Abstract
The selective alteration of the cellular genome by laser microbeam irradiation has been extensively applied in cell biology. We report here the use of the third harmonic (355 nm) of an yttrium-aluminum garnet laser to facilitate the direct transfer of the neo gene into cultured human HT1080-6TG cells. The resultant transformants were selected in medium containing an aminoglycoside antibiotic, G418. Integration of the neo gene into individual human chromosomes and expression of the gene were demonstrated by Southern blot analyses, microcell-mediated chromosome transfer, and chromosome analyses. The stability of the integrated neo gene in the transformants was shown by a comparative growth assay in selective and nonselective media. Transformation and incorporation of the neo gene into the host genome occurred at a frequency of 8 X 10(-4)-3 X 10(-3). This method appears to be 100-fold more efficient than the standard calcium phosphate-mediated method of DNA transfer.
Full text
PDF

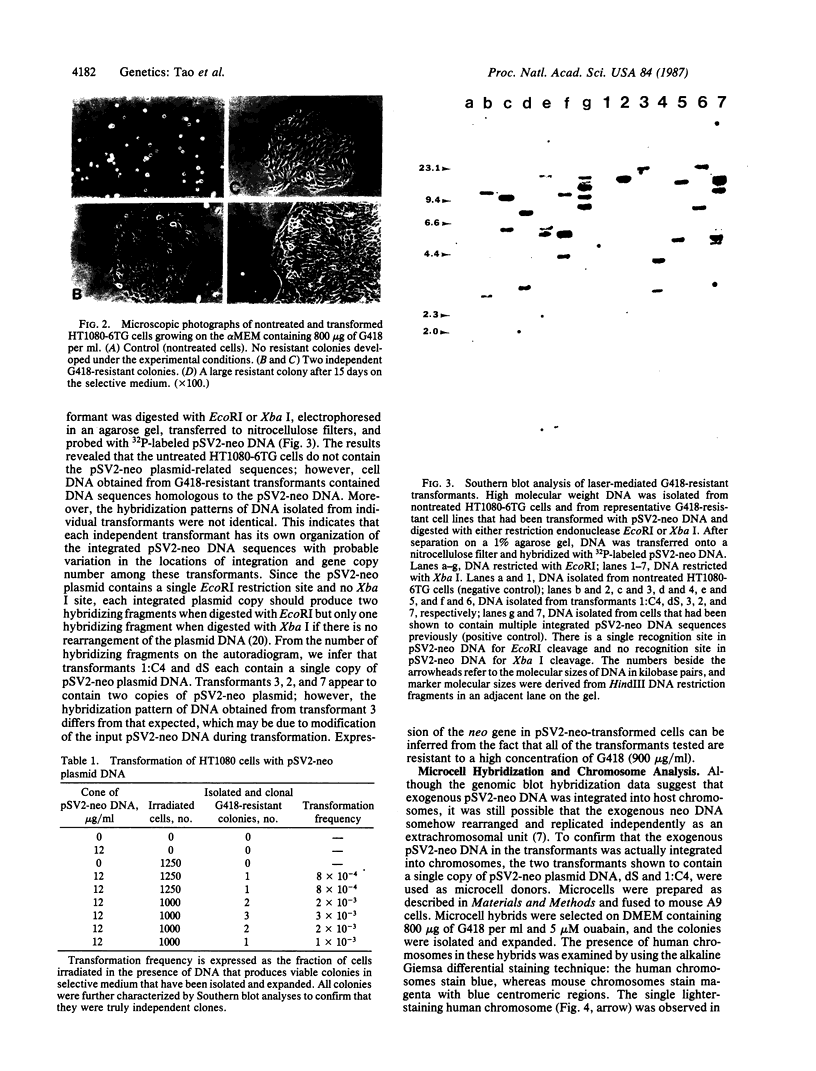
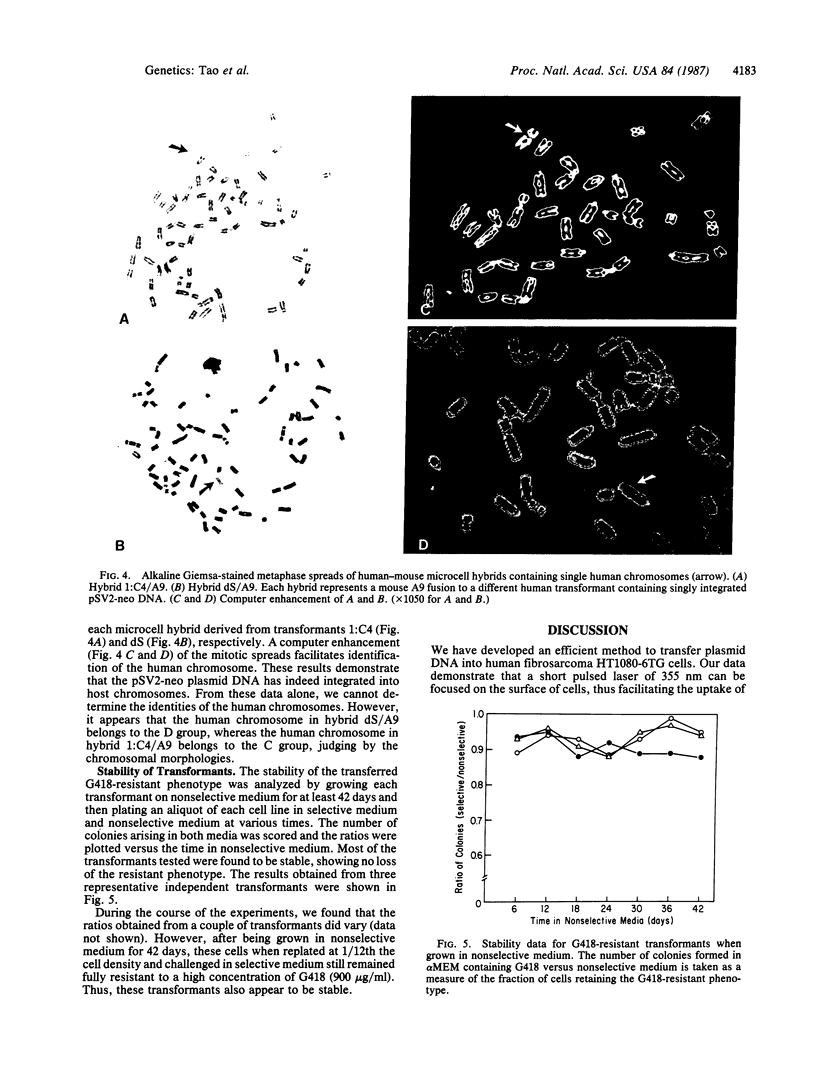

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berns M. W., Aist J., Edwards J., Strahs K., Girton J., McNeill P., Rattner J. B., Kitzes M., Hammer-Wilson M., Liaw L. H. Laser microsurgery in cell and developmental biology. Science. 1981 Jul 31;213(4507):505–513. doi: 10.1126/science.7017933. [DOI] [PubMed] [Google Scholar]
- Berns M. W., Chong L. K., Hammer-Wilson M., Miller K., Siemens A. Genetic microsurgery by laser: establishment of a clonal population of rat kangaroo cells (PTK2) with a directed deficiency in a chromosomal nucleolar organizer. Chromosoma. 1979 Jun 21;73(1):1–8. doi: 10.1007/BF00294839. [DOI] [PubMed] [Google Scholar]
- Berns M. W. Directed chromosome loss by laser microirradiation. Science. 1974 Nov 22;186(4165):700–705. doi: 10.1126/science.186.4165.700. [DOI] [PubMed] [Google Scholar]
- Brenner S., Branch A., Meredith S., Berns M. W. The absence of centrioles from spindle poles of rat kangaroo (PtK2) cells undergoing meiotic-like reduction division in vitro. J Cell Biol. 1977 Feb;72(2):368–379. doi: 10.1083/jcb.72.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R. High efficiency transformation by direct microinjection of DNA into cultured mammalian cells. Cell. 1980 Nov;22(2 Pt 2):479–488. doi: 10.1016/0092-8674(80)90358-x. [DOI] [PubMed] [Google Scholar]
- Croce C. M. Loss of mouse chromosomes in somatic cell hybrids between HT-1080 human fibrosarcoma cells and mouse peritioneal macrophages. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3248–3252. doi: 10.1073/pnas.73.9.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Friend K. K., Chen S., Ruddle F. H. Differential staining of interspecific chromosomes in somatic cell hybrids by alkaline Giemsa stain. Somatic Cell Genet. 1976 Mar;2(2):183–188. doi: 10.1007/BF01542631. [DOI] [PubMed] [Google Scholar]
- Graessmann M., Graessman A. "Early" simian-virus-40-specific RNA contains information for tumor antigen formation and chromatin replication. Proc Natl Acad Sci U S A. 1976 Feb;73(2):366–370. doi: 10.1073/pnas.73.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Klobutcher L. A., Miller C. L., Ruddle F. H. Chromosome-mediated gene transfer results in two classes of unstable transformants. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3610–3614. doi: 10.1073/pnas.77.6.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield J. W. The use of drug-resistant markers to study the hybridization of mouse fibroblasts. Exp Cell Res. 1966 Jan;41(1):190–196. doi: 10.1016/0014-4827(66)90558-1. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L., Hammer R. E., Trumbauer M. E., Rosenfeld M. G., Birnberg N. C., Evans R. M. Dramatic growth of mice that develop from eggs microinjected with metallothionein-growth hormone fusion genes. Nature. 1982 Dec 16;300(5893):611–615. doi: 10.1038/300611a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Wigler M. Genetic and physical linkage of exogenous sequences in transformed cells. Cell. 1980 Nov;22(1 Pt 1):309–317. doi: 10.1016/0092-8674(80)90178-6. [DOI] [PubMed] [Google Scholar]
- Peterson S. P., Berns M. W. Evidence for centriolar region RNA functioning in spindle formation in dividing PTK2 cells. J Cell Sci. 1978 Dec;34:289–301. doi: 10.1242/jcs.34.1.289. [DOI] [PubMed] [Google Scholar]
- Rasheed S., Nelson-Rees W. A., Toth E. M., Arnstein P., Gardner M. B. Characterization of a newly derived human sarcoma cell line (HT-1080). Cancer. 1974 Apr;33(4):1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Saxon P. J., Srivatsan E. S., Leipzig G. V., Sameshima J. H., Stanbridge E. J. Selective transfer of individual human chromosomes to recipient cells. Mol Cell Biol. 1985 Jan;5(1):140–146. doi: 10.1128/mcb.5.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffl F., Baumann G. Thermo-induced transcripts of a soybean heat shock gene after transfer into sunflower using a Ti plasmid vector. EMBO J. 1985 May;4(5):1119–1124. doi: 10.1002/j.1460-2075.1985.tb03748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Walter R. J., Berns M. W. Computer-enhanced video microscopy: digitally processed microscope images can be produced in real time. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6927–6931. doi: 10.1073/pnas.78.11.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Zambryski P., Joos H., Genetello C., Leemans J., Montagu M. V., Schell J. Ti plasmid vector for the introduction of DNA into plant cells without alteration of their normal regeneration capacity. EMBO J. 1983;2(12):2143–2150. doi: 10.1002/j.1460-2075.1983.tb01715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]