Abstract
Face processing undergoes a fairly protracted developmental time course but the neural underpinnings are not well understood. Prior fMRI studies have only examined progressive changes (i.e., increases in specialization in certain regions with age), which would be predicted by both the Interactive Specialization (IS) and maturational theories of neural development. To differentiate between these accounts, the present study also examined regressive changes (i.e., decreases in specialization in certain regions with age), which is predicted by the IS but not maturational account. The fMRI results show that both progressive and regressive changes occur, consistent with IS. Progressive changes mostly occurred in occipital-fusiform and inferior frontal cortex whereas regressive changes largely emerged in parietal and lateral temporal cortices. Moreover, inconsistent with the maturational account, all of the regions involved in face viewing in adults were active in children, with some regions already specialized for face processing by 5 years of age and other regions activated in children but not specifically for faces. Thus, neurodevelopment of face processing involves dynamic interactions among brain regions including age-related increases and decreases in specialization and the involvement of different regions at different ages. These results are more consistent with IS than maturational models of neural development.
Beginning in infancy, the face is an important social stimulus. Infants show a preference for the face versus other stimuli soon after birth (Cassia, Turati, & Simion, 2004) and, early on, infants discriminate their mothers’ faces from those of strangers (Bushnell, Sai, & Mullin, 1989). However, typical face processing in infancy differs from typical face processing in adulthood in terms of both perceptual and social information processing (Bhatt, Bertin, Hayden, & Reed, 2005; Carver et al., 2003).
In addition, face processing improves with age in childhood (Pascalis & Slater, 2003) and may not reach adult-like levels until well into adolescence (Carey, Diamond, & Woods, 1980; Ellis, Shepard, & Bruce, 1973). However, there is debate about the exact nature of the developmental change (Carey & Diamond, 1994; McKone & Boyer, 2006; Mondloch, Geldart, Maurer, & Le Grand, 2003; Pellicano, Rhodes, & Peters, 2006). Earlier studies suggested that children are more sensitive to facial features than to the spacing among features (second-order structure) and that second-order processing takes more time to mature (Carey & Diamond, 1994; Schwarzer, 2000). However, recent findings show that even infants as young as five months of age are sensitive to second-order structure in faces (Bhatt, Bertin, Hayden, & Reed, 2005). Moreover, when floor and ceiling effects are controlled, young children do not show particular deficits in second-order processing (McKone & Boyer, 2006). Although this debate continues, it is widely accepted that face processing continues to develop throughout childhood. Therefore, the present study focuses on developmental changes specifically in the time window of 5 to 12 years of age.
Recent functional magnetic resonance imaging (fMRI) studies on the development of face processing (Grill-Spector, Golarai, & Gabrieli, 2008) have largely focused on age-related changes in the fusiform face area (FFA; Kanwisher, McDermott, & Chun, 1997). Nearly all of these studies report an increase in specialization of the FFA for faces from childhood to adulthood (Aylward et al., 2005; Golarai et al., 2007; Passarotti, Smith, DeLano, & Huang, 2007; Scherf et al., 2007). Children younger than 7–8 years of age do not show strong responses to faces in the FFA, and when the FFA is detected in younger children, it is smaller than in adults or older children (Gathers, Bhatt, Corbly, Farley, & Joseph, 2004; Golarai et al., 2007; Passarotti et al., 2003; Scherf, Behrmann, Humphreys, & Luna, 2007). Importantly, some studies (Golarai et al., 2007; Scherf et al., 2007) report no increases in specialization in other functionally defined brain regions like the occipital face area (OFA; Rossion et al., 2003) or the lateral occipital complex (LOC; Malach et al., 1995), suggesting that these regions already show a preferential response for the relevant category in childhood. Nevertheless, because younger children do not appear to strongly activate the FFA in response to faces, developmental fMRI studies should attempt to characterize the entire set of brain regions or networks that are recruited in younger children for face processing, rather than focusing only on the FFA. To address this, the present study examines patterns of activation for face processing throughout the brain in younger and older children and adults.
Examining an extended network for face processing can also help discriminate among theoretical accounts of brain development, like the maturational and interactive specialization (IS) accounts described by Johnson (2005). The major features of the two views that are addressed in the present study are outlined in Table 1. In the maturational account, the emergence of a specific cognitive function in a brain region depends on gene expression in that region. Johnson notes that in the maturational view “circuits that support components of the adult system are assumed to come ‘on-line’ at various ages” and that “specific cognitive mechanisms are either present or absent at a given age” (p. 14). Johnson also notes that the maturational view assumes a one-to-one correspondence between brain region and cognitive function. With respect to face processing, the maturational view would predict that regions specialized for face processing in adults emerge based on the combined influence of gene expression and experience on cortical circuitry (Johnson, 2005, 2007), with the influence of experience less emphasized by some authors (Kadosh & Johnson, 2007). In either case, the specialized function of particular brain regions emerges over time in a linear, deterministic fashion, assuming the involved brain regions remain intact throughout development.
Table 1.
Predictions for the Interactive Specialization and Maturational viewpoints
| Viewpoint(s) | Feature | Prediction for developmental fMRI studies of face processing | Supported by previous studies? | Supported by present study? |
|---|---|---|---|---|
| Maturational and IS | Increased tuning of a brain region for preferred stimulus or cognitive function | 1. Specialization for faces increases with age (p. 168 for IS view); i.e., progressive changes with development. | Yes, specialization index increases with age | Yes, specialization index increases with age |
| Maturational | One-to-one correspondence between brain structure and function | 2. Only one or a few regions show increased specialization for face processing with development (p. 16) | Yes, but only tested a few regions | No, regions other than the FFA and OFA are specialized for face processing and show developmental changes |
| The brain-to-function mapping is static over development | 3. A region will either be associated with face processing or not associated with face processing across development (p. 14); hence, minimal to no activation in a region is expected prior to specialization | Not adequately tested because a specialization index of 0 cannot distinguish between lack of activation and activation that is broadly tuned (i.e., not specific for faces) | No, because no regions showed absence of activation in childhood followed by face specialized activation in adulthood | |
| IS | Brain networks or systems support a given cognitive function | 4. Multiple regions will show specialization for faces with development (p. 16) | Not adequately tested because only a few brain regions have been investigated (typically) | Yes, multiple brain regions show specialization for faces |
| The brain-function mapping is dynamic over development | 5. Regions that show specialization for face processing in adults may be partially activated in a wide variety of circumstances in children prior to specialization (p. 16) | Not adequately tested because a specialization index of 0 cannot distinguish between lack of activation and activation that is broadly tuned (i.e., not specific for faces) | Yes, because brain regions that are specialized for faces in adults show non-specific activation in childhood (i.e., associated with different cognitive functions) | |
| The same behavior is supported by different neural substrates at different ages during development | 6. Different regions or networks may show specialization for faces in children and adults (p. 167); regions specialized in children will show regressive changes and the brain will eventually develop into the adult pattern | Not tested because no prior studies have examined regressive changes | Yes, regressive changes were observed |
Evidence for the maturational viewpoint is provided in a case study of a 16-year old boy who suffered brain damage at one day of age in the occipital and occipito-temporal cortex (Farah, Rabinowitz, Quinn, & Liu, 2000). The child never developed typical face recognition capabilities despite years of experience with faces and otherwise normal development. His object recognition capabilities, in contrast, were intact. Farah et al. concluded that “the human genome contains sufficiently explicit information about faces and nonface objects [and] experience with these categories is not necessary for their functional delineation and differential brain localization” (p. 117). This study supports one aspect of the maturational viewpoint–that brain regions involved in face processing are specified in the genome. If those regions are damaged, then even years of experience with faces cannot override the original loss of integrity of that region. Hence, specialization for faces will never develop.
Johnson (2005) notes that the maturational viewpoint has dominated much of the field in terms of relating developmental changes in behavior to brain function. We note that focusing only on regions like the FFA and not on large-scale activation patterns is also more in line with the maturational view than with other accounts of brain development. In contrast to the maturational view, the IS account posits that all regions of a network (e.g., the face processing network) are at least partially activated early on and may initially serve as a general purpose mechanism for processing many types of stimuli (de Haan, Humphreys, & Johnson, 2002; Johnson, 2001). In addition, “different brain regions may be critical for the same task at different ages” (Johnson, 2005, p. 11). According to the IS account, cortical specialization results from activity-dependent interactions among regions as they “compete with each other to acquire their role in new computational abilities” (Johnson, 2005, p. 16). The IS account also predicts that the emergence of a new cognitive function will be the result of changes in activity over numerous brain regions rather than only a few regions, as in the maturational account.
As noted earlier, most studies have focused on developmental increases in specialization for faces in specific circumscribed regions (e.g., FFA or OFA). This approach, however, does not adequately test some critical assertions of the IS account. First, the IS account stresses that specialization for certain cognitive functions is the result of the interaction of many brain regions. With development, acquisition of a new cognitive computation involves functional reorganization so that “ … the same behavior could potentially be supported by different neural substrates at different ages during development” (p. 167; Johnson, 2005). This implies that children could recruit different regions from adults for face-specialized processing (Table 1, Prediction 4). Few developmental fMRI studies of face processing have examined brain regions that are activated in children but not in adults (cf. Gathers et al., 2004; Passarotti et al., 2003), yet this is a critical prediction of the IS account. The maturational account assumes specialized cognitive functions unfold over time in one or a few brain regions (Table 1, Prediction 2). Consequently, children would not recruit different regions from adults in this view but may, instead, “have reduced versions of the adult mind” (Johnson, 2005, p. 13).
Second, simply showing that a particular region like the FFA becomes more specialized with age is also not a sufficient test of the IS account because the maturational account would make the same prediction (Table 1, Prediction 1). One of the critical distinctions is characterizing the response of the region prior to specialization in adulthood. If maturation of a brain region occurs in the time window tested in a given study, the region might be expected to show minimal or no activation in childhood prior to specialization in adulthood, which would be consistent only with the maturational view (Table 1, Prediction 3). The IS account would predict that a brain region is activated in some fashion, albeit non-specifically, in childhood prior to specialization of the region in adulthood (Table 1, Prediction 5). However, this outcome could also be supported by the maturational view if maturation of the region was complete or nearly complete by the age of the youngest participants in the study. Therefore, Prediction 5 in Table 1 would not necessarily differentiate the two accounts, whereas Prediction 3 (i.e., no activation prior to specialization) would only be consistent with the maturational view.
We note that a specialization index (as used in some prior developmental studies) cannot distinguish between non-specific and absence of activation in a region. A specialization index of 0 (or close to 0) could reflect either outcome. Therefore, approaches are needed to make the critical distinctions among (1) lack of activation in children, (2) non-specific but significant activation in children, and (3) specialized activation in children (albeit less specialized than in adults). The present study will make these critical distinctions.
The present goal is to offer operational definitions for specific assertions of the IS and maturational accounts of brain development. To make the critical distinctions outlined above, the present study will compare fMRI signal for each condition relative to every other condition and relative to baseline. Specialization for faces is defined in two ways: “face-preferential” and “face-specific.” With a “face-preferential” response, a brain region shows a significantly greater response to faces than to either natural or manufactured objects and a greater response relative to baseline. A preferential response, as used in the present study, is most similar to the response measured by the first paper that defined the FFA (Kanwisher et al., 1997) in which the FFA was determined by the contrast of faces versus non-face objects. In addition to including this contrast, a “preferential” response, as presently defined, goes one step further by eliminating voxels in which the face response does not yield a greater response than the resting baseline condition (Joseph, Partin, & Jones, 2002).
On the surface, the present definition of face-preferential responses may not seem very restrictive. However, Gathers et al. (2004) systematically explored different definitions of specialized activation, including “preferential” activation. In these studies, “selective” activation was defined as a greater response to faces than to all other categories tested and baseline, and no differential response among the other categories and baseline. This very stringent definition does not yield the FFA, even in adults (Joseph & Gathers, 2002). Nevertheless, the present study will also explore a more restrictive definition of specialized activation than face-preferential but less restrictive than face-selective activation. We term this profile of activation “face-specific.” A face-specific response requires that faces activate a region greater than baseline and greater than both natural and manufactured objects (cf. face-preferential activation which requires activation greater than only one of the two non-face categories). In the present study, the FFA does not show a face-specific response but does show a face-preferential response in adults. Therefore, we use face-preferential activation to isolate brain regions in all age groups because it is more inclusive and yields regions like the FFA which can be investigated further with additional analyses. In addition, using a “preferential” response to isolate regions provides a basis for more brain regions to explore in children, who may not show the same degree of specialization as adults.
The present paper also explores activation profiles other than face-preferential and face-specific. It is important to distinguish between “no activation” in a brain region versus “non-specific” activation. We define “not activated” as absence of a statistically significant response above baseline to any of the conditions in the study (face, natural or manufactured objects). We also define two types of non-specific activation. “Conjoined” activation is defined as statistically significant activation for faces, natural and manufactured objects relative to baseline, but no statistically significant differences among faces, natural and manufactured objects (Joseph & Gathers, 2002). Conjoined activation is necessarily mutually exclusive with both face-specific and face-preferential activation because faces must be greater than at least one other category with face-specific or face-preferential activation but this cannot be the case for conjoined activation. A second type of non-specific activation (which we will term “non-specific”) is defined as a response to any single category (face, natural or manufactured objects) relative to baseline, but the activation does not meet the stricter criteria for a face-preferential, face-specific or conjoined response. This means that the brain region is activated in some fashion but not preferentially for faces and not equivalently for all categories. As an example, a region could show a response to faces greater than baseline, but this would not qualify as a face-preferential or face-specific response because the activation is not also greater than at least one other category. Nevertheless, the brain region is activated in some fashion whereas with “not activated” status, no condition activates the region greater than baseline.
Once the brain regions are characterized as face-specific, face-preferential, conjoined, non-specific, or non-activated, we also will examine changes in specialization with age using a specialization index similar to what has been used in other studies (Golarai et al., 2007). The present analysis will extend previous findings by characterizing the changes across age as either progressive or regressive. A progressive change means that the face-specialization index in a brain region increases with age; a regressive change means that the face-specialization index decreases with age. Most developmental fMRI studies of face processing to date have only examined progressive changes, but observations in other cognitive domains like lexical processing have noted that both progressive and regressive changes occur across development (e.g., Brown et al., 2005). Most importantly, the maturational account would predict a shift from minimal or no activation in childhood to specialized activation in adulthood, or progressive changes in most brain regions recruited for face processing (Table 1, Prediction 1). In contrast, the IS account would predict significant reorganization from childhood to adulthood such that both progressive and regressive changes might emerge in multiple brain regions, as a reflection of regions competing to support specific cognitive functions. Within the time window of development explored in the present study, progressive changes such as increases in myelination and white matter occur (De Bellis et al., 2001; Giedd, 2004; cf. Nagel et al., 2006) and regressive changes, such as decreases in gray matter volume or synaptic density, have also been reported in certain brain regions (Gogtay et al., 2004; Sowell et al., 2004). Although fMRI BOLD responses are not a direct measure of these cellular changes, it is important to characterize large-scale brain activation changes throughout development to set the stage for linking BOLD activation to potential underlying cellular events.
Many definitions of specialization have been used in the literature and conclusions about the development of specialized neural substrates for face processing will depend heavily upon the definition that is adopted. A specialization index typically reflects the difference in percent fMRI signal change to faces and non-faces, scaled to the total response for faces and non-faces (Golarai et al., 2007). In the present study, we will use a specialization index that reflects the incremental difference in fMRI signal for faces versus non-faces and baseline scaled to the average response for non-faces and baseline:
This formula uses mean intensity values rather than percent signal change values based on a recent criticism of selectivity indices (Simmons, Bellgowan, & Martin, 2007) that points out that when using percent signal change values (which can be negative), adjustments to baseline are necessary to avoid inflated estimates of specialization. The present formula uses mean intensity values, which are positive. In addition, the formula includes the baseline response in the calculation, thereby addressing concerns of adjusting for baseline responses (see Methods for validation of this formula)
This index can be likened to a percent signal change for faces versus all other conditions. Consequently, an index of 0 would mean that faces are not activated greater than all of the other conditions and baseline combined. We note that the present index will only be applied to regions that have already been defined as having a face-specific or face-preferential response (in at least one age group) in the first analysis phase. Because none of the regions in the present study showed a significantly greater response to a non-face category than to faces, the present specialization index gives a measure of the degree of preferential response to faces compared to all other conditions combined.
With all of these operational definitions in place, the main predictions are provided in Table 1. Outcomes that are consistent with either account are that specialization for faces in regions like the FFA will increase with age (Prediction 1) and that earlier in development, regions like the FFA could be partially or non-specifically activated (Prediction 5). For Prediction 1, we expect an increase in the specialization index with age (progressive changes) and for Prediction 5, children will show conjoined or non-specific activation for regions that show face-preferential or face-specific responses in adulthood. Outcomes that are consistent with the maturational account are that one or only a few regions will show increased specialization for faces with age (Prediction 2) and a region specialized for faces in adulthood could be non-activated in childhood (Prediction 3). Specifically, Prediction 3 would suggest that a region will show no activation greater than baseline for any condition in childhood prior to showing face-preferential or face-specific activation in adulthood. Outcomes that are consistent with the IS account are that multiple regions will show specialization for faces throughout development (Prediction 4) and that different regions or networks of regions could be more strongly activated in childhood than in adulthood–these regions will show regressive changes and no longer be specialized for face processing by adulthood (Prediction 6). Thus, Prediction 6 suggests that the specialization index will decrease with age as an index of regressive changes. We will also explore the different types of regressive change–do the regions that show face-preferential or face-specific responses in childhood become obsolete for face processing in adulthood (i.e., are not activated in adulthood), show partial activation (i.e., show non-specific or conjoined activation in adulthood) or simply decrease in degree of specialization (i.e., could still show a face-preferential or face-specific response but less than that observed in childhood)?
Method
Participants
Fifty-seven 5–12 year old children (31 males, mean age 8.8 years) and 43 adults (20 males, mean age 26.9 years, 39 right-handed) with no significant medical histories or conditions were compensated for their participation. Four children did not complete the study. Data from six other children were omitted due to excessive head motion (see criteria below). Data from the remaining 47 children (22 males, mean age of 9.3 years, 42 right-handed) were divided into two age groups, based on the median age: older children (9.8–12 years; n=24) and younger children (5–9.7 years; n=23). All children had normal visual acuity, were fluent in English, with receptive and expressive language skills within normal age limits according to the Peabody Picture Vocabulary Test (Dunn & Dunn, 1997) and the Expressive Vocabulary Test (Williams, 1997). Children provided written assent accompanied by parental consent and adults provided written consent in accord with the guidelines established by the university’s Institutional Review Board.
Design
The 9-minute face localizer task consisted of nine task blocks (three each of human faces, F, natural objects, N, and manufactured objects, M) interleaved with eight fixation blocks (17.8s each). Within each task block, 30 randomly-ordered, gray-scale stimuli appeared for 1000 ms each followed by a fixation cross for 400 ms. Face stimuli were 30 photographs scanned from a high school yearbook. Photos of 30 natural and 30 manufactured objects were acquired from different sources (Photodisc CD, Photodisc, Seattle, WA, MacMillan Visual Dictionary, Corbeil & Archambault, 1992). Fixation stimuli consisted of a black crosshair centered on a white background. Participants were isntructed to press a button with their index finger each time they viewed a stimulus, but not to press the button during fixation blocks. Stimuli were projected onto a screen using a high-resolution LCD projector connected to a Dell computer running E-prime 1.0 (Psychology Software Tools, Pittsburg, PA). Participants viewed the stimuli (visual angle of 6.74 degrees) through a mirror attached to the head coil. Because the data used in the present analysis were pooled from previous experiments, some of the participants performed an object-matching task in separate functional runs in addition to the face localizer task (Gathers et al., 2004) whereas other participants performed an additional face-matching and object-matching task (Joseph, Gathers et al., 2006). However, only the data from the face localizer run were used in the present analysis.
fMRI Data Acquisition
Data were collected using a Siemens Vision 1.5 Tesla magnet equipped with a quadrature head coil and T2*-weighted gradient echo sequence. Seven brain volumes per task block and three brain volumes per fixation block consisted of 46 axial slices acquired in ascending order to allow for whole brain coverage (TR = 6000 ms, TE = 40 ms, flip angle = 90°, 64 × 64 matrix, FOV = 228 × 228 mm2, thickness = 3mm, gap = 0.6 mm). High-resolution T1-weighted MP-RAGE anatomical scans (150 sagittal slices 1 mm thick for adults and 76 sagittal slices 2 mm for the children, FOV = 256 × 256 mm2) were also collected for each participant.
fMRI Data Analysis
Using FMRIB’s FSL package (http://www.fmrib.ox.ac.uk/fsl), images in each participant’s time series were motion corrected and functional runs were discarded when uncorrected head motion exceeded half a voxel (1.7 mm). As reported in the studies from which the present data were pooled, corrected head motion did not differ between adults and children (Gathers et al., 2004; Joseph, Gathers et al., 2006). Images in the data series were spatially smoothed with a 3D Gaussian kernel (FWHM = 7.5 mm), and temporally smoothed using a high-pass filter (360 seconds).
Customized square waveforms (on/off) representing each of the three conditions (F, Faces, M, Manufactured, N, Natural) were convolved with a double-gamma hemodynamic response function. Hemodynamic parameters were estimated for each explanatory variable (F, M, N) and statistical contrast maps of interest (F>M, F>N, F>fixation, N>M, N>F, N>fixation, M>N, M>F, M>fixation,) were generated. The six movement parameters (3 rotation values in radians and 3 translation values in millimeters) were added as covariates of no interest to model the variance in the fMRI signal induced by the head motion. Contrast maps were normalized into common stereotaxic space using linear registration before mixed-effects group analyses were performed.
We conducted one group analysis for each of the three age groups. The group contrast maps were then logically combined (Joseph et al., 2002) at the voxel level to yield face-preferential regions of interest. Each of nine group statistical maps (Table 2) was thresholded at an uncorrected probability of .05 for a one-tailed test, converted into binary masks then combined using fslmaths. This yielded voxels in which faces elicited more activation than fixation and faces showed greater activation than either natural objects or manufactured objects or both. Cluster detection (Xiong, Gao, Lancaster, & Fox, 1995) was then applied to the face-preferential maps in each age group such that parameter estimate (PE) values for faces versus fixation differed significantly from zero (p < 0.001, 2-tailed). We only considered smaller regions if that region was part of the core or extended face network (Fairhall & Ishai, 2007).
Table 2.
Alpha Levels used to Test Activation Profiles in the Present Study
| Profile | F>fix | F>M | F>N | M>fix | M>F | M>N | N>fix | N>F | N>M |
|---|---|---|---|---|---|---|---|---|---|
| Face-preferential | <.025 | <.025 | <.025 | na | na | na | na | na | na |
| Face-specific | <.017 | <.017 | <.017 | na | na | na | na | na | na |
| Conjoined | <.008 | >.008 | >.008 | <.008 | na | >.008 | <.008 | na | na |
| Non-specific | <.025 | na | na | <.025 | na | na | <.025 | na | na |
| Not activated | >.025 | >.025 | >.025 | >.025 | >.025 | >.025 | >.025 | >.025 | >.025 |
Note. F, Faces; fix, fixation baseline; M, Manufactured Objects; N, Natural Objects. Underline indicates that any of the underlined conditions could be significant in a given row; otherwise, all other conditions must be significant. “na” means that the particular contrast is not relevant for testing that profile of activation.
In each face-preferential ROI for each age group, mean intensity values for each condition (face, manufactured, natural, and fixation baseline), were extracted for each participant. To determine whether a given region’s response was best characterized as face-preferential, face-specific, conjoined, non-specific or not activated, and whether these profiles differed across age in a given region, pair-wise t-tests were conducted on mean intensity values. Note that a face-preferential region defined in one age group could show a different profile in a different age group and vice-versa. The alpha levels and relevant contrasts needed for each profile of activation are outlined in Table 2. For the face-preferential profile, the F>fixation and either the F>M or the F>N contrast had to be significant at a Bonferroni-corrected alpha level of .025. This alpha level was determined by the fact that at least two of the three relevant contrasts must be significant (alpha = .05/2). Face-specific activation was defined as a significant difference for F>fixation, F>M, and F>N contrasts at an alpha level of .017 (alpha = .05/3). For conjoined activation the F>fixation, M>fixation, N>fixation contrasts all had to be significant at an alpha level of .008 (alpha = .05/6) but the F>M, F>N, M>N contrasts could not be significant at this same alpha level . For non-specific activation, at least one of the contrasts versus baseline (F>fixation, M>fixation, N> fixation) was significant at an alpha level of .025 but neither the F>N nor the F>M contrast was significant at this level nor did the contrasts meet the criteria for face-preferential, face-specific or conjoined. For a non-activated profile, none of the contrasts was significant at .025.
In each region of interest, we also computed a specialization index as described in the introduction. To validate the present formula, we compared the present selectivity index to a baseline adjusted formula as proposed by Simmons et al. (2007) and to the formula that (Golarai et al., 2007) used in their developmental fMRI study of face processing. We used fMRI signal in the right FFA for this purpose. The present specialization index was significantly correlated with the baseline adjusted formula proposed by Simmons et al. [r (90) = .85, p < .0001] but was not correlated with the formula used by Golarai et al. [r (90) = .15, p < .16]. Hence, the specialization index that we presently use is comparable to a more appropriate baseline-adjusted formula.
Results
Behavioral Results
Although the fMRI task involved passive viewing, participants pressed a button at the onset of each picture to indicate they were attending to the picture. An analysis of percent button presses as a function of stimulus type (face, natural, manufactured) and age (adult, older, younger) indicated that younger children (M = 97%, SD = 7%) performed as well as adults (M = 97%, SD = 6%) with older children slightly lower (M = 94%, SD =21%). However, none of the main effects or interactions was significant..
Face-preferential, Face-specific, Conjoined, Non-specific and No activation Profiles
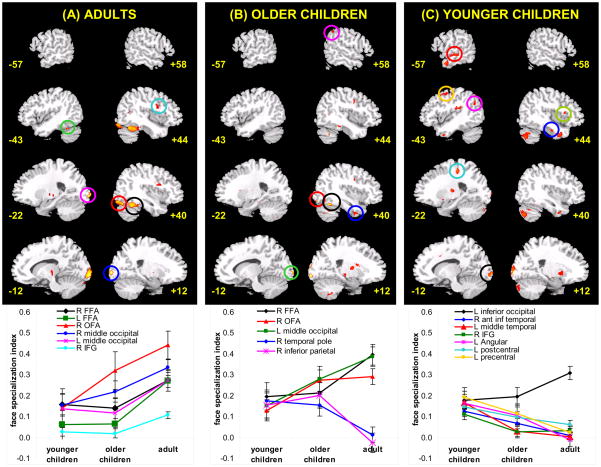
The voxel-wise analysis of face-preferential activation in each age group revealed extended networks of brain regions (Table 3), consistent with other studies (e.g., Blonder et al., 2004; Fairhall & Ishai, 2007; Haxby, Hoffman, & Gobbini, 2000). These regions were used as regions-of-interest (ROIs) in the remaining analyses. Each of the regions in Table 3 showed a face-preferential response for the age group in which it emerged, according to both the voxel-wise analysis and the post-hoc t-tests conducted on mean intensity values. The general distribution of activation in each age group (Figure 1) shows that younger children recruit left hemisphere regions more heavily than do older children and adults. Older children show a distribution of activation more similar to adults than to younger children; however, some features are similar in older and younger children.
Table 3.
Results of the analysis of percent signal change and mean intensity values using logical combination
| Percent Signal Change | Mean Intensity Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MNI coordinates | Age Effect | Condition Effect | Age × Cond Interaction | Response Profile |
||||||
| Region | x | y | z | F(2, 87) | F(2, 174) | F(4, 174) | All | Adult | Older | Younger |
| Adult face-preferential regions | ||||||||||
| R Fusiform (FFA) | 44 | −60 | −22 | 0.7 | 7.2* | 1.9 | pref | |||
| L Fusiform (FFA) | −40 | −52 | −24 | 0.5 | 1.5 | 3.6* | pref | conj | conj | |
| R Inf Occipital (OFA) | 38 | −82 | −14 | 9.5* | 3.3* | 0.5 | pref | |||
| L Fusiform (OFA) | −28 | −82 | −16 | 2.2 | 0.7 | 1.2 | conj | |||
| L Mid Occipital | −22 | −98 | −2 | 12.5* | 16.6* | 0.6 | spec | |||
| R Mid Occipital | 22 | −96 | −2 | 6.9* | 15.3* | 0.8 | spec | |||
| R Inf Oper Frontal | 42 | 2 | 26 | 0.8 | 2.8 | 3.1* | pref | non | non | |
| R Inf Orb Frontal | 32 | 28 | −16 | 0.2 | 5.4* | 0.7 | spec | |||
| R Mid Temporal | 52 | −64 | 8 | 0.4 | 2.0 | 2.8* | pref | non | conj | |
| R Sup Temporal | 48 | −40 | 14 | 0.1 | 6.8* | 0.9 | spec | |||
| MNI coordinates | Age Effect | Condition Effect | Age × Cond Interaction | Response Profile |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | x | y | z | F(2, 87) | F(2, 174) | F(4, 174) | All | Adult | Older | Younger |
| L Hippocampus | −20 | −8 | −18 | 1.7 | 1.6 | 2.2 | conj | |||
| Older Children face-preferential regions | ||||||||||
| R Fusiform (FFA) | 40 | −54 | −20 | 3.6* | 5.3* | 1.6 | pref | |||
| R Inf Occipital(OFA) | 34 | −86 | −12 | 6.9* | 8.9* | 0.9 | spec | |||
| L Calcarine | −12 | −104 | 2 | 6.5* | 13.3* | 1.1 | spec | |||
| R Ant Fusiform | 42 | −38 | −16 | 1.3 | 3.9* | 0.4 | pref | |||
| R Temporal pole | 36 | 0 | −42 | 3.6* | 7.8* | 3.0* | -- | non | pref | |
| R Inf Parietal | 60 | −48 | 44 | 2.7 | 4.6* | 2.9* | -- | pref | -- | |
| L Insula | −24 | 16 | 10 | 2.2 | 4.3* | 1.5 | pref | |||
| R Precentral | 42 | −2 | 34 | 0.8 | 6.5* | 1.0 | spec | |||
| R Hippocampus | 24 | −20 | −6 | 0.4 | 10.5* | 0.4 | pref | |||
| Younger Children face-preferential regions | ||||||||||
| R Fusiform (FFA) | 38 | −50 | −24 | 1.5 | 5.3* | 1.0 | pref | |||
| MNI coordinates | Age Effect | Condition Effect | Age × Cond Interaction | Response Profile |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | x | y | z | F(2, 87) | F(2, 174) | F(4, 174) | All | Adult | Older | Younger |
| L Calcarine | −16 | −100 | −6 | 8.4* | 16.8* | 0.5 | spec | |||
| R Middle Occipital | 24 | −98 | 2 | 5.6* | 12.3* | 0.2 | spec | |||
| R Inf Temporal | 46 | −10 | −30 | 3.7* | 7.8* | 3.7* | non | non | pref | |
| L Temporal Pole | −44 | 2 | −36 | 2.0 | 5.5* | 0.6 | pref | |||
| L Mid Temporal | −60 | −24 | −6 | 6.8* | 5.2* | 5.4* | -- | conj | spec | |
| R Inf Oper Frontal | 46 | 16 | 12 | 1.4 | 6.0* | 1.4 | pref | |||
| L Precentral | −44 | −2 | 50 | 0.7 | 3.7* | 1.5 | pref | |||
| L Angular | −46 | −68 | 32 | 3.7* | 5.1* | 3.2* | -- | non | pref | |
| L PostCentral | −32 | −24 | 52 | 1.6 | 4.4* | 1.9 | (pref) | |||
| L Postcentral | −42 | −16 | 52 | 0.8 | 4.9* | 1.9 | (pref) | |||
Note. pref, face-preferential; spec, face-specific; conj, conjoined response; non, non-specific response; --, not activated.
Parentheses indicated a marginally significant face-preferential response.
p < .05
Figure 1.
Brain regions showing a face-preferential response in each age group: (A) Adults, (B) Older Children, and (C) Younger Children (p < .001, uncorrected). In each age group, the regions that showed a significant age effect for the face specialization index are circled and the color of the circle corresponds to the age effect indicated in the graphs at the bottom. Error bars are standard errors.
The voxel-wise analysis alone reveals regions that show a face-preferential response in each age group separately, but it does not reveal whether activation shifts from minimal or no activation in childhood to specialized activation in adulthood, an outcome that would be consistent with the maturational but not the IS account. The analysis below enabled us to distinguish between no activation versus non-specific activation in childhood. In addition, the regions that were defined as face-preferential were determined by combining group activation maps but did not consider individual-subject face-preferential responses; consequently, we also conducted the analyses described below to consider individual-subject variability.
The percent signal change for each of the three experimental conditions (face, natural, manufactured) relative to baseline was computed for each participant and each ROI, then submitted to a two-way ANOVA with age (younger, older, adult) as a between-subjects factor and condition as a within-subjects factor. These analyses were conducted to determine whether a Condition × Age interaction occurred, because this interaction was not tested at the voxel-level of analysis. This interaction indicates that the particular region’s preferential activation for faces changes with age. Within ROIs that showed a significant Age × Condition interaction, pair-wise t-tests were then conducted on mean intensity values (see Table 2) to determine whether different age groups showed different types of responses: face-preferential, face-specific, conjoined, non-specific or non-activated.
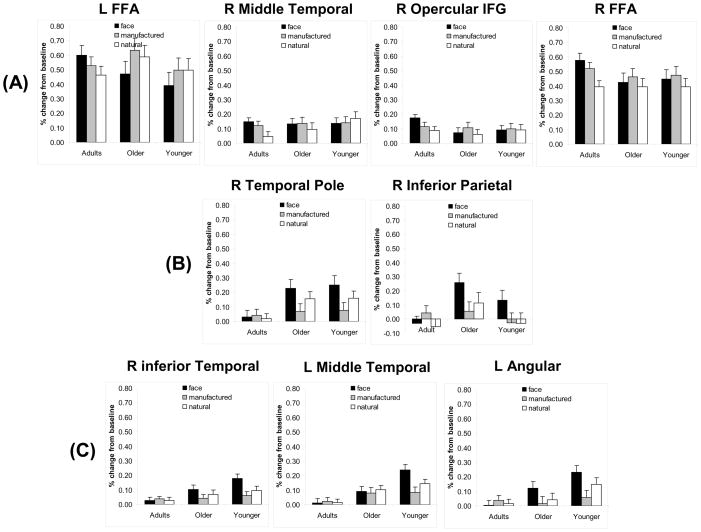
This analysis (Table 3 and Figure 2) revealed main effects of age and, most importantly, Age × Condition interactions in several regions. No brain regions showed a shift from being non-activated in childhood to face-preferential or face-specific activation in adulthood; therefore, within the developmental time window examined in the present study, a critical prediction of the maturational account was not supported. Three brain regions, however, revealed a change from non-specific activation in childhood to face-preferential activation in adulthood–the left FFA, right middle temporal gyrus and right inferior frontal gyrus (identified as face-preferential in adults). Whereas the left FFA showed a conjoined response in both younger and older children, the right middle temporal gyrus and right inferior frontal gyri showed either non-specific or conjoined responses in children (Figure 2A). Note that the shift from non-specific to face-preferential or face-specific activation would be consistent with either the maturational or IS accounts. The analysis using a specialization index is critical for further differentiating the two accounts.
Figure 2.
Percent signal change for each condition versus baseline for each region that showed a significant Age × Condition interaction in the analysis of percent signal change values for face-preferential regions in (A) adults, (B) older children and (C) younger children. In adults, the right FFA region is shown because previous studies have focused on this region. However, this region did not show a significant Age × Condition interaction.
Right FFA Activation
Because the right FFA has been the focus of previous developmental studies, the response to different categories in this region is illustrated in Figure 2A. This graph shows that only adults yield a face-preferential response. However, the Age × Condition interaction did not reach significance (p = .11); therefore, the preferential response to faces does not change across the age range that we tested. Of note, the right FFA showed only a face-preferential response but did not meet the criteria for the more restrictive face-specific response because faces did not activate the FFA more than manufactured objects (also see Haxby et al., 2001).
We also examined face-preferential activation in individual participants in both native space and standardized MNI space. In native space, 67% of adults, 30% of older children and 50% of younger children showed face-preferential activation in the right FFA. In standard space, 61% of adults, 39% of older children and 41% of younger children showed activation in the right FFA. Because the two studies that tested children under 9 years of age show that this age group does not robustly activate the right FFA (Gathers et al., 2004; Scherf et al., 2007), we also examined the younger age group in more detail. Indeed, in native space, only 25% of the children from 5 to 8 years of age showed a right FFA, but 65% of children from 8.3 to 9.7 years of age showed a right FFA. In standard space, the outcome was similar: 25% of 5-to-8-year olds and 50% of 8.3-to-9.7-year olds showed right FFA activation.
We also analyzed right FFA cluster size in standard space as a function of age group. Individuals who did not show activation in the right FFA or whose standardized cluster size (z-score) was greater than 2.5 relative to their age group were treated as missing values (only one adult was omitted as an outlier). In addition, three adults had very large clusters of activation that were considered outliers–these were also treated as missing values. Although the average cluster size was larger in adults (M = 205 voxels, SD = 309 voxels, N = 22) than older children (M = 35 voxels, SD = 31 voxels; N = 9) and younger children (M = 40 voxels, SD = 44 voxels, N = 9), the age effect did not reach significance [F(2, 40) = 2.3, p = .09].
Face Specialization Index
Within each face-preferential region in Table 3, the average mean intensity value for each participant and each of four conditions (face, natural, manufactured, fixation) was used to compute a specialization index. An ANOVA with age group as a between-subjects variable was then conducted for the specialization index. Table 4 shows all the regions that showed a significant main effect of age and whether the change in specialization was progressive (i.e., increased with age) or regressive (i.e., decreased with age).
Table 4.
Results of the analysis of face-specialization index
| MNI coordinates | Age Effect | |||||
|---|---|---|---|---|---|---|
| Region | Cluster Size | x | y | z | F(2, 87) | Change |
| Adult face-preferential regions | ||||||
| R Fusiform (FFA) | 262 | 44 | −60 | −22 | 3.2* | progressive |
| L Fusiform (FFA) | 18 | −40 | −52 | −24 | 4.6* | progressive |
| R Inf Occipital (OFA) | 169 | 38 | −82 | −14 | 3.5* | progressive |
| L Middle Occipital | 325 | −22 | −98 | −2 | 5.5* | progressive |
| R Middle Occipital | 202 | 22 | −96 | −2 | 4.3* | progressive |
| R Inf Opercular Frontal | 114 | 42 | 2 | 26 | 7.2* | progressive |
| L Fusiform (OFA) | 25 | −28 | −82 | −16 | 2.1 | none |
| L Hippocampus | 24 | −20 | −8 | −18 | 1.3 | none |
| R Inf Orbital Frontal | 15 | 32 | 28 | −16 | 0.1 | none |
| R Middle Temporal | 57 | 52 | −64 | 8 | 1.7 | none |
| R Superior Temporal | 23 | 48 | −40 | 14 | 1.0 | none |
| Older children face-preferential regions | ||||||
| R Fusiform (FFA) | 17 | 40 | −54 | −20 | 4.1* | progressive |
| R Inf Occipital (OFA) | 329 | 34 | −86 | −12 | 3.4* | progressive |
| L Middle Occipital | 27 | −12 | −104 | 2 | 4.7* | progressive |
| R Temporal Pole | 47 | 36 | 0 | −42 | 4.2* | regressive |
| R Inf Parietal | 25 | 60 | −48 | 44 | 5.4* | regressive |
| R Hippocampus | 26 | 24 | −20 | −6 | 0.8 | none |
| L Insula | 34 | −24 | 16 | 10 | 2.9 | none |
| R Precentral | 31 | 42 | −2 | 34 | 0.05 | none |
| R Fusiform | 26 | 42 | −38 | −16 | 0.6 | none |
| Younger children face-preferential regions | ||||||
| L Inf Occipital | 154 | −16 | −100 | −6 | 4.0* | progressive |
| R Anterior Inf Temporal | 247 | 46 | −10 | −30 | 6.8* | regressive |
| L Middle Temporal | 619 | −60 | −24 | −6 | 12.0* | regressive |
| R Inf Opercular Frontal | 41 | 46 | 16 | 12 | 3.3* | regressive |
| L Angular | 121 | −46 | −68 | 32 | 5.8* | regressive |
| L PostCentral | 244 | −32 | −24 | 52 | 3.3* | regressive |
| L Precentral | 86 | −44 | −2 | 50 | 4.3* | regressive |
| R Calcarine | 168 | 24 | −98 | 2 | 2.4 | none |
| L Postcentral | 32 | −42 | −16 | 52 | 0.2 | none |
| L Inf Temporal | 45 | −44 | 2 | −36 | 0.1 | none |
| L Inf Temporal | 15 | −36 | 10 | −34 | 2.0 | none |
| R Fusiform (FFA) | 17 | 38 | −50 | −24 | 0.7 | none |
Note. FFA, fusiform face area; Inf, inferior; L, left; MNI, Montreal Neurological Institute; OFA, occipital face area; R, right
All of the brain regions in adults that exhibited age effects showed progressive changes (Figure 1A), including the right and left FFA, right OFA, bilateral middle occipital gyrus and the right inferior frontal gyrus (opercular portion). Interestingly, the right FFA did not show the biggest developmental change nor did it show the greatest specialization for faces in adults. Instead, the right OFA (red line in Figure 1A) showed the greatest specialization for faces in adults [t(42) = −2.17, p = .035 for the right FFA v. right OFA paired t-test] and the greatest magnitude of change in specialization with age. The left FFA (green line in Figure 1A) also showed a marked developmental increase in specialization (i.e., a significant age effect on the specialization index). Finally, the right middle occipital gyrus (bright blue line in Figure 1A) showed as much or more specialization for faces in adults compared to the right FFA (as indicated by the higher values of the specialization index compared to the right FFA).
In older children (Figure 1B), all of the visual processing regions showed increased specialization with age, including the right FFA, right OFA, and left calcarine sulcus. The right temporal pole and right inferior parietal cortex showed regressive changes – children showed specialization for faces but adults did not.
Nearly all of the face-preferential regions in younger children exhibited regressive changes with age except the left inferior occipital gyrus, which continued to become specialized for faces with age (Figure 1C). The regressive changes were in the temporal lobe (right anterior inferior temporal gyrus, left middle temporal gyrus), parietal lobe (left postcentral and left angular gyrus) or frontal lobe (left precentral gyrus, right inferior frontal gyrus). Most regressive changes occurred in the left hemisphere.
Importantly, many regions that showed progressive changes in terms of the face-specialization index did not show an Age × Condition interaction in the analysis of percent signal change (Table 3), indicating that a face-preferential response is present even in the youngest age group but that specialization increases with age. This occurred in the right FFA, right OFA and bilateral middle occipital gyrus (Figure 1A). In contrast, other components of the face network shift from non-specialized to specialized in the age range we tested (the left FFA and right IFG-opercular portion). Previous studies that used a specialization index would not have revealed the shift from non-specific activation in childhood to specialized activation by adulthood in these two regions.
In addition, previous developmental fMRI studies of face processing have not examined regressive changes with development. In other words, it is unknown whether any brain regions show a specialized response for faces in children which then shifts toward non-specific or no activation in adults. The present study revealed that bilateral inferior parietal regions, left middle temporal cortex, right anterior inferior temporal gyrus, and right temporal pole all showed regressive changes (Figures 2B and 2C).
Discussion
The present study evaluated maturational versus IS accounts of brain development (Johnson, 2005) by documenting brain networks underlying face processing in younger children, older children, and adults. Although several brain regions showed increased specialization for faces with development (consistent with prior studies), this outcome would be predicted by both the IS and maturational accounts. When the differential predictions of the two accounts are considered (Table 1), the results favored the IS account. First, multiple brain regions (and not just the right FFA) showed increased specialization for faces with development. This is in line with the idea that networks of regions are implicated in the development of specialized cognitive capacities. Second, regions that show specialization for face processing in adults were nonspecifically activated by non-face categories in children prior to specialization. This idea is consistent with the IS account, but not the maturational account, in that the mapping of a cognitive function to brain regions is fluid and dynamic over development. Third, different brain networks were activated for faces in children and adults and some regions that were active in children showed regressive changes with development. This outcome is consistent with the proposal of the IS account that the same behavior may be supported by different neural substrates at different ages during development. Finally, the prediction of the maturational account that the specialization of function in adulthood in a given brain region is preceded by minimal or no activation in childhood was not confirmed. Consequently, the maturational view does not explain the patterns of brain activation in the developmental time window examined in the present study.
Many analyses were critical for testing these differential predictions, but prior studies have not used such analyses. By focusing primarily on the right FFA, prior studies were not able to test the assertion of the IS account that specialized capacities like face processing rely on an extended network of regions that interact throughout development. The present study showed that, indeed, an extended network of regions undergoes developmental change. Also, prior studies on the development of face processing have relied on changes in a specialization index with age. However, a specialization index alone cannot distinguish between minimal/no activation in a region versus non-specific activation in a region prior to specialization. The present analysis of mean intensity values was necessary to distinguish between these two different outcomes. By using this analysis, the present study showed that all regions specialized for face processing in adulthood are at least partly active in children. Some regions are already specialized for faces by 5 years of age but other regions are non-specifically activated and continue to be tuned for faces with development. Finally, no prior study on the development of face processing has considered regressive changes with age, although this has been explored in other cognitive domains (Brown et al., 2005). The present findings indicate that many brain regions recruited in children for face processing show reduced specialization for faces by adulthood. Each of these findings is discussed in more detail below.
Specialization of the right FFA with development
The main focus of prior developmental fMRI studies of face processing has been the specialization of the FFA across development. The present analysis of the specialization index confirms previous findings of an increase in the fine tuning of the FFA for faces with development (Aylward et al., 2005; Golarai et al., 2007; Passarotti et al., 2007; Scherf et al., 2007). In addition, the present findings also revealed that the FFA shows a preferential response to faces in both adults and older children (Gathers et al., 2004; Golarai et al., 2007; Passarotti et al., 2003). One point of departure, however, was that the younger children in the present study showed activation in a region consistent with the right FFA whereas Gathers et al. and Scherf et al. reported that the younger group of children in their studies did not show FFA activation. However, the present individual-subject analysis clarifies this issue–the youngest children in the study (8 years or younger) were less likely to show right FFA activation than children more than 9 years of age or adults.
The analysis conducted on percent signal change (Table 3) showed that the Age × Condition interaction in the adult right FFA fell short of significance, indicating that degree of preference for faces does not change across age. However, inspection of Figure 2A reveals that only adults seem to demonstrate a face-preferential response in the right FFA–neither age group of children shows a greater response to faces than to at least one other category. In fact, the pair-wise contrasts of conditions support this conclusion. The lack of an Age × Condition interaction on percent signal change in the right FFA is likely due to greater variability among children than adults.
Consistent with some prior studies (Gauthier, Tarr, Anderson, Skudlarski, & Gore, 1999; Hanson, Matsuka, & Haxby, 2004; Haxby et al., 2001; Joseph & Gathers, 2002), the right FFA did not show exclusive activation for faces in adults; in fact, the preferential response for faces was only relative to one object category but not both categories. Most studies that localize the FFA compare faces with objects but not with different subclasses of objects, nor do these studies require that faces produce a greater response than multiple subclasses of objects. Although one study examined face responses to different classes of stimuli like scenes, body parts and cars (Schwarzlose, Swisher, Dang, & Kanwisher, 2008) that study did not compare faces to different subclasses of objects like natural and manufactured objects. When such comparisons are made (e.g., Haxby et al., 2001), the FFA shows responses to these non-face categories. Because such comparisons are necessarily more restrictive in terms of the degree of specialization for faces, it is not surprising that the FFA showed a less exclusive “face-preferential” response rather than a more exclusive “face-specific” response. In fact, the finding that activation to faces in the right FFA was only greater than one other category would be expected by accounts that posit distributed representations of different classes of objects (including faces) in the ventral temporal lobes (Hanson et al., 2004).
Progressive and regressive changes in specialization with development
The present analysis of extended brain networks for face processing in all age groups also enabled us to examine both progressive and regressive changes. Prior studies have mostly focused on progressive changes by restricting analyses to brain regions like the FFA that are specialized in adults (which can only reveal, at best, progressive changes). The present study showed that in addition to the right FFA, other regions also showed progressive changes, including the right OFA, left FFA, bilateral middle occipital cortex and the right inferior frontal gyrus. Few prior studies have examined brain regions that are specialized for faces in children that are not specialized in adults though regressive changes have been examined in other tasks (Brown et al., 2005). Including such regions in the present analysis reveals potential regressive changes in face processing development.
Regressive changes in cortical regions are as fundamental to the IS view as progressive changes. The IS approach explains that cortical regions achieve specialization of function through “competitive interactions” with other regions to reduce redundancy and promote efficiency (Johnson, 2005). The struggle for specialization may involve cellular changes in synaptic gain or loss. Findings of regressive changes support the idea that some areas of the network may lose out, at a cellular and a functional level, to prevent duplication and promote specialization. Although a direct neurobiological correlate of progressive and regressive changes has not been established, some have suggested that regressive changes may be driven by synaptic loss (Huttenlocher & Dabholkar, 1997), gray matter reduction (Sowell et al., 2001), and selective pruning of synaptic contacts (Changeux & Dehaene, 1989). Progressive changes may reflect increased myelination with development which continues to increase throughout childhood and adolescence in some brain regions (Gogtay et al., 2004; Sowell et al., 2004).
In the present conceptualization, regressive changes are more fundamental to the IS than the maturational account. However, according to Johnson (2005), the maturational viewpoint would predict increases in intra-regional connectivity but the IS view would predict that inter-regional connectivity increases can influence the intra-regional connectivity (Table 10.1; Johnson, 2005). In other words, regressive changes in the maturational view would occur in the same brain regions as the progressive changes, but the IS view predicts that some brain regions will show regressive changes whereas others will show progressive changes. Hence, the present finding that some regions show regressive changes and others show progressive changes is not consistent with the maturational viewpoint.
Regressive changes were indeed observed in the present study in the right and left parietal lobe and left middle temporal gyrus. Parietal and middle temporal/superior temporal sulcus activations are reported in other developmental fMRI studies of face processing (Aylward et al., 2005; Joseph, Gathers et al., 2006; Passarotti et al., 2003; Passarotti et al., 2007; Scherf et al., 2007) and these regions are considered part of the extended face network (Haxby et al., 2001). Studies in adults have linked these regions to featural face processing (Joseph, Gathers et al., 2006; Lobmaier, Klaver, Loenneker, Martin, & Mast, 2008; Maurer et al., 2007), which is more likely to occur in children than adults (Rhodes, Brake, & Atkinson, 1993). However, this conclusion is largely speculative because the right OFA may be involved in featural face processing (Pitcher, Walsh, Yovel, & Duchaine, 2007). Differentiating among these possibilities awaits direct testing of featural and configural processing changes across development.
Other regions in temporal, parietal and frontal cortex showed regressive changes with age (Figure 2C) but all of the progressive changes for face processing emerged in occipital or occipito-temporal regions, with the exception of the right inferior frontal gyrus (opercular portion). The mirror neuron system (MNS), a subcomponent of the social brain, includes the right IFG. Mirror neurons, which allow one to see and represent others actions in one’s own mind, are linked to imitation, intention, and empathy (Iacoboni et al., 2005; Leslie, Johnson-Frey, & Grafton, 2004). It is possible, then, that the emergence of the right opercular IFG for face processing with development reflects processing of faces in a social context.
The regressive changes in children, however, may be linked to more general information processing mechanisms that change with development. One possibility is that children were not attending to the stimuli to the same extent that adults were. However, the analysis of button presses indicates no age differences in attending to the stimuli. Another possibility is that children may require more resources to attend to the faces than would adults. This could lead to the recruitment of additional brain regions (Cabeza, Anderson, Locantore, & McIntosh, 2002). However, the younger children seem to recruit a qualitatively different network of regions, primarily in the left hemisphere, rather than additional regions that might be used in a compensatory manner. The speculation that left-hemisphere recruitment reflects developmental changes in face processing, rather than related to other cognitive mechanisms, will needed to be tested in future research.
Summary and Conclusion
The present study showed that the right FFA is not the only brain region to undergo increases in specialization for face processing with age. The right OFA also showed marked increases in specialization for faces with age. This finding is not surprising in light of other studies that have underscored the importance of the right OFA in mature face recognition (Pitcher et al., 2007; Rossion et al., 2003; Steeves et al., 2009). Also, a right opercular IFG region shifts from non-specialized to specialized activation with development. This region is considered to be part of the extended network of face processing (Fairhall & Ishai, 2007) and both functional and anatomical connectivity studies have emphasized the importance of right inferior frontal and right FFA connectivity in typical face processing (Fairhall & Ishai, 2007; Thomas et al., 2008). Finally, the present study revealed a combination of both regressive and progressive changes among brain regions involved in face processing, consistent with the IS account of brain development. Future studies may provide additional support for the IS account by using functional or effective connectivity analyses that explore the interaction of components of the face processing network across development. Another future direction will be to compare the interplay of regressive and progressive developmental changes across different domains of cognition and behavior.
Acknowledgments
This research was supported by the National Institutes of Health (R01-MH063817; R01-HD052724; R01-HD042451; F31-MH067461; P20-RR015592; P50-DA005312) and the National Science Foundation (BCS-0224240).
We thank R. Avison, A. Bognar, C. Corbly, S. Whitaker and X. Liu for their technical assistance and S. Heydinger for his help with participant recruitment. We also thank the volunteers and their families.
Footnotes
Two prior published reports are related: Gathers et al., (2004), NeuroReport, 15, 1549-1553, and Joseph et al., (2006), Cognitive Affective, & Behavioral Neuroscience, 6, 223-235.
Contributor Information
Jane E. Joseph, Department of Anatomy and Neurobiology, University of Kentucky
Ann D. Gathers, Department of Anatomy and Neurobiology, University of Kentucky
Ramesh S. Bhatt, Department of Psychology, University of Kentucky
References
- Aylward EH, Park JE, Field KM, Parsons AC, Richards TL, Cramer SC, et al. Brain activation during face perception: Evidence of a developmental change. Journal of Cognitive Neuroscience. 2005;17:308–319. doi: 10.1162/0898929053124884. [DOI] [PubMed] [Google Scholar]
- Bhatt RS, Bertin E, Hayden A, Reed A. Face processing in infancy: Developmental changes in the use of different kinds of relational information. Child Development. 2005;76:169–181. doi: 10.1111/j.1467-8624.2005.00837.x. [DOI] [PubMed] [Google Scholar]
- Blonder LX, Smith CD, Davis CE, Kesler/West ML, Garrity TF, Avison MJ, et al. Regional brain response to faces of humans and dogs. Cognitive Brain Research. 2004;20:384–394. doi: 10.1016/j.cogbrainres.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cerebral Cortex. 2005;15(3):275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Bushnell IWR, Sai F, Mullin JT. Neonatal recognition of mother’s face. British Journal of Developmental Psychology. 1989;7:3–15. [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17(3):1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Carey S, Diamond R. Are faces perceived as configurations more by adults than by children? Visual Cognition. 1994;1(2/3):253–274. [Google Scholar]
- Carey S, Diamond R, Woods B. Development of face recognition-A maturational component? Developmental Psychology. 1980;16:257–269. [Google Scholar]
- Carver LJ, Dawson G, Panagiotides H, Meltzoff AN, McPartland J, Gray J, et al. Age-related differences in neural correlates of face recognition during the toddler and preschool years. Developmental Psychobiology. 2003;42(2):148–159. doi: 10.1002/dev.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassia VM, Turati C, Simion F. Can a nonspecific bias toward top-heavy patterns explain newborns’ face preference? Psychological Science. 2004;15(6):379–383. doi: 10.1111/j.0956-7976.2004.00688.x. [DOI] [PubMed] [Google Scholar]
- Changeux JP, Dehaene S. Neuronal models of cognitive functions. Cognition. 1989;33:63–109. doi: 10.1016/0010-0277(89)90006-1. [DOI] [PubMed] [Google Scholar]
- Corbeil JC, Archambault A, editors. The MacMillan visual dictionary. New York: MacMillan; 1992. [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, et al. Sex differences in brain maturation during childhood and adolescence. Cerebral Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- de Haan M, Humphreys K, Johnson MH. Developing a brain specialized for face perception: A converging methods approach. Developmental Psychobiology. 2002;40(3):200–212. [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test. 3. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Ellis HD, Shepard J, Bruce A. The effects of age and sex upon adolescents’ recognition of faces. Journal of Genetic Psychology. 1973;123:173–174. doi: 10.1080/00221325.1973.10533202. [DOI] [PubMed] [Google Scholar]
- Fairhall SL, Ishai A. Effective connectivity within the distributed cortical network for face perception. Cerebral Cortex. 2007;17:2400–2406. doi: 10.1093/cercor/bhl148. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Rabinowitz C, Quinn GE, Liu GT. Early commitment of neural substrates for face recognition. Cognitive Neuropsychology. 2000;17(1/2/3):117–123. doi: 10.1080/026432900380526. [DOI] [PubMed] [Google Scholar]
- Gathers AD, Bhatt RS, Corbly CR, Farley AB, Joseph JE. Developmental shifts in cortical loci for face and object recognition. NeuroReport. 2004;15(10):1549–1553. doi: 10.1097/01.wnr.0000133299.84901.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC. Activation of the middle fusiform ‘face area’ increases with expertise in recognizing novel objects. Nature Neuroscience. 1999;2(6):568–573. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- Giedd J. Structural magnetic resonance imaging of the adolescent brain. Annals New York Academy of Sciences. 2004;1021:75–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd J, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G, Ghahremani DG, Whitfield-Gabrieli S, Reiss A, Eberhardt JL, Gabrieli JD, et al. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nature Neuroscience. 2007;10(4):512–522. doi: 10.1038/nn1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Golarai G, Gabrieli J. Developmental neuroimaging of the human ventral visual cortex. Trends in Cognitive Sciences. 2008;12:152–162. doi: 10.1016/j.tics.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SJ, Matsuka T, Haxby JV. Combinatorial codes in ventral temporal lobe for object recognition: Haxby (2001) revisited: is there a “face” area? NeuroImage. 2004;23:156–166. doi: 10.1016/j.neuroimage.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293(5539):2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000b;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. The Journal of Comparative Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one’s own mirror neuron system. PLoS Biology. 2005;3(3):0529–O0535. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH. Functional brain development in humans. Nature Reviews Neuroscience. 2001;2:475–483. doi: 10.1038/35081509. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Developmental Cognitive Neuroscience. Malden, MA: Blackwell Publishing; 2005. [Google Scholar]
- Johnson MH. Developing a social brain. Acta Paediatrica Oslo Norway. 2007;96(1):3–5. doi: 10.1111/j.1651-2227.2006.00037.x. [DOI] [PubMed] [Google Scholar]
- Joseph JE, Gathers AD. Natural and manufactured objects activate the “fusiform face area”. Neuroreport. 2002;13(7):935–938. doi: 10.1097/00001756-200205240-00007. [DOI] [PubMed] [Google Scholar]
- Joseph JE, Gathers AD, Liu X, Corbly CR, Whitaker SK, Bhatt RS. Neural developmental changes in processing inverted faces. Cognitive Affective, & Behavioral Neuroscience. 2006;6(3):223–235. doi: 10.3758/cabn.6.3.223. [DOI] [PubMed] [Google Scholar]
- Joseph JE, Partin DJ, Jones KM. Hypothesis testing for selective, differential, and conjoined brain activation. Journal of Neuroscience Methods. 2002;118:129–140. doi: 10.1016/s0165-0270(02)00122-x. [DOI] [PubMed] [Google Scholar]
- Kadosh KC, Johnson MH. Developing a cortex specialized for face perception. Trends in Cognitive Science. 2007;11(9):367–369. doi: 10.1016/j.tics.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. The Journal of Neuroscience. 1997;17(11):4301–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie KR, Johnson-Frey SH, Grafton ST. Functional imaging of face and hand imitation: Towards a motor theory of empathy. NeuroImage. 2004;21:601–607. doi: 10.1016/j.neuroimage.2003.09.038. [DOI] [PubMed] [Google Scholar]
- Lobmaier JS, Klaver P, Loenneker T, Martin E, Mast FW. Featural and configual face processing strategies: Evidence from a functional magnetic resonance imaging study. Neuroreport. 2008;19(3):287–291. doi: 10.1097/WNR.0b013e3282f556fe. [DOI] [PubMed] [Google Scholar]
- Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, et al. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proceedings of the National Academy of Sciences USA. 1995;92:8135–8139. doi: 10.1073/pnas.92.18.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer D, Craven KM, Le Grand R, Mondloch CJ, Springer MV, Lewis CL, et al. Neural correlates of processing facial identity based on features versus their spacing. Neuropsychologia. 2007;45:1438–1451. doi: 10.1016/j.neuropsychologia.2006.11.016. [DOI] [PubMed] [Google Scholar]
- McKone E, Boyer BL. Sensitivity of 4-year olds to featural and second-order relational changes in face distinctiveness. Journal of Experimental Child Psychology. 2006:1–29. doi: 10.1016/j.jecp.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Mondloch CJ, Geldart S, Maurer D, Le Grand R. Developmental changes in face processing skills. Journal of Experimental Child Psychology. 2003;86:67–84. doi: 10.1016/s0022-0965(03)00102-4. [DOI] [PubMed] [Google Scholar]
- Morton J, Johnson MH. CONSPEC and CONLERN: A two-process theory on infant face recognition. Psychological Review. 1991;98:164–181. doi: 10.1037/0033-295x.98.2.164. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Medina KL, Yoshii J, Schweinsburg AD, Moadab I, Tapert SF. Age-related changes in prefrontal white matter volume across adolescence. Neuroreport. 2006;13:1427–1431. doi: 10.1097/01.wnr.0000233099.97784.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascalis O, Slater A. The development of face processing in infancy and early childhood. Hauppauge, NY: Nova Science Publishers, Inc; 2003. [Google Scholar]
- Passarotti AM, Paul BM, Bussiere JR, Buxton RB, Wong EC, Stiles J. The development of face and location processing: An fMRI study. Developmental Science. 2003;6(1):100–117. [Google Scholar]
- Passarotti AM, Smith J, DeLano M, Huang J. Developmental differences in the neural bases of the face inversion effect show progressive tuning of face-selective regions to the upright orientation. NeuroImage. 2007;34:1708–1722. doi: 10.1016/j.neuroimage.2006.07.045. [DOI] [PubMed] [Google Scholar]
- Pellicano E, Rhodes G, Peters M. Are preschoolers sensitive to configural information in faces? Developmental Science. 2006;9(3):270–277. doi: 10.1111/j.1467-7687.2006.00489.x. [DOI] [PubMed] [Google Scholar]
- Pitcher D, Walsh V, Yovel G, Duchaine B. TMS evidence for the involvement of the right occipital face area in early face processing. Current Biology. 2007;17:1568–1573. doi: 10.1016/j.cub.2007.07.063. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Brake S, Atkinson AP. What’s lost in inverted faces? Cognition. 1993;47:25–57. doi: 10.1016/0010-0277(93)90061-y. [DOI] [PubMed] [Google Scholar]
- Rossion B, Caldara R, Seghier M, Schuller A, Lazeyras F, Mayer E. A network of occipito-temporal face-sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain. 2003;126:2381–2395. doi: 10.1093/brain/awg241. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Behrmann M, Humphreys K, Luna B. Visual category-selectivity for faces, places and objects emerges along different developmental trajectories. Developmental Science. 2007;10(4):F15–F30. doi: 10.1111/j.1467-7687.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- Schwarzer G. Development of face processing: the effect of face inversion. Child Development. 2000;71(2):391–401. doi: 10.1111/1467-8624.00152. [DOI] [PubMed] [Google Scholar]
- Schwarzlose RF, Swisher JD, Dang S, Kanwisher N. The distribution of category and location information across object-selective regions in human visual cortex. Proceedings of the National Acadamy of Sciences. 2008;105:4447–4452. doi: 10.1073/pnas.0800431105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Bellgowan PS, Martin A. Measuring selectivity in fMRI data. Nature Neuroscience. 2007;10(1):4–5. doi: 10.1038/nn0107-4. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. The Journal of Neuroscience. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. The Journal of Neuroscience. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves J, Dricot L, Goltz HC, Sorger B, Peters J, Milner AD, et al. Abnormal face identity coding in the middle fusiform gyrus of two brain-damaged prosopagnosic patients. Neuropsychologia. 2009;47:2584–2592. doi: 10.1016/j.neuropsychologia.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Thomas C, Moya L, Avidan G, Humphreys K, Jung KJ, Peterson MA, et al. Reduction in white matter connectivity, revealed by diffusion tensor imaging, may account for age-related changes in face perception. Journal of Cognitive Neuroscience. 2008;20(2):268–284. doi: 10.1162/jocn.2008.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KT. Expressive Vocabulary Test. Circle Pines, Minnesota: American Guidance Service, Inc; 1997. [Google Scholar]
- Xiong J, Gao JH, Lancaster JL, Fox PT. Clustered pixels analysis for functional MRI activation studies of the human brain. Human Brain Mapping. 1995;3:287–301. [Google Scholar]