Abstract
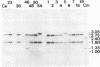
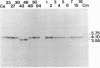
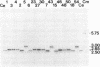
The inheritance of six polymorphic loci mapping in the rRNA-encoding (rDNA) region of the inverted repeat sequence of chloroplast DNA (cpDNA) was scored in hybrid subclones derived from reciprocal interspecific crosses between the green algae Chlamydomonas eugametos and Chlamydomonas moewusii. In order to enhance the detection of cells that had undergone recombination between parental cpDNAs, hybrids were selected that inherited a chloroplast antibiotic-resistance marker contributed by the mating-type-minus(mt-) parent, the parent that normally contributes fewer cpDNA molecules. The major findings of this study can be summarized as follows. (i) The majority of the hybrids (14/17) were recombinant for cpDNA markers in the 10-kilobase-pair rDNA region under study. (ii) Only one allele of each polymorphic cpDNA locus was ever detected in the hybrids, thus suggesting that newly recombined rDNA sequences in one copy of the inverted repeat are rapidly spread to the other by a copy-correction mechanism. (iii) Chloroplast streptomycin-resistance (sr-2) and erythromycin-resistance (er-nM1) loci, although showing little or no genetic linkage, were mapped to the 16S and 23S rRNA gene regions of the cpDNA, respectively, by virtue of their perfect coinheritance with polymorphic markers within these genes. (iv) cpDNA markers associated with a putative intron of the C. eugametos 23S rRNA gene were inherited by all 17 hybrids. Such a result is similar to that observed for certain alleles of the large rRNA gene of yeast mitochondria in crosses between ω+ and ω- strains.
Keywords: chloroplast DNA recombination, DNA polymorphism
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavalier-Smith T. Electron microscopic evidence for chloroplast fusion in zygotes of Chlamydomonas reinhardii. Nature. 1970 Oct 24;228(5269):333–335. doi: 10.1038/228333a0. [DOI] [PubMed] [Google Scholar]
- Chiu W. L., Sears B. B. Recombination between chloroplast DNAs does not occur in sexual crosses of Oenothera. Mol Gen Genet. 1985;198(3):525–528. doi: 10.1007/BF00332951. [DOI] [PubMed] [Google Scholar]
- Dujon B. Sequence of the intron and flanking exons of the mitochondrial 21S rRNA gene of yeast strains having different alleles at the omega and rib-1 loci. Cell. 1980 May;20(1):185–197. doi: 10.1016/0092-8674(80)90246-9. [DOI] [PubMed] [Google Scholar]
- Infanger A., Bertrand H. Inversions and recombinations in mitochondrial DNA of the (SG-1) cytoplasmic mutant in two Neurospora species. Curr Genet. 1986;10(8):607–617. doi: 10.1007/BF00418128. [DOI] [PubMed] [Google Scholar]
- Jacquier A., Dujon B. An intron-encoded protein is active in a gene conversion process that spreads an intron into a mitochondrial gene. Cell. 1985 Jun;41(2):383–394. doi: 10.1016/s0092-8674(85)80011-8. [DOI] [PubMed] [Google Scholar]
- Kuroiwa T., Kawano S., Nishibayashi S., Sato C. Epifluorescent microscopic evidence for maternal inheritance of chloroplast DNA. Nature. 1982 Jul 29;298(5873):481–483. doi: 10.1038/298481a0. [DOI] [PubMed] [Google Scholar]
- Lee R. W., Lemieux C. Biparental Inheritance of Non-Mendelian Gene Markers in CHLAMYDOMONAS MOEWUSII. Genetics. 1986 Jul;113(3):589–600. doi: 10.1093/genetics/113.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux C., Turmel M., Seligy V. L., Lee R. W. Chloroplast DNA recombination in interspecific hybrids of Chlamydomonas: Linkage between a nonmendelian locus for streptomycin resistance and restriction fragments coding for 16S rRNA. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1164–1168. doi: 10.1073/pnas.81.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macreadie I. G., Scott R. M., Zinn A. R., Butow R. A. Transposition of an intron in yeast mitochondria requires a protein encoded by that intron. Cell. 1985 Jun;41(2):395–402. doi: 10.1016/s0092-8674(85)80012-x. [DOI] [PubMed] [Google Scholar]
- Maguire G. A., Docherty K., Hales C. N. Sugar transport in rat liver lysosomes. Direct demonstration by using labelled sugars. Biochem J. 1983 Apr 15;212(1):211–218. doi: 10.1042/bj2120211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella C. A., Lambowitz A. M. Unidirectional gene conversion associated with two insertions in neurospora crassa mitochondrial DNA. Genetics. 1979 Nov;93(3):645–654. doi: 10.1093/genetics/93.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medgyesy P., Fejes E., Maliga P. Interspecific chloroplast recombination in a Nicotiana somatic hybrid. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6960–6964. doi: 10.1073/pnas.82.20.6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mets L. J., Geist L. J. Linkage of a Known Chloroplast Gene Mutation to the Uniparental Genome of CHLAMYDOMONAS REINHARDII. Genetics. 1983 Nov;105(3):559–579. doi: 10.1093/genetics/105.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon P. E., Nicolas P., Schürmann P., Stutz E. Streptomycin-resistance of Euglena gracilis chloroplasts: identification of a point mutation in the 16S rRNA gene in an invariant position. Nucleic Acids Res. 1985 Jun 25;13(12):4299–4310. doi: 10.1093/nar/13.12.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers A. M., Grant D. M., Rabert D. K., Harris E. H., Boynton J. E., Gillham N. W. Mutants of Chlamydomonas reinhardtii with physical alterations in their chloroplast DNA. Plasmid. 1982 Mar;7(2):133–151. doi: 10.1016/0147-619x(82)90073-7. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D., Malnoe P. Anatomy of the chloroplast ribosomal DNA of Chlamydomonas reinhardii. Cell. 1978 Oct;15(2):661–670. doi: 10.1016/0092-8674(78)90034-x. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D., van Dillewijn J. Transformation of the green alga Chlamydomonas reinhardii with yeast DNA. Nature. 1982 Mar 4;296(5852):70–72. doi: 10.1038/296070a0. [DOI] [PubMed] [Google Scholar]
- Sager R. Genetic analysis of chloroplast DNA in Chlamydomonas. Adv Genet. 1977;19:287–340. doi: 10.1016/s0065-2660(08)60247-3. [DOI] [PubMed] [Google Scholar]
- Sigmund C. D., Ettayebi M., Morgan E. A. Antibiotic resistance mutations in 16S and 23S ribosomal RNA genes of Escherichia coli. Nucleic Acids Res. 1984 Jun 11;12(11):4653–4663. doi: 10.1093/nar/12.11.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sor F., Fukuhara H. Erythromycin and spiramycin resistance mutations of yeast mitochondria: nature of the rib2 locus in the large ribosomal RNA gene. Nucleic Acids Res. 1984 Nov 26;12(22):8313–8318. doi: 10.1093/nar/12.22.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausberg R. L., Butow R. A. Gene conversion at the var1 locus on yeast mitochondrial DNA. Proc Natl Acad Sci U S A. 1981 Jan;78(1):494–498. doi: 10.1073/pnas.78.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn A. R., Butow R. A. Nonreciprocal exchange between alleles of the yeast mitochondrial 21S rRNA gene: kinetics and the involvement of a double-strand break. Cell. 1985 Apr;40(4):887–895. doi: 10.1016/0092-8674(85)90348-4. [DOI] [PubMed] [Google Scholar]