκ, clone TB 28-2), anti-immunoglobulin G (IgG) (clone G18-145), anti-IgM (clone G20-127), and KI-67 (clone B56) were purchased from Becton/Dickinson (BD) Biosciences (San Jose, CA); CD20 (clone B9E9), CD34 (clone 581), CD58 [lymphocyte function-associated antigen 3 (LFA-3), clone AICD58] and CD138 (clone B-A38) were obtained from Beckman Coulter (Fullerton, CA); CCR10 (clone 314305) was from R&D Systems (Minneapolis, MN), CD19 (clone HIB19) was from eBiosciences (San Diego, CA), and both anti-IgA (polyclonal goat antibody) and anti-IgG (polyclonal goat Ab) were from Southern Biotech (Birmingham, AL).
Immunophenotypic studies
Leukapheresis samples and PBMCs were labelled with Abs conjugated to various fluorochromes. The number of CD34+ cells was estimated by flow cytometry using the FC500 (Beckman Coulter) or FACSAria (BD Biosciences) flow cytometer. B lymphocytes and PCs were identified using a seven-colour combination of fluorochrome-conjugated Abs. In order to analyse the phenotypes of B lymphocytes and PCs, we used a two-step strategy. First, the cellular phenotype was determined based on the expression of cytoplasmic immunoglobulin, CD19, CD20, CD38 and CD138. Cells were labelled with CD19, CD20, CD38 and CD138 mAbs, fixed and permeabilized with the Cytofix/Cytoperm kit (BD Biosciences), and then labelled with anti-kappa (APC) and anti-lambda (FITC) mAbs. This first step makes it possible to define immunoglobulin-secreting cells as CD19+CD20−CD38++ (Fig. 1). In the second step, the full phenotypes of B lymphocytes and PCs were determined upon gating on CD19+ CD20+ CD38−/+ and CD19+CD38++ cells, respectively. Fluorescence emissions were analysed in a FACSAria flow cytometer, driven by the FACSDiva 6.1 software (BD Biosciences). Data were analysed with the Infinicyt 1.3 software (Cytognos SL, Salamanca, Spain). The fluorescence intensity of the cell populations was compared using the staining index (SI) provided by the following formula: [mean fluorescence intensity (MFI) obtained from the given mAb minus the MFI obtained with a control mAb]/[2 times the standard deviation (SD) of the MFI obtained with the same control mAb].16
Figure 1.
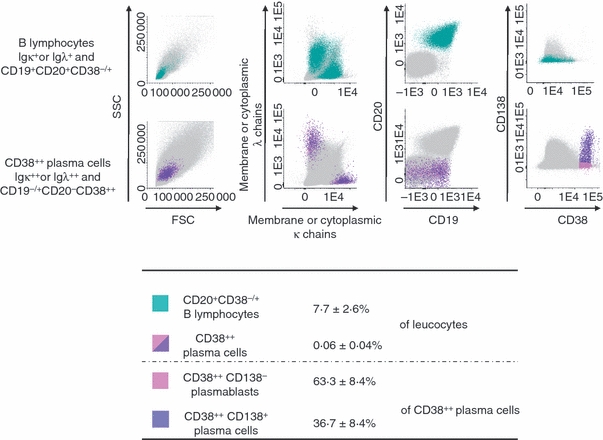
B lymphocytes and plasma cells present in leukapheresis products from healthy donors. Flow cytometric discrimination between B lymphocytes and plasma cells present in leukapheresis products from healthy donors stained for CD19, CD20, CD38, CD138, and membrane/cytoplasmic immunoglobulin kappa and lambda light chains (m/cyIgκ or m/cyIgλ) was performed. After gating out cell doublets, B lymphocytes were defined as m/cyIgκ+ or m/cyIgλ+ cells with a CD19+CD20+CD38−/+CD138− immunophenotypic profile (green dots). Plasma cells were gated as m/cyIgκ- or m/cyIgλ-expressing cells, which were CD20− and showed high expression of CD38 (cIg+CD19−/+CD20−CD38++cells). Mobilized plasma cells comprised both CD138− cells (pink dots) and CD138+cells (purple dots). Other cell populations in the sample are shown as grey dots. Data from one experiment representative of six experiments are shown. The table indicates mean percentages of B lymphocytes and CD38++ plasma cells in leucocytes, and mean percentages of CD138− plasmablasts and CD138+ plasma cells in CD38++ plasma cells.
Statistical methods
Mean values, SDs, medians and ranges were calculated for continuous variables with the spss statistical software package (SPSS 13.0 Inc., Chicago, IL). Student’s t-test (n > 6) or the Wilcoxon test (n ≥ 5) was used to evaluate the statistical significance of differences observed between groups for paired and unpaired variables. Correlation studies were performed using the Pearson test. P values ≤0.05 were considered to be associated with statistical significance.
Results
Mobilization of plasma cells into the PB of healthy individuals treated with G-CSF
Eleven healthy donors were treated with G-CSF in order to mobilize HSCs into the PB and collect them. PCs and B lymphocytes were identified using the first step labelling technique, based on CD19, CD20 and CD38 expression and staining of membrane and cytoplasmic immunoglobulin light chain (m/cyIgLC) (Fig. 1). After G-CSF treatment for 5 days, median values of 7·6 PCs/μl (CD19+ CD20−CD38++m/cyIgLC++ cells; range 0·3–17·1 cells/μl), 649·8 B lymphocytes/μl (CD19+CD20+ CD38−/+m/cyIgLC+; range 120·5–1437·6 cells/μl) and 78 CD34+ cells/μl (range 18–138·4 cells/μl) were detected in the PB (Table 1). As it was not possible to harvest the PB of healthy donors who were treated with G-CSF at various times before or after the leukapheresis procedure because of ethical considerations, the counting of circulating cells in steady-state conditions was performed in another series of age-related healthy donors. Median values of 1·3 PCs/μl, 154·2 B lymphocytes/μl and 1·8 CD34+ cells/μl were detected in the PB of 11 healthy individuals in steady-state conditions using the same labelling and flow cytometry gating strategies (Table 1). These counts are within the range of those reported in other studies.13,14,17 Thus, a 5-day treatment with G-CSF of healthy adults induced a significant 6-fold increase in the number of circulating PCs (P =0.002), a 4·2-fold increase in the number of circulating B lymphocytes (P =0.0002) and a 44-fold increase in the number of circulating CD34+ cells (P =0.000003) (Table 1). We then looked for an extensive phenotype of these circulating PCs.
Table 1.
Granulocyte colony-stimulating factor (G-CSF) induces the mobilization of plasma cells
| Leucocytes | CD38++ Plasma cells | CD20+ CD38−/+B lymphocytes | CD34+ HSCs | n | |
|---|---|---|---|---|---|
| Peripheral blood cell count (cells/μl) | |||||
| Steady-state conditions | 5700 (4000–8700) | 1·3(0·2–7·9) | 154·2(76·0–253·5) | 1·8 (1·0–3·4) | 11 |
| Day 5 after G-CSF treatment start | 40000 (25300–61500) | 7·6 (0·3–17·1) | 649·8 (120·5–1437·6) | 78·0 (18·0–138·4) | 11 |
| Fold increase attributable to G-CSF treatment;P value (Wilcoxon test) | 7·00.000003 | 6·00.002 | 4·20.0002 | 44·00.000003 | |
| Leukapheresis cell count (cells/μl) | 193 000 (84300–348600) | 123·5 (34·4–286·0) | 15 091·6 (7477·4–37805·6) | 1 681·8 (646·2–5070·6) | 26 |
| Collected cells (106 cells/leukapheresis) | 58 285 (25964–121522) | 34·4 (5·3–100·3) | 3 875·1 (2121·2–11659·2) | 509·4 (219·7–1410·7) | 26 |
| Cells injected into patient (106 cells/kg) | 750 (267–1620) | 0·4 (0·1–1·4) | 55·3 (13·4–185·8) | 5·8 (1·8–25·0) | 26 |
Results are expressed as median values and ranges in brackets.
Phenotypic characterization of circulating PCs after G-CSF treatment
The PC phenotype was assessed using the second step labelling strategy. Mobilized PCs secreted both kappa (mean of 51·3% of all PCs) and lambda (mean of 48·7% of all PCs) light chains (Fig. 1). Mobilized PCs comprised mainly cyIgG+ cells (55·3%), cyIgM+ cells (29·4%) and cyIgA+ cells (15·3%) (Table 2). Immunoglobulin heavy chain classes in mobilized PCs were in inverse proportions to those of mobilized CD19+CD20+B lymphocytes, which comprised 83·7% IgM+, 9·8% IgG+ and 6·4% IgA+ cells (median values). Mobilized CD38++ PCs comprised 62·2 ± 14% CD138− plasmablasts and 37·8 ± 14% CD138+ PCs (n = 26). Both CD138− plasmablasts and CD138+ PCs showed high levels of expression of CD27, CD38 and CD43, but lower reactivity for CD45 and HLA class II than B lymphocytes (P≤ 0.05; Fig. 2). CD138− plasmablasts and CD138+ PCs showed clear phenotypic differences (Fig. 2). CD138+ PCs displayed a higher SI (versus CD138− plasmablasts) for cytoplasmic immunoglobulin κ light chains (3·2 SI fold increase; n = 6; P= 0.0005) and CD27 (2·5 SI fold increase; n = 6; P= 0.001), and a lower SI for CD45 (1·3 SI fold decrease; n = 6; P= 0.0004). HLA class II (including HLA-DR) expression was low and similar in CD138− plasmablasts versus CD138+ PCs (Fig. 2).
Table 2.
Expression of immunoglobulin heavy chain isotypes by granulocyte colony-stimulating factor (G-CSF)-mobilized (day +5) plasma cells and B lymphocytes
| CD38++ plasma cells | CD20+CD38−/+ B lymphocytes | n | |
|---|---|---|---|
| IgG (%) | 55·3 (28·0–76·5) | 9·8 (5·0–13·4) | 6 |
| IgM (%) | 29·4 (13·7–57·1) | 83·7 (76·7–91·2) | 6 |
| IgA (%) | 15·3 (9·0–39·6) | 6·4 (2·6–12·4) | 6 |
Values are expressed as median values and ranges in brackets.
Figure 2.
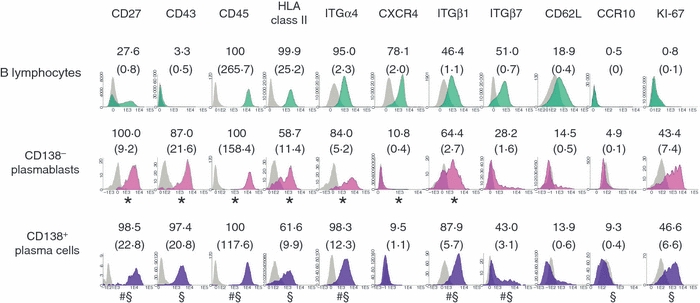
Expression of activation and homing molecules by peripheral blood (PB) mobilized B lymphocytes and mobilized CD138− plasmablasts or CD138+ plasma cells. Detailed immunophenotypic profiles of B lymphocytes and both CD138− plasmablasts and CD138+ plasma cells in leukapheresis samples from healthy donors analysed by multiparameter flow cytometry were obtained. The phenotypic profiles of B lymphocytes and both CD138− plasmablasts and CD138+ plasma cells were defined after gating on CD19+CD20+CD38−/+CD138− B lymphocytes (green histograms), and both CD19−/+CD20−CD38++CD138− (pink histograms) and CD19−/+CD20−CD38++CD138+ (purple histograms) cells, respectively. Grey histograms display the corresponding negative control for the same cell populations. Data from one experiment representative of between six and 30 experiments are shown. Values displayed in each panel indicate the mean percentage of positive cells from the gated cell population and the mean staining index obtained for each specific monoclonal antibody (mAb) used is shown in brackets. *, # or § indicates a significant difference in the staining index (P≤ 0.05; paired Student’s t-test) between B lymphocytes and CD138− plasmablasts (*), between CD138− plasmablasts and CD138+ plasma cells (#), and between B lymphocytes and CD138+ plasma cells (§).
Regarding homing receptors, CD138+ PCs displayed a higher SI (versus CD138− plasmablasts) for the α4 integrin (2·4 SI fold increase; n = 6; P= 0.002) and CXCR4 was systematically absent on both mobilized CD138− plasmablasts and CD138+PCs while positive on B lymphocytes present in the same sample; CD138− plasmablasts and CD138+PCs constantly expressed ITGβ1 and variable levels of ITGβ7, whereas CD62L was poorly expressed on mobilized CD138− plasmablasts and CD138+PCs. Finally, both mobilized CD138− plasmablasts and CD138+PCs were constantly negative for CXCR5, CCR2, CCR10, VCAM1 (CD106), α5 integrin (CD49e), LFA-3 (CD58) and CD70, as well as for the CD56 and CD117 markers, which are aberrantly expressed by malignant PCs from a variable proportion of myeloma patients (data not shown).18 Based on KI-67 antibody staining of cycling cells, mobilized B lymphocytes showed a quiescent KI-67-negative phenotype (0·8 ± 0·3% KI-67+cells) while mobilized CD138− plasmablasts or CD138+PCs displayed an activated phenotype with 43·4 ± 30·1% and 46·6 ± 31·0% KI-67+cells, respectively (n = 6; P≤ 0.02; Fig. 2).
Number of grafted plasma cells and CD34+ cells
Median values of 34 × 106 PCs, 3875 × 106 B lymphocytes and 509 × 106 CD34+ cells were collected in one leukapheresis product, in the absence of a direct correlation between the PC, B-lymphocyte and CD34 cell counts in leukapheresis products (Table 1; n = 26). Median values of 0·4 × 106 PCs/kg, 55·3 × 106 B lymphocytes/kg and 5·8 × 106 CD34+ cells/kg were infused into the patients for allogenic stem cell transplantation (Table 1).
Discussion
What is the organ origin of the circulating PCs? After their generation in the lymph nodes, newly generated PCs exit into the lymphatic system and then the PB and home mainly to the BM, spleen or MALT.1 Whereas some evidence exists indicating that BM HSCs and PCs share the same niche in mice, this has not been demonstrated in humans. It is noteworthy that the percentage of CD34+ HSCs in the BM was similar to that of BM PCs (i.e. 0·5%), as were the counts of circulating CD34+ cells and PCs (Table 1). Regarding CD34+ HSCs, the treatment of healthy individuals with G-CSF results in two processes: a 3-fold amplification of the pool of BM CD34+ HSCs 19, and the mobilization of these BM HSCs into the PB. This resulted in a 44-fold increase in the counts of circulating CD34+ cells, while G-CSF treatment increased 4·2–7·0-fold other leucocytes such as PCs and B lymphocytes. This argues against the idea that PCs share the same niche as HSCs in humans. An alternative possibility is that the 6·2-fold difference between the increase in circulating HSCs and that of PCs after G-CSF treatment can be explained by the lack of PC expansion by G-CSF. The effect of a G-CSF treatment on the count of BM PCs has not been reported. As BM PCs, and PCs in general, do not express the G-CSF receptor (see http://amazonia.transcriptome.eu/index.php?zone=PlasmaCell)20,21 and, in con-trast to BM CD34+ HSCs, they do not expand in vitro22, it may be anticipated that G-CSF treatment will not expand BM PCs in vivo. Thus, the increase in circulating PCs could be mainly attributable to mobilization of tissue PCs into the PB. Mobilization of CD34+HSCs is mediated by cleavage of SDF-1 and adhesion molecules by proteases produced by G-CSF-activated BM neutrophils.23 As CXCR4+ PCs are recruited into the BM through SDF-1-expressing cells 12, one could anticipate that cleavage of SDF-1 induced by G-CSF treatment could also release BM PCs into the blood. In addition, MALT PCs are located close to a proliferation inducing ligand-producing neutrophils and SDF-1-producing cells and activation of these MALT neutrophils by G-CSF could also promote the release of PCs from these tissues.24
The PCs that are induced to circulate after G-CSF mobilization displayed a phenotype that was close to that of circulating PCs in healthy individuals in steady-state conditions or to that of PCs generated from memory B cells in vitro.13,20 Comparison of the heavy chain isotype distribution in circulating PCs in steady-state or G-CSF-mobilization conditions indicates that G-CSF mobilization increased the percentage of IgG-circulating PCs (from 31 to 55·3%) and decreased that of IgA-circulating PCs (from 42·0 to 15·3%). The percentage of IgM-circulating PCs remained similar.13 As IgA-secreting PCs home preferentially to mucosae rather than to the BM,1 the decrease in the IgA-secreting PC frequency suggests that mobilized PCs could not preferentially originate from a mucosal site. An interesting feature is the low CD62L expression by mobilized PCs. CD62L plays an important role in leucocyte–endothelial cell interaction. It is essential to mediate lymphocyte adhesion and transmigration into the lymph nodes from high endothelial venules to the parenchyma, and also contributes to the recruitment of leucocytes from the blood to areas of inflammation.25 CD62L is highly expressed by circulating PCs detected in steady-state conditions or 7 days after TT vaccination,13–15 while it is absent on PCs from the BM, spleen or tonsil.14,15 CD62L is also expressed by newly generated PCs in vitro. 20 The role of CD62L in PC migration into the BM is not known, and the homing of mobilized PCs in the BM remains to be demonstrated. The lack of CD62L expression by mobilized PCs suggests that these PCs could originate from the BM or tissue PCs that are induced to recirculate, and they do not correspond to newly generated PCs. These findings, together with the relatively high expression of KI-67 found for mobilized PCs, indicate that these cells are not quiescent and that the mobilization process of tissue PCs into the PB could require activation of BM/tissue PCs and their entry into the G1 cell cycle phase.
The overall number of PCs in a healthy individual has been estimated to be around 109.1 These PCs may survive for decades at least and are responsible for the long-term humoral memory. Based on these calculations, the number of infused PCs would represent around one-thirtieth of the overall PC count in an adult. It is interesting to consider that these cells can home to the BM and other tissues and contribute to maintain some of the donor's humoral memory in the grafted patient.
Acknowledgments
This work was supported by grants from the Ligue Nationale Contre le Cancer (équipe labellisée 2009), Paris, France, from INCA (n° RPT09001FFA), and from MSCNET European strep (N°E06005FF). Cytometry analyses were run on the cytometry platform of the Institute of Research in Biotherapy (http://irb.montp.inserm.fr/en/index.php?page=Plateau&IdEquipe=3, Montpellier Rio Imaging).
Glossary
Abbreviations:
- BM
bone marrow
- HSC
haematopoietic stem cell
- IgLCκ
immunoglobulin light chain kappa
- IgLCλ
immunoglobulin light chain lambda
- m/cyIgLC
membrane and cytoplasmic immunoglobulin light chain
- mAb
monoclonal antibody
- PB
peripheral blood
- PC
plasma cell
- SDF-1
stromal cell-derived factor-1
Disclosures
The authors report no potential conflicts of interest.
Author contributions
AC contributed to the carrying out of the experiments, the design of the research, and the writing of the paper. MPA and AO contributed to the writing of the paper. ML contributed to the carrying out of the experiments. TK, ZYL and JFR provided the donor samples. BK contributed to the design of the research and the writing of the paper.
References
- 1.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–50. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 2.Tarlinton D, Radbruch A, Hiepe F, Dorner T. Plasma cell differentiation and survival. Curr Opin Immunol. 2008;20:162–9. doi: 10.1016/j.coi.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 3.DiLillo DJ, Hamaguchi Y, Ueda Y, Yang K, Uchida J, Haas KM, Kelsoe G, Tedder TF. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol. 2008;180:361–71. doi: 10.4049/jimmunol.180.1.361. [DOI] [PubMed] [Google Scholar]
- 4.Odendahl M, Mei H, Hoyer BF, et al. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood. 2005;105:1614–21. doi: 10.1182/blood-2004-07-2507. [DOI] [PubMed] [Google Scholar]
- 5.Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–18. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- 7.Garrett RW, Emerson SG. Bone and blood vessels: the hard and the soft of hematopoietic stem cell niches. Cell stem cell. 2009;4:503–6. doi: 10.1016/j.stem.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Cashen AF, Lazarus HM, Devine SM. Mobilizing stem cells from normal donors: is it possible to improve upon G-CSF? Bone Marrow Transplant. 2007;39:577–88. doi: 10.1038/sj.bmt.1705616. [DOI] [PubMed] [Google Scholar]
- 9.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–7. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 10.Massberg S, Schaerli P, Knezevic-Maramica I, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–10. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 12.Hargreaves DC, Hyman PL, Lu TT, et al. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med. 2001;194:45–56. doi: 10.1084/jem.194.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caraux A, Klein B, Paiva B, et al. Circulating human B and plasma cells. Age-associated changes in counts and detailed characterization of circulating normal CD138- and CD138 + plasma cells. Haematologica. 2010;95:1016–20. doi: 10.3324/haematol.2009.018689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mei HE, Yoshida T, Sime W, Hiepe F, Thiele K, Manz RA, Radbruch A, Dorner T. Blood-borne human plasma cells in steady state are derived from mucosal immune responses. Blood. 2009;113:2461–9. doi: 10.1182/blood-2008-04-153544. [DOI] [PubMed] [Google Scholar]
- 15.Medina F, Segundo C, Campos-Caro A, Gonzalez-Garcia I, Brieva JA. The heterogeneity shown by human plasma cells from tonsil, blood, and bone marrow reveals graded stages of increasing maturity, but local profiles of adhesion molecule expression. Blood. 2002;99:2154–61. doi: 10.1182/blood.v99.6.2154. [DOI] [PubMed] [Google Scholar]
- 16.Maecker HT, Frey T, Nomura LE, Trotter J. Selecting fluorochrome conjugates for maximum sensitivity. Cytometry A. 2004;62:169–73. doi: 10.1002/cyto.a.20092. [DOI] [PubMed] [Google Scholar]
- 17.Serke S, Sauberlich S, Huhn D. Multiparameter flow-cytometrical quantitation of circulating CD34(+)-cells: correlation to the quantitation of circulating haemopoietic progenitor cells by in vitro colony-assay. Br J Haematol. 1991;77:453–9. doi: 10.1111/j.1365-2141.1991.tb08609.x. [DOI] [PubMed] [Google Scholar]
- 18.Rawstron AC, Orfao A, Beksac M, et al. Report of the European Myeloma Network on multiparametric flow cytometry in multiple myeloma and related disorders. Haematologica. 2008;93:431–8. doi: 10.3324/haematol.11080. [DOI] [PubMed] [Google Scholar]
- 19.Zaucha JM, Knopinska-Posluszny W, Bieniaszewska M, Mysliwski A, Hellmann A. The effect of short G-CSF administration on the numbers and clonogenic efficiency of hematopoietic progenitor cells in bone marrow and peripheral blood of normal donors. Ann Transplant. 2000;5:20–6. [PubMed] [Google Scholar]
- 20.Jourdan M, Caraux A, De Vos J, et al. An in vitro model of differentiation of memory B cells into plasmablasts and plasma cells including detailed phenotypic and molecular characterization. Blood. 2009;114:5173–81. doi: 10.1182/blood-2009-07-235960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Carrour T, Assou S, Tondeur S, et al. Amazonia!: an online resource to google and visualize public human whole genome expression data. Open Bioinformatics J. 2010;4:5–10. [Google Scholar]
- 22.Merville P, Dechanet J, Desmouliere A, Durand I, de Bouteiller O, Garrone P, Banchereau J, Liu YJ. Bcl-2 + tonsillar plasma cells are rescued from apoptosis by bone marrow fibroblasts. J Exp Med. 1996;183:227–36. doi: 10.1084/jem.183.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–81. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 24.Huard B, McKee T, Bosshard C, et al. APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J Clin Invest. 2008;118:2887–95. doi: 10.1172/JCI33760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sperandio M. Selectins and glycosyltransferases in leukocyte rolling in vivo. FEBS J. 2006;273:4377–89. doi: 10.1111/j.1742-4658.2006.05437.x. [DOI] [PubMed] [Google Scholar]


