Abstract
Adolescent initiation of ethanol consumption is associated with subsequent heightened probability of ethanol-use disorders. The present study examined the relationship between motivational sensitivity to ethanol initiation in adolescent rats and later ethanol intake. Experiment 1 determined that ethanol induces locomotor activation shortly after administration but not if tested at a later post-administration interval. In Experiment 2, adolescents were assessed for ethanol-induced locomotor activation on postnatal day 28. These animals were then evaluated for ethanol-mediated conditioned taste aversion and underwent a 16-day-long ethanol intake protocol. Ethanol-mediated aversive effects were unrelated to ethanol locomotor stimulation or subsequent ethanol consumption patterns. Ethanol intake during late adolescence was greatest in animals initiated to ethanol earliest at postnatal day 28. Females that were more sensitive to ethanol’s locomotor-activating effects showed a transient increase in ethanol self-administration. Blood ethanol concentrations during initiation were not related to ethanol-induced locomotor activation. Adolescent rats appeared sensitive to the locomotor-stimulatory effects of ethanol. Even brief ethanol exposure during adolescence may promote later ethanol intake.
Keywords: adolescence, rat, ethanol, reinforcement, conditioned taste aversion, ethanol intake
Introduction
The use of alcohol during adolescence may have unique implications for the development of alcohol use disorders (Anthony and Petronis, 1995; DeWit et al., 2000). For example, Grant and Dawson (1997) found that subjects who started drinking before age 15 were four-times more likely to develop alcohol dependence than those who started after age 21. Therefore, understanding the factors promoting vulnerability to problematic adolescent alcohol consumption and the factors that underlie the facilitative effects of alcohol initiation upon later drug affinity is important (Schramm-Sapyta et al., 2008). One of these factors is the hedonic nature (appetitive or aversive) of the first experience with the drug. Adolescents who perceive the drug as more rewarding may be at higher risk for alcohol use disorders (Schramm-Sapyta et al., 2006). This perspective gives animal researchers a framework with which to examine why certain subjects progress rapidly from controlled use of alcohol to abuse and dependence, while others continue controlled drinking despite repeated drug exposure.
Recent studies show that adolescents exhibit age-specific patterns of responding to several acute effects of ethanol (e.g., sedation, motor coordination, hypothermia, narcosis; Spear and Varlinskaya, 2005; White et al., 2002) that normally should serve to preclude further engagement in ethanol intake. Age-related differences in motivational sensitivity to ethanol are also apparent. Adolescent rats are seemingly more sensitive than adults to the rewarding effects of ethanol (Pautassi et al., 2008; Philpot et al., 2003) yet less sensitive to the aversive consequences of the drug (Anderson et al., 2008; Varlinskaya and Spear, 2008).
The level of activity in an inescapable novel environment has long been hypothesized to predict drug self-administration in rodents (Nadal et al., 2002). Following activity assessment, animals can be classified as either high or low responders (HR and LR, respectively) by calculating the median split. This procedure has been widely employed in adult animals (Klebaur & Bardo, 1999; Nadal et al., 2005) and developing animals (Arias et al., 2009b). These subpopulations differ in their susceptibility to the aversive (Arias et al., 2009c) and appetitive (Nocjar et al. 1999) motivational effects of ethanol and ethanol intake (Cools and Gingras, 1998; Nadal et al., 2002). Similarly, ethanol-induced locomotor activation has been considered a measure of ethanol’s appetitive effects (Pautassi et al., 2009). The underlying rationale is that ethanol-induced forward locomotion and its reinforcing effects derive from a common neurobiological mechanism, namely, activation of the mesocorticolimbic dopaminergic system (Orsini et al., 2004). Ethanol-induced acute locomotor activation is quite common in mice (Chuck et al., 2006), but less so in rats (Cunningham et al., 1993). Recently, however, Arias et al. (2008, 2009b) found ethanol-induced behavioral activation in preweanling rats given high but not low doses of ethanol (2.5 and 0.5 g/kg, respectively). The literature on the acute locomotor-activating effects of ethanol in adolescent animals is scarce and controversial. Recent work suggests that adolescent mice may be more sensitive than adult mice to these effects (Hefner et al., 2007; Stevenson et al., 2008). However, in another study, adult mice exhibited significantly more ethanol-induced locomotion than their adolescent counterparts (Faria et al., 2008).
The exact nature of the relationship between motivational sensitivity to ethanol and ethanol intake is still unclear (Green and Grahame, 2008). One approach to analyze this phenomenon involves the characterization of subpopulations of heterogeneous rats expressing differential susceptibility to ethanol’s effects and the subsequent assessment of their affinity for ethanol intake. The work by Schramm-Sapyta et al. (2008) represents an important step in this direction. These researchers assessed individual differences among heterogeneous adolescent rats in terms of novelty-seeking, stress hormone levels, and initial ethanol consumption. Early ethanol intake, but not the other behavioral and hormonal markers, predicted later ethanol affinity.
We examined the relationship between measures of motivational sensitivity to ethanol and ethanol intake in adolescent rats. Specifically, the experiments analyzed susceptibility to ethanol intake in subpopulations of rats that differed in their experience with ethanol as well as their motivational response to the drug. Animals were screened for ethanol-induced behavioral activation, and their sensitivity to the aversive effects of ethanol was measured using a conditioned taste aversion (CTA) test. Animals were then tested in an ethanol intake protocol. A within-subjects design was employed, in which animals were sequentially assessed in a set of measures reflecting hedonic sensitivity to ethanol and affinity toward the drug. Based on previous research (Arias et al, 2008, 2009b; Risinger et al., 1994; Truxell et al., 2007) the hypotheses were that alcohol initiation during early adolescence would increase later ethanol consumption, that adolescents would exhibit ethanol-induced locomotor activation, and that animals that were more sensitive to these effects would show an even greater proclivity to engage in ethanol self-administration. Individual differences in ethanol metabolism may help explain differences in ethanol’s behavioral effects and ethanol intake (Walker and Ehlers, 2009). Therefore, a final experiment assessed blood ethanol concentrations (BECs) in heterogeneous adolescent rats classified as either high- or low-responders in terms of ethanol-induced locomotion.
General Methods
Subjects
A total of 138 Wistar rats, both males and females, derived from 38 litters were used (Experiment 1: 51 animals, 13 litters; Experiment 2: 59 animals; 12 litters; Experiment 3: 28 animals; 13 litters). These animals were born and reared at the Instituto Ferreyra (INIMEC-CONICET, Córdoba, Cba, Argentina). Births were examined daily, and the day of parturition was considered postnatal day 0 (PD0). Pups were housed with the dam in standard maternity cages. The colony was maintained at 22–23°C with a 12 h/12 h light/dark cycle. Weaning was performed on PD21, and then animals were housed (45 × 30 × 20 cm cage) in groups of 5–8 until the start of the Experiment on PD28. During PD25–27, animals were handled twice per day for 2 min. To eliminate confounds between litter and treatment effects, no more than one subject per litter was assigned to the same condition (Holson and Pearce, 1992). The different experimental phases include repeated tests on the same animals. That is, the same animals subjected to CTA testing on PD29–34 had ethanol-induced activity on P28 and were assessed for ethanol intake on PD37–52. The experimental procedures complied with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and were approved by the Institutional Animal Care and Use Committee.
Ethanol administration procedures
All ethanol administrations were conducted via the intragastric (i.g.) route by introducing a 12 cm section of polyethylene-50 tubing into the pup’s oral cavity. The tubing was connected to a 5 ml syringe mounted with a 25 gauge needle (Becton Dickinson, Rutheford, NJ). About 6 cm of tubing were guided into the subjects’ stomach prior to the delivery of ethanol. The dose (2.5 g/kg) was achieved by administering 0.015 ml/g of a 21% v/v ethanol solution (Porta Hnos, Córdoba, Argentina; vehicle: tap water).
Conditioning and testing procedures
Housing conditions
Animals were housed with a same-sex partner throughout most of the conditioning and testing procedures. We aimed to minimize the potential stressful effects of isolation, which can, by itself, alter ethanol affinity. Animals were isolated, however, during the experimental days in which individual intake scores were recorded. Specifically, isolation was employed during the CTA protocol (PD29–34) and the forced access phase of the ethanol intake protocol (PD41–44). Isolation was required during these days to allow gathering intake data from the graded tubes.
Ethanol-induced locomotor activity on PD28
On PD28, the rats were weighed (portable Ohaus L2000, Ohaus, Pine Brook, NJ) and given ethanol (2.5 g/kg) or its vehicle. Rats were then returned to a holding chamber (a standard housing tub lined with pine shavings) where they remained for 5 or 30 min.
Locomotor activity was evaluated in square wooden chambers (30 × 30 × 30 cm). The wall of these containers was opaque, and the floor was lined with black rubber. Locomotor activity was recorded at a post-administration time of 5–11 min (early interval, Experiments 1 and 2) or 30–36 min (late interval, Experiment 1). The dependent variables were total duration of horizontal forward locomotion (s) during the 7 min test and total duration (s) of vertical behavior. Vertical behavior (i.e., wall climbing) was measured when the adolescents stood on their rear limbs with the forepaws placed on the walls of the chamber. Locomotion was defined as the movement of the four paws at a given time. The dependent variables were recorded in real-time by experimenters blind to the training conditions of the animals. Two separate experimenters injected the animals and observed the locomotion, respectively.
Illumination in the testing room was provided by two fluorescent lamps, positioned on the ceiling, about 2.5 m from the testing chambers. Evaluation began by gently placing each adolescent rat in the center of the chamber.
Ethanol-induced conditioned taste aversion
We closely followed the procedure created by Anderson et al. (2008) and Varlinskaya and Spear (2008). On day 1 of the experimental protocol (PD29), the adolescents were housed in individual cages (30 × 23 × 25 cm) and given ad libitum access to food and water. On the morning of PD30, the water bottle was replaced by a graded tube containing 50% of the water they had ingested during the previous 24 h period. On day 3 (PD31), animals were weighed and then returned to their cage. The water tube was then replaced by a tube containing a 0.1% saccharin solution (Parker Davis, Buenos Aires, Argentina). Animals were given 30 min access to the solution, saccharin intake was measured, and animals were administered ethanol (2.5 g/kg) or its vehicle. On day 4 (PD32), the adolescents remained in their cages with ad libitum access to food and water, and on day 5 (PD33) they were again given only 50% of the volume of water they had ingested on day 1. Conditioned taste aversion was assessed on day 6 (PD34). Subjects were given 60 min access to a graded tube containing a 0.1% saccharin solution. Saccharin intake was recorded at the termination of this test and expressed as milliliters consumed per 100 grams of body weight (ml/100 g).
Ethanol intake assessment
The ethanol intake protocol began on PD37 and was completed on PD52. The protocol was composed by four phases, each one 4 days long. Phase 1 involved two-bottle choice tests (ethanol vs. water), and in Phase 2 animals were given 24 h access to ethanol as the sole fluid. Phase 3 was an “ethanol deprivation” phase, in which ethanol was not available. Phase 4 then repeated the procedure of Phase 1.
By using this protocol, we aimed to assess ethanol intake under a variety of experimental conditions to maximize the possibility of finding treatment-related differences in ethanol preference. Another aim was to conduct all intake tests during the adolescent stage of development. Phases 1 and 4 of the protocol were designed on the basis of previous two-bottle choice studies conducted in our laboratory (Pepino et al., 2004; Ponce et al., 2004, 2008) which had proven useful to detect early ethanol exposure effects.
The protocol was also inspired by a recent study that employed the alcohol deprivation effect (Bell et al., 2008) to assess ethanol intake in adolescent rats. Although the latter and other alcohol deprivation effect studies employed highly concentrated ethanol solutions (i.e., 10–30% v/v), the present study utilized drug concentrations in the 3–6% v/v range. The rationale for using these concentrations was that several studies conducted in heterogeneous, nonselected Wistar (Ponce et al., 2004, 2008) and Long-Evans hooded rats (Youngentob et al., 2007) indicate that these animals drink very little ethanol when having access to 7% v/v or higher ethanol concentrations.
During the assessment, the dependent variables under analysis were ethanol intake (g/kg) and percent selection ([consumption of ethanol/overall liquid ingestion] × 100). A detailed account of the intake assessment protocol follows:
Phase 1 (first instance of two-bottle choice tests, PD37–40)
Daily 2 h intake sessions were conducted. Each session was preceded by 22 h of fluid deprivation. Adolescents were given simultaneous access to graded tubes filled with tap water or a specific ethanol solution. On the first testing day, a 3% v/v ethanol solution was available together with the water. This solution was increased by 1% v/v of ethanol per day until reaching 6% v/v ethanol. The volume consumed from each tube was assessed at 20, 60, and 120 min. The animals were returned, in same-sex pairs, to their holding cages after each daily intake test session. The positions of the tubes were varied across sessions.
Phase 2 (24 h, forced-access intake phase, PD41–44)
Immediately after termination of the last 2 h choice session, animals were transferred to individual holding cages lined with pine shavings. In those cages and for the next 4 days, animals were given access to ethanol as the only fluid available and had free access to regular food chow. Specifically, animals had simultaneous access to three graded tubes containing 3, 4, and 5% ethanol diluted in tap water. The rationale for offering several ethanol concentrations is that, in adult animals, this condition usually promotes greater ethanol intake and facilitates the increase in ethanol intake typically found after a period of drug deprivation (Rodd-Henricks et al., 2001). Ethanol consumption was measured once per day at 0830 h.
Phase 3 (ethanol deprivation phase, PD45–48)
At 0830 h on PD44, animals were housed in same-sex pairs in standard holding cages lined with pine shavings and had ad libitum access to water and food. The animals remained undisturbed in these conditions for the next 4 days. This phase was intended as an “ethanol-withdrawal” phase and was introduced to test whether it would facilitate greater ethanol intake during Phase 4 (compared with Phase 1) and allow the expression of ethanol initiation effects.
Phase 4 (second instance of two-bottle choice tests, PD49–52)
This phase repeated the procedures of Phase 1.
Determination of blood ethanol concentrations on PD28
Adolescent rats used for determination of BECs (Experiment 3) were handled twice per day for 2 min (PD25–27). On PD28, they were administered 2.5 g/kg ethanol and assessed in terms of ethanol-induced locomotion. Blood ethanol concentrations were determined from blood samples taken immediately after the behavioral assessment at a post-administration time of 12 min. Blood samples consisted of trunk blood (2 ml samples) obtained through decapitation. The vials containing the blood were stored at −70°C and analyzed by means of a Hewlett-Packard gas chromatographer (Model 5890). Blood ethanol concentrations were expressed as milligrams of ethanol per deciliter of body fluid (mg/dl = mg%).
Data analysis
Results are expressed as mean ± SEM. Body weights were analyzed using a three-way analysis of variance (ANOVA; sex × ethanol dose × time at testing; Experiment 1) or a three-way mixed ANOVA (between group factors: sex × ethanol dose on PD28 × ethanol dose on PD31; days of assessment was the repeated-measure; Experiment 2).
Ethanol-induced forward locomotion and wall climbing were analyzed by separate three-way (Experiment 2) or four-way (Experiment 1) mixed-factor ANOVAs. The between-group factors were sex (male or female), ethanol dose on PD28 (0.0 or 2.5 g/kg), and testing interval in Experiment 1 (early or late, 5–11 min or 30–36 min post-administration). The within-group measure was time at test (bins 1–7; bin duration: 1 min).
In Experiment 2, considering the cumulative 7 min session for forward locomotion data, adolescent animals treated with ethanol on PD28 were divided into low- and high-responders (LR, HR) by a median split procedure. Forward locomotion among water-treated, LR and HR male and female rats was analyzed using a two-way mixed ANOVA (between-group factors: sex, class assignment-factor [LR-HR]; within-group factor: time at test). Conditioned taste aversion was defined as a significant decrease in saccharin intake in animals treated with ethanol compared with counterparts given vehicle. Conditioning and testing sessions differed in length (30 and 60 min, respectively). Conditioned taste aversion was evaluated as a function of the rat’s prior experience with ethanol during the activation test on PD28. Therefore, saccharin intake (ml/100 g) during conditioning and testing (PD31 and PD34, respectively) was analyzed separately using 2 × 2 × 2 ANOVAs (ethanol dose on PD28 [2.5 or 0.0 g/kg] × ethanol dose at CTA training [2.5 or 0.0 g/kg] × sex [male or female]).
A three-way mixed ANOVA examined water consumption scores (ml/100 g) during the days in which animals had access to water and varying ethanol solutions (sessions 1, 2, 3, and 4 of Phases 1 and 4 of the intake protocol). Ethanol treatment on PD28 (0.0 or 2.5 g/kg) and PD31 (0.0 or 2.5 g/kg) and sex (male or female) were between-group factors, and days of assessment (sessions 3, 4, 5, and 6) and Phase (1 or 4) were repeated measures.
Ethanol intake during Phases 1 and 4 involved the same testing procedure consisting of four daily two-bottle choice sessions. Therefore, ethanol intake and percent selection during Phases 1 and 4 were analyzed using mixed ANOVAs that included sex (male or female), ethanol dose on PD28 (i.e., before the assessment of ethanol-induced activity, 0.0 or 2.5 g/kg), and ethanol dose on PD31 (at CTA training, 0.0 or 2.5 g/kg) as between-group factors. Phase (1 and 4) and session within each phase (session 1, 2, 3, and 4) were the repeated measures.
Total g/kg of ethanol consumed during each daily session of Phase 2 was analyzed using ANOVA (sex × ethanol dose on PD28 × ethanol dose at PD31 × session).
When studying the relationship between ethanol-induced forward locomotion on PD28 and CTA or ethanol intake, ANOVAs were also used. These analyses were similar to those specified above, with the difference that the factor “ethanol dose on PD28” was replaced by a between-subjects factor with three levels (LR, HR, water-treated). Pearson correlation tests (two-tailed) were also used to determine the relationship between activity on PD28, saccharin consumption, and ethanol intake scores.
Blood ethanol concentrations (Experiment 3) were analyzed using a two-way ANOVA. Sex (male or female) and class-assignment as a function of median of ethanol-induced locomotion (LR or HR) were the between-group factors.
Following the execution of the omnibus ANOVAs, the loci of significant main effects or interactions were further examined using follow-up ANOVAs and pair-wise comparisons (Fisher’s LSD post hoc tests or planned comparisons). Orthogonal planned comparisons were specifically employed to analyze the loci of significant interactions involving repeated measures.
More in detail, Fisher’s LSD was used for the analysis of simple main effects or interactions comprising “between” factors. On the other hand, we employed orthogonal planned comparisons to analyze the significant interactions involving between-by-within factors. The rationale for using this approach was that there is no unambiguous choice of the appropriate error terms for post-hoc comparisons involving between-group and within-group interactions (Winer, 1991). Values of p < 0.05 were considered statistically significant.
Experiment 1
This experiment determined if a relatively high dose of ethanol (2.5 g/kg) can induce behavioral activation in adolescent rats. A 2 (sex: male or female) × 2 (dose: 0.0 or 2.5 g/kg ethanol) × 2 (testing interval: early or late) factorial design was used, with 6–7 animals in each experimental group. Animals were given ethanol or its vehicle, with behavior in the testing chamber recorded at post-administration times of 5–11 or 30–36 min. Testing was conducted at post-administration times similar to those at which ethanol’s activating and depressant effects had been detected in infants (Arias et al., 2008). Based on a preliminary, pilot study (Acevedo et al., 2009), we decided to discard the use of a lower dose of ethanol. In that study we found that 0.5 g/kg ethanol exerted neither activating nor depressant motor effects in male and female adolescent rats.
Results
Body weights did not differ as a function of experimental condition. The ANOVA revealed only that, as expected, males had greater body weight than females (97.50 ± 1.97 g and 89.27 ± 2.57 g, respectively).
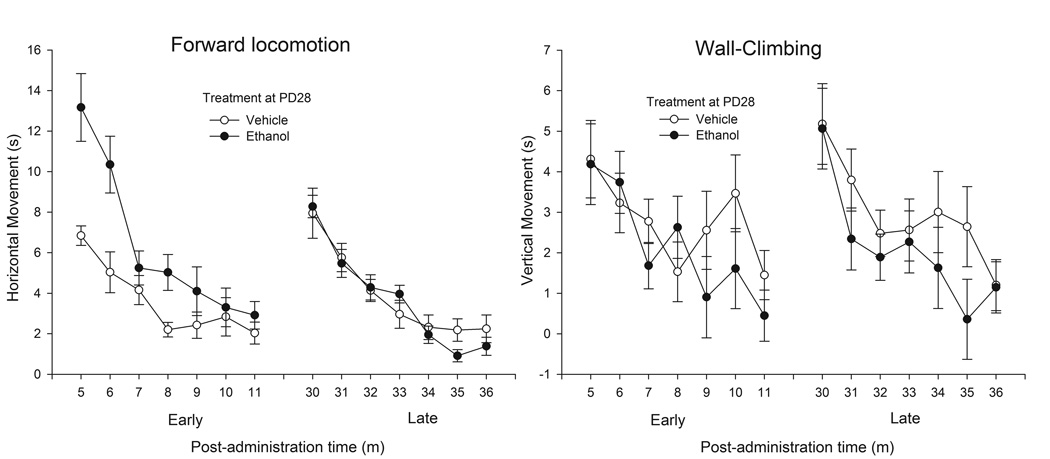
Figure 1 depicts behavioral activation (forward locomotion and wall climbing, left and right panels, respectively) in adolescent rats given 2.5 g/kg ethanol or vehicle. Ethanol evoked forward locomotion in the male and female adolescent rats, but only when tested during the early phase of intoxication (5–11 min). This effect was particularly noticeable during the first four bins of assessment. The duration of vertical climbing behavior showed a progressive decrease as testing progressed, a pattern that was substantially similar across drug treatment, sex, and post-administration time. These impressions were confirmed by the ANOVAs. The ANOVA for vertical climbing behavior yielded only a significant main effect of bin (F6,246 = 9.31, p < 0.001). The analysis of horizontal, forward locomotion yielded significant main effects of dose, testing interval, and bin of evaluation (F1,41 = 7.37, F1,41 = 6.31, F6,246 = 48.08, respectively; p < 0.05). The bin × dose and time at testing × dose interactions also achieved significance (F6,246 = 3.46, F1,41 = 9.81, respectively; p < 0.001). Ethanol-treated animals exhibited significantly more forward locomotion than controls during the early testing interval (bins 1, 2, and 4).
Figure 1.
Locomotor activity (forward locomotion and wall-climbing, left and right sections, respectively, expressed in seconds) in 28-day-old male and female adolescent rats as a function of ethanol treatment (0.0 [vehicle] or 2.5 g/kg) and post-administration bin of assessment (5–11 min or 30–36 min; early and late intervals, respectively). Data were collapsed across sex (male or female). The sex factor did not exert a significant main effect or significantly interact with the remaining variables. The vertical bars indicate SEM.
Experiment 2
This experiment assessed the relationship between different measures of motivational sensitivity to ethanol, as well as the relationship between these measures and ethanol consumption during adolescence. A 2 (sex: male or female) × 2 (ethanol treatment on PD28: 2.5 or 0.0 g/kg) × 2 (ethanol treatment on PD31: 2.5 or 0.0 g/kg) factorial design was employed. Eight groups were thus created. Each of these groups had a minimum of six and a maximum of nine animals. On PD28, animals were tested for behavioral activation induced by ethanol (2.5 g/kg). Testing was conducted at a post-administration time at which that dosage evoked activating effects in the previous Experiment (i.e., 5–11 min). Conditioned taste aversion training and testing were conducted between PD31–34. Animals were given a single pairing of self-administered saccharin and ethanol’s effects (2.5 g/kg) with saccharin intake measured 48 h after the pairing. Animals were then assessed for ethanol intake.
Results
Body weights
Table 1 presents data for body weights across several points of the experimental protocol. The corresponding ANOVA indicated that, as expected, body weights showed a progressive increase across days, and males had significantly greater body weight than females (significant main effects of sex and day of assessment; F1,51 = 77.28, F12,612 = 813.55, respectively; p < 0.001). Ethanol treatment on PD28 and PD31 did not alter this pattern of results. Body weight was not significantly different between adolescents classified as high- or low-responders in terms of ethanol-induced locomotor activity.
Table 1.
Adolescent body weights during Experiment 2.
| PD28 | PD30 | PD37 | PD38 | PD39 | PD40 | PD41 | PD42 | PD43 | PD44 | PD49 | PD50 | PD51 | PD52 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | 69.7 ± 0.9 | 69.3 ± 1.2 | 84.0 ± 1.4 | 80.8 ± 1.4 | 79.3 ± 1.2 | 78.5 ± 1.2 | 96.6 ± 2.0 | 98.8 ± 2.0 | 105.9 ± 2.1 | 107.3 ± 1.8 | 112.4 ± 1.8 | 109.5 ± 1.7 | 104.7 ± 4.5 | 108.5 ± 1.9 |
| Males | 77.2 ± 1.2 | 77.4 ± 1.4 | 93.3 ± 1.4 | 89.6 ± 1.4 | 88.2 ± 1.3 | 87.5 ± 1.3 | 107.0 ± 1.8 | 111.2 ± 1.8 | 117.5 ± 1.8 | 120.7 ± 2.0 | 132.4 ± 2.7 | 129.9 ± 2.7 | 127.3 ± 3.3 | 128.9 ± 2.7 |
Body weight of male and female adolescent rats in Experiment 2. These animals were assessed for ethanol-induced locomotor activation (PD28), trained for ethanol-mediated conditioned taste aversion (PD31–33), and underwent a 16-day-long ethanol intake protocol (PD37–52). Values are expressed as mean ± SEM.
Ethanol-induced behavioral activation on PD28
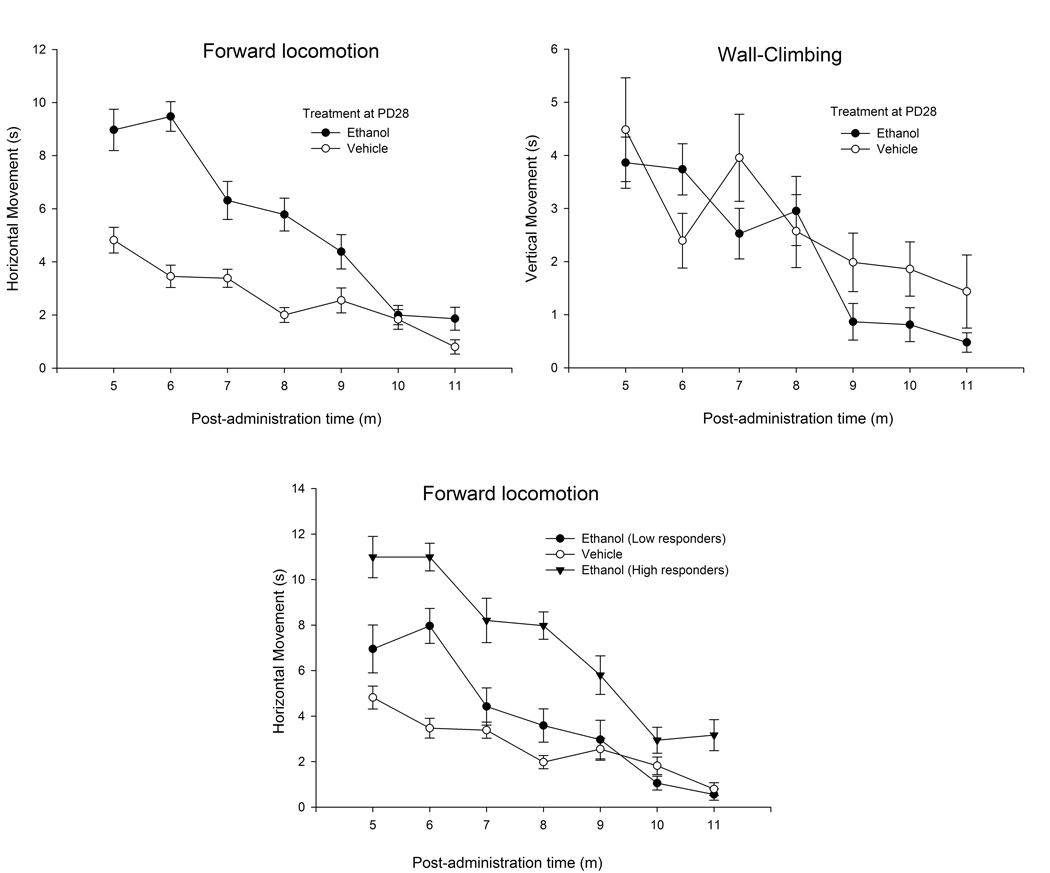
Horizontal and vertical activity is shown in Fig. 2a (upper section, left and right panels, respectively). Administration of ethanol evoked substantial forward locomotion, significantly more than that observed in animals treated with only vehicle. Vertical climbing behavior showed a progressive decline across the session. The inferential analysis confirmed these observations. The ANOVA for horizontal behavior indicated significant main effects of ethanol dose and bin of testing and a significant ethanol dose × bin of testing interaction (F1,55 = 38.88, F6,330 = 43.13, F6,330 = 9.01, respectively; p < 0.001). Planned comparisons indicated significant differences between ethanol- and water-treated animals during bins 1, 2, 3, 4, and 5. The ANOVA for vertical climbing behavior only indicated a significant main effect of bin of testing (F6,330 = 8.54; p < 0.001). Sex exerted neither a significant main effect on horizontal or vertical movements nor significant interactions with the remaining variables. The ethanol-treated animals were divided into low- and high-responders (LR, HR) by a median split procedure. One-way ANOVA indicated the effectiveness of this allocation (F2,56 = 77.12, p < 0.0001). Horizontal (forward) locomotion during the session for ethanol-treated LR and HR animals is shown in Fig. 2 (lower panel).
Figure 2.
Upper panel: Locomotor activity during a post-administration time of 5–11 min (forward locomotion and wall-climbing, left and right sections, respectively, expressed in seconds) in 28-day-old male and female adolescent rats given ethanol (2.5 g/kg, i.g.) or its vehicle (tap water). Lower panel: Locomotor activity (forward-locomotion) during a post-administration time of 5–11 min in 28-day-old male and female adolescent rats given ethanol (2.5 g/kg, i.g.) or its vehicle (tap water). In this panel, ethanol-treated adolescents were divided into high- and low-responders by a split-median procedure. Data were collapsed across sex (male or female). The sex factor did not exert a significant main effect or significantly interact with the remaining variables. The vertical bars indicate SEM.
Ethanol-mediated conditioned taste aversion on PD34
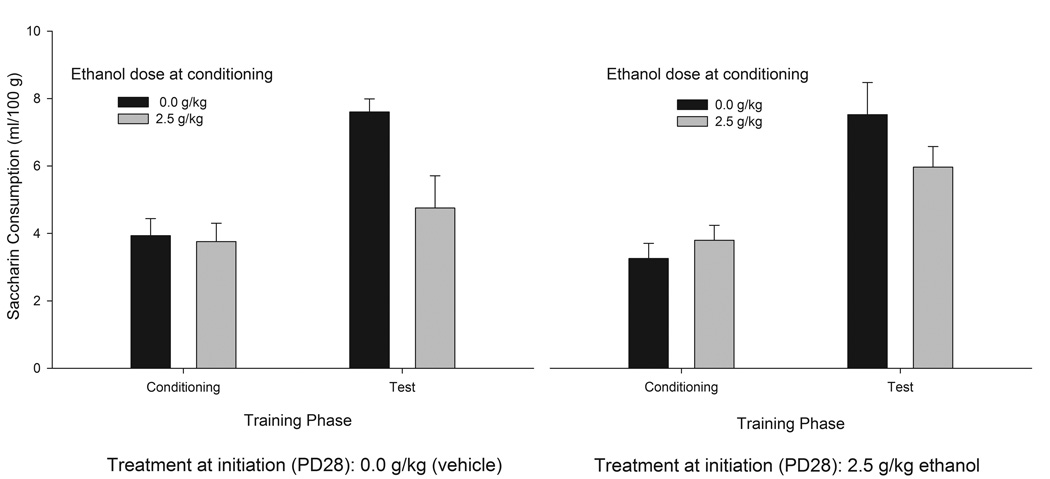
The ANOVA for saccharin intake during training indicated the absence of baseline differences in saccharin consumption as a function of sex or previous ethanol experience on PD28. Saccharin intake at test was significantly lower in animals given the saccharin-ethanol pairing on PD31 than in vehicle-treated controls (F1,51 = 11.29; p < 0.005). Thus, ethanol induced CTA in the adolescents. The ANOVA indicated no significant main effect of sex or ethanol treatment on PD28 on the subsequent ability of ethanol to induce CTA. That is, the conditioned response did not significantly differ between males and females or between adolescents with or without initiation of ethanol intake on PD28. Moreover, ANOVA indicated that HR and LR subjects exhibited similar patterns of saccharin intake at training and at test. Fig. 3 depicts saccharin intake during training and testing as a function of ethanol treatment on PD28 and PD31.
Figure 3.
Saccharin intake (ml/100 g) during conditioning and test sessions in male and female adolescent rats as a function of ethanol treatment during initiation (PD28) and conditioning (PD31). On PD28, the rats were treated with ethanol (2.5 g/kg, i.g.) or its vehicle (tap water, 0.0 g/kg). During conditioning (PD31), saccharin intake was paired with ethanol administration (2.5 g/kg, i.g.) or its vehicle (tap water, 0.0 g/kg). The length of conditioning and test sessions was 30 and 60 min, respectively. Data were collapsed across sex (male or female). The sex factor did not exert a significant main effect or significantly interact with the remaining variables. The vertical bars indicate SEM.
Pearson product-moment correlation conducted for the overall sample of animals as well as for each subgroup of high- and low-responders indicated a lack of association between ethanol-induced forward locomotion on PD28 and saccharin intake on PD31.
Ethanol-intake assessment from PD37 to PD52
Water intake
Water intake during Phases 1 and 4 of the protocol was not affected by ethanol dose. The ANOVA revealed that females drank significantly more water than males, but only during the first phase (significant main effect of sex and significant sex × phase interaction; F1,51 = 4.23, F1,51 = 4.65, respectively; p < 0.05). Overall water intake was higher on the last day of each phase and increased significantly from Phase 1 to Phase 4 (significant main effects of day and phase; F3,153 = 25.75, F1,51 = 24.57, respectively; p < 0.0001). The increase in water intake between phases was particularly clear during sessions 2 and 4 (significant phase × day interaction; F3,153 = 6.19, p < 0.001). Table 2 presents data for water intake in males and females across the sessions of Phases 1 and 4.
Table 2.
Water intake (ml/100 g) in male and female adolescents during Phases 1 and 4 of the intake protocol (Experiment 2).
| Phase 1 | Phase 4 | |||||||
|---|---|---|---|---|---|---|---|---|
|
Session 1 (PD37) |
Session 2 (PD38) |
Session 3 (PD39) |
Session 4 (PD40) |
Session 1 (PD49) |
Session 2 (PD50) |
Session 3 (PD51) |
Session 4 (PD52) |
|
| Females | 5.95 ± 0.51 | 7.32 ± 0.50 | 7.13 ± 0.41 | 8.42 ± 0.15 | 5.15 ± 0.33 | 5.53 ± 0.30 | 6.45 ± 0.29 | 6.10 ± 0.30 |
| Males | 4.85 ± 0.41 | 6.32 ± 0.45 | 6.54 ± 0.31 | 7.52 ± 0.27 | 5.18 ± 0.30 | 5.75 ± 0.15 | 5.70 ± 0.18 | 6.16 ± 0.30 |
Water intake (ml/100 g) by males and females during Phases 1 and 4 of the intake protocol. During each daily 2 h intake session, adolescents were given simultaneous access to tap water and a given ethanol solution. Values are expressed as mean ± SEM.
Ethanol intake during Phases 1 and 4 (first and second instances of two-bottle choice tests, PD37–40 and PD49–52, respectively)
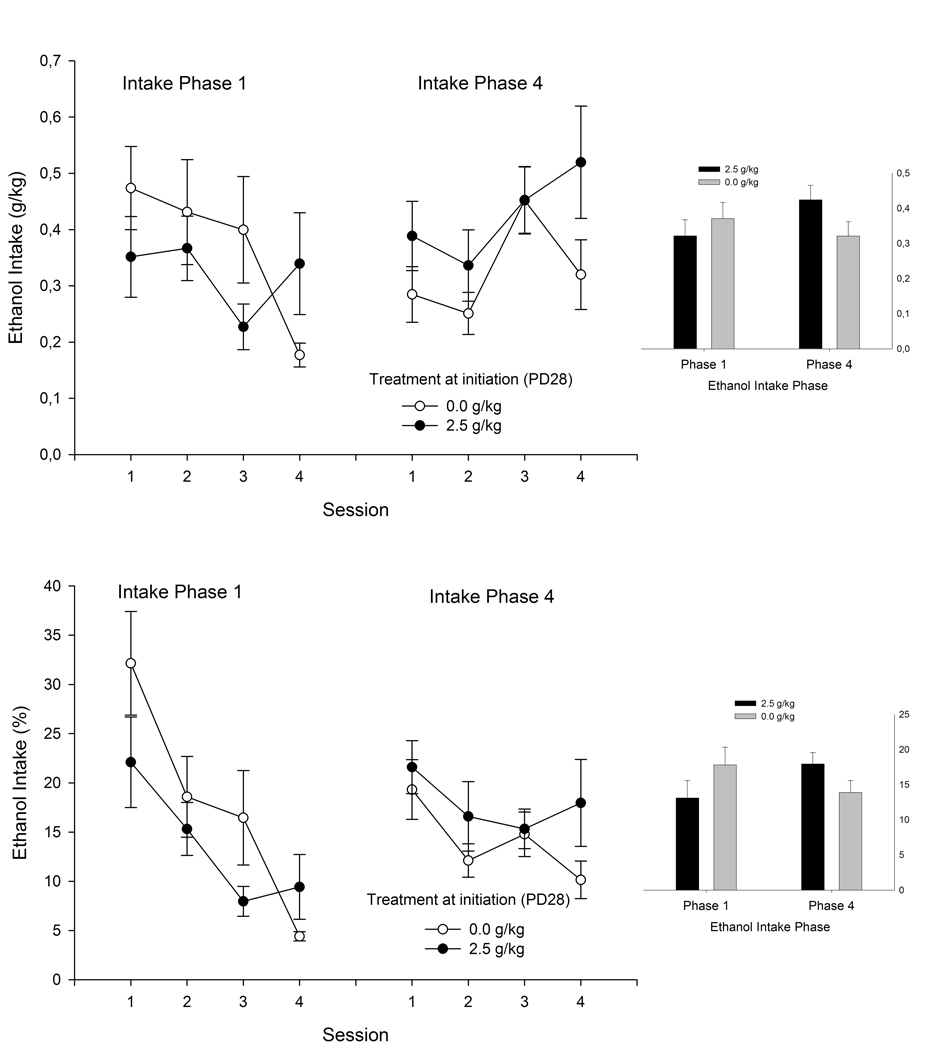
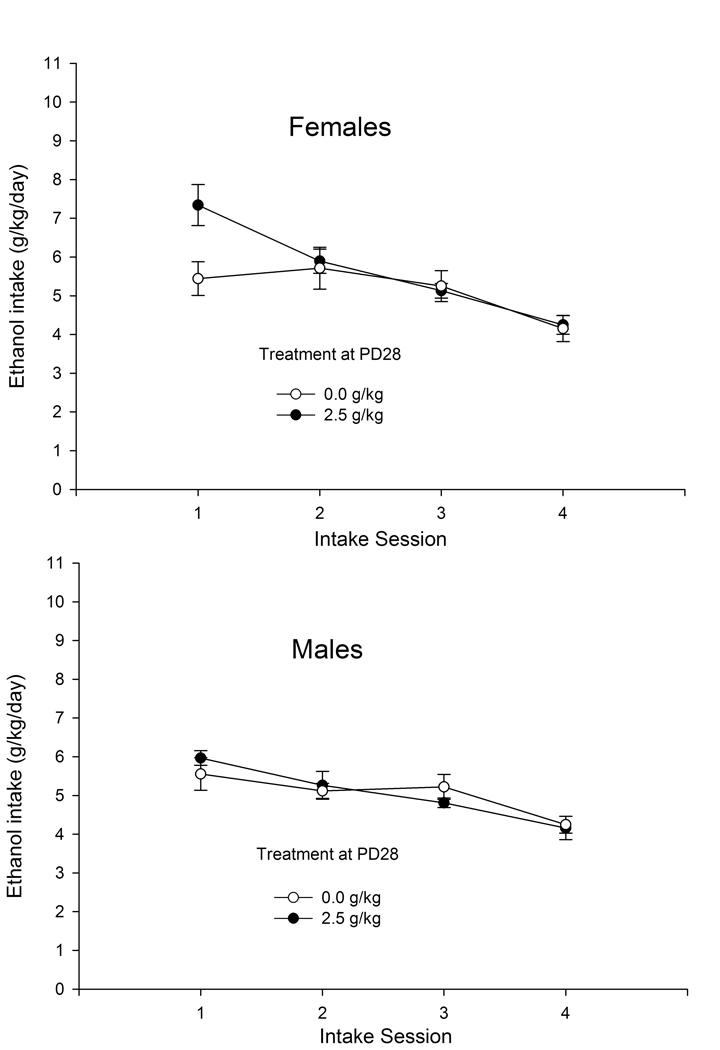
Fig. 4 depicts ethanol intake (g/kg and percent selection) during Phases 1 and 4. During Phase 1, animals exhibited a progressive decrease in ethanol intake across testing days. This effect was not observed during Phase 4, in which ethanol intake either increased (g/kg) or remained fairly stable (percent selection). Ethanol treatment on PD28 (i.e., “initiation”) appeared to affect ethanol intake. Although initiated and non-initiated animals drank roughly the same amount of ethanol during Phase 1, adolescents initiated with ethanol on PD28 exhibited more ethanol intake during Phase 4 and when offered 6% ethanol.
Figure 4.
Ethanol intake (grams per kilogram and its corresponding percentage, upper and lower sections, respectively) in male and female adolescent rats during Phases 1 and 4 of the ethanol intake protocol as a function of day of assessment (sessions 1, 2, 3, and 4) and ethanol treatment during initiation. Initiation occurred on PD28 and consisted of a single administration of ethanol (2.5 g/kg, i.g.) or its vehicle (0.0 g/kg, tap water). Then, during PD37–52, the adolescents were subjected to a procedure for the assessment of ethanol consumption, which consisted of four phases. The graph depicts ethanol intake during Phases 1 and 4. Each of these phases was composed of four sessions, in which animals had access to water and a given ethanol solution (3, 4, 5, or 6% ethanol, for sessions 1, 2, 3, and 4, respectively). Data were collapsed across sex. The sex factor did not exert a significant main effect or significantly interact with the remaining variables. The smaller bar graphs depict ethanol intake during Phases 1 and 4, averaged across sessions. The vertical bars indicate SEM.
The ANOVAs indicated that ethanol treatment on PD31 (during CTA training) and sex did not significantly affect ethanol intake. The ANOVA for g/kg indicated that the phase × day interaction was significant, as well as the phase × initiation and day × initiation interactions (F3,153 = 4.40, F1,51 = 5.03, F3,153 = 2.85, respectively; p < 0.05). The ANOVA for percent selection indicated that the phase × day and phase × initiation interactions achieved significance (F3,153 = 4.32, F1,51 = 5.48, respectively; p < 0.05). For both dependent variables, pair-wise comparisons indicated that ethanol intake during Phase 1 significantly decreased from day 1 (when animals were offered 3% ethanol) to day 4 (when 6% ethanol was given). Pair-wise comparisons also indicated a corresponding significant increase during Phase 4. Post hoc tests confirmed that ethanol intake significantly increased from Phase 1 to 4 in ethanol-initiated animals, but not in counterparts treated with vehicle on PD28, and revealed that average consumption of 6% ethanol was greater in ethanol-initiated animals than in adolescents treated with vehicle on PD28.
Ethanol intake during Phase 2 (24 h, forced-access intake phase, PD41–44)
ANOVAs indicated significant main effects of sex (F1,51 = 3.86, p < 0.05) and day of assessment (F3,153 = 23.52, p < 0.0001) and a significant day of assessment × ethanol dose interaction on PD28 (F3,153 = 3.96, p < 0.01). Ethanol treatment on PD31 (during CTA training) did not significantly affect intake scores. Fig. 5 depicts intake values in ethanol-initiated and non-initiated male and female adolescents (upper and lower sections, respectively). Ethanol initiation implies the ethanol dosage administered on PD28.
Figure 5.
Ethanol intake (g/kg) in female and male adolescent rats during Phase 2 of the intake protocol as a function of day of assessment (sessions 1, 2, 3, and 4) and ethanol treatment during initiation. Initiation occurred on PD28 and consisted of a single administration of ethanol (2.5 g/kg, i.g.) or its vehicle (0.0 g/kg, tap water). Then, during PD37–52, the adolescents were subjected to a procedure for the assessment of ethanol consumption, which consisted of four phases. The graph depicts ethanol intake during Phase 2. This phase lasted for 4 days, in which animals were given continuous, 24 h access to ethanol as the only fluid available in the homecage. Data in this figure are collapsed across ethanol treatment on PD31 (2.5 or 0.0 g/kg). This factor did not exert a significant main effect or significantly interact with the remaining variables. The vertical bars indicate SEM.
According to the planned comparisons, ethanol intake during the last 24 h cycle was significantly lower than on any other day. Pair-wise comparisons indicated that females drank significantly more ethanol than males and, perhaps more importantly, that initial ethanol consumption was significantly greater in ethanol-initiated adolescents than in controls given vehicle. Specifically, ethanol administration on PD28 significantly increased the total g/kg of ethanol consumed during the first 24 h cycle of Phase 2. Although this effect appeared to be greater in females than males (Fig. 5), the three-way interaction (sex × session × initiation) did not achieve significance.
Ethanol intake as a function of high or low sensitivity to ethanol-induced locomotor activity during initiation on PD28
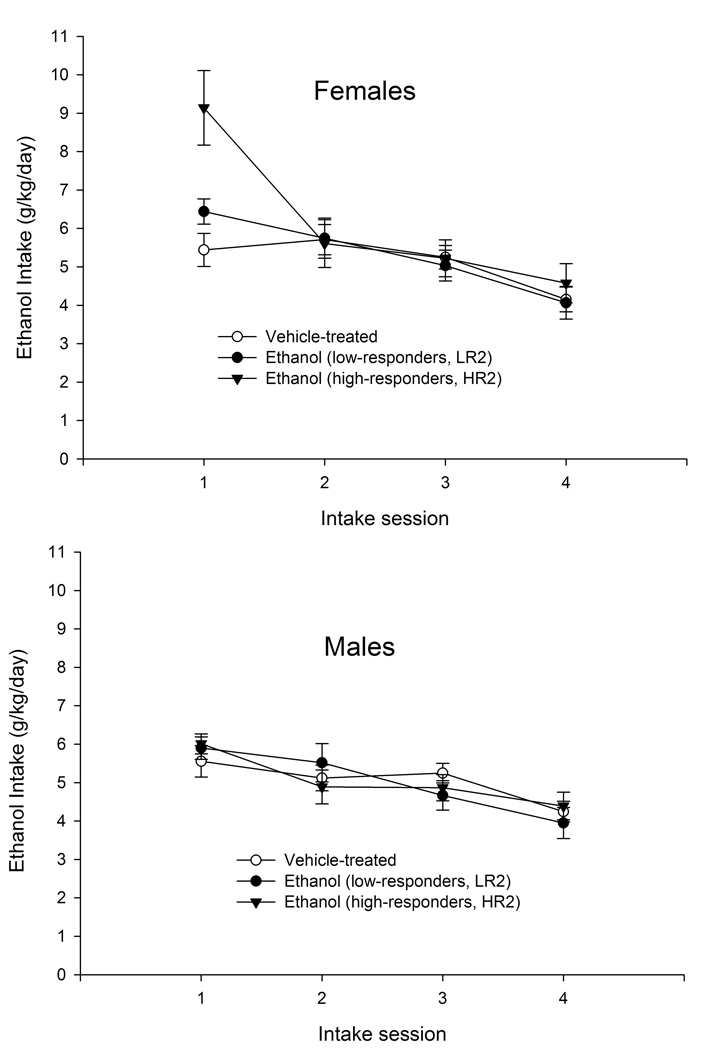
The ANOVAs for ethanol intake and percent selection across Phases 1 and 4 of the intake protocol revealed very similar drinking between high- and low-responders. High responders, however, drank more 6% ethanol (i.e., g/kg on day 4 of each phase) than low-responders or non-initiated animals (F1,47 = 6.26, F1,47 = 13.96, respectively; p < 0.01). Mean intake (g/kg) of 6% ethanol across Phases 1 and 4 in HR, LR, and water-treated (non-initiated) animals has been depicted in Figure 6.
Figure 6.
Left panel: Ethanol intake (g/kg) in female and male adolescent rats during Phase 2 of the intake protocol as a function of day of assessment (sessions 1, 2, 3, and 4) and sensitivity to ethanol treatment at initiation. Right panel: Mean intake of 6% ethanol (g/kg) in adolescent rats during phases 1 and 4 of the intake protocol as a function of sensitivity to ethanol treatment at initiation. Initiation occurred on PD28 and consisted of a single administration of ethanol (2.5 g/kg, i.g.) or its vehicle (0.0 g/kg, tap water). In these panels, ethanol-treated adolescents were divided into high- and low-responders by a split-median procedure that considered the total amount of forward locomotion evoked by ethanol during initiation on PD28. During PD37–52, the adolescents were subjected to a procedure for the assessment of ethanol consumption, which consisted of four phases. The graph in the left panel depicts ethanol intake during Phase 2. This phase lasted for 4 days, in which animals were given continuous, 24 h access to ethanol as the only fluid available in the homecage. The graph in the right panel depicts mean ethanol intake of 6% ethanol across Phases 1 and 4. During these phases adolescents were given daily two-bottle choice tests. On the first session, a 3% v/v ethanol solution was available together with the water. This solution was increased by 1% v/v of ethanol per day until reaching 6% v/v ethanol on session 4. Data in this figure are collapsed across ethanol treatment on PD31 (2.5 or 0.0 g/kg). The vertical bars indicate SEM.
The ANOVA for g/kg consumed during Phase 2 (between-group factors: sex, treatment at CTA training, and class-assignment; within-group factors: ethanol intake on PD41, 42, 43, and 44) yielded a significant class-assignment × day of assessment interaction (F6,141 = 3.52, p < 0.005) as well as a significant four-way interaction between the latter factors and ethanol treatment on PD31 and sex (F6,141 = 2.98, p < 0.01).
To better understand the significant four-way interaction yielded by the overall analysis, follow-up ANOVAs (between-group factors: class-assignment and treatment on PD31; within-group factors: ethanol intake on PD41, 42, 43, and 44) were performed for each sex. In males, only a significant main effect of session was observed (F3,87 = 11.32, p < 0.001). Males, regardless of treatment at initiation, drank gradually less ethanol as the phase progressed. In females, the ANOVA indicated significant main effects of the within-group factor (session) and the class-assignment factor (F3,54 = 15.65, F2,54 = 4.44, respectively; p < 0.05). The interaction between these two factors also achieved significance (F6,54 = 2.88, p < 0.05). Subsequent pair-wise comparisons indicated that during the first 24 h cycle of this phase, HR female animals drank significantly more ethanol than any other group of females. Low responders and water-treated adolescents did not differ between each other. These results are depicted in Fig. 6.
Pearson correlation tests indicated the absence of a significant association between saccharin consumption during the CTA test and ethanol intake scores. These correlations were conducted for the total sample of subjects as well as for each group defined by the combination of treatments on PD28 and PD31.
Experiment 3
This experiment analyzed BECs in male and female adolescent rats characterized as high- or low-responders in terms of ethanol-induced locomotor activity. The aim was to assess whether differences in ethanol metabolism could account for the behavioral differences observed between HR and LR animals. Animals (n = 28, 18 males and 10 females) were given ethanol (2.5 g/kg) and then assessed in terms of locomotor activation. Animals were sacrificed at a post-administration time of 13 min, and their BECs were assessed using gas chromatography.
Results
Ethanol induced significantly more forward locomotion in high- than in low-responders (F1,24 = 53.65, p < 0.0001). Mean and standard error values were the following: LR (16.11 ± 2.52), HR (42.22 ± 2.52). Similar to the previous experiments, high- and low-responders did not differ in ethanol-induced vertical climbing behavior. Sex did not exert a main effect or significantly interact with the group-assignment factor.
The two-way between-group ANOVA for BECs indicated a lack of significant main effects or significant interactions. Sex exerted neither a significant main effect nor a significant interaction with the remaining factor. Mean and SEM, respectively, in LR and HR adolescents were the following: males (144.20 ± 14.91 mg% and 122.05 ± 12.39 mg%), females (135.20 ± 14.97 mg% and 108.16 ± 16.18 mg%). Under the present conditions, BECs at the time of testing appeared to be similar in high- and low-responders.
Discussion
Previous studies found that adolescent mice (Stevenson et al., 2008) and infant rats (Arias et al., 2009a, b) exhibit ethanol-induced locomotor activation. The evidence for this effect in adolescent rats was, however, scarce. One of the main findings of the present study is that adolescent, heterogeneous Wistar rats show robust and reliable sensitivity to ethanol-mediated forward locomotion. The activating effects of ethanol were specific for horizontal, forward locomotion, were similar across males and females, and emerged when testing occurred soon after administration (5–11 min). Another important result was that ethanol exposure during early adolescence (i.e., ethanol initiation on PD28) significantly affected ethanol affinity during late adolescence.
In Experiments 2 and 3, characterizing subpopulations of animals with differential susceptibility to ethanol’s effects was possible (i.e., high- and low-responders). This differential sensitivity was apparently not related to differences in ethanol metabolism.
Recently, Arias et al. (2008) found that 2.5 g/kg ethanol evoked forward locomotion in 2-week-old rats tested 5–10 min post-administration but not if testing was delayed until 30–35 min or 60–65 min. The activating effect was blocked by nonspecific opioid antagonism (Arias et al., 2009b). Ethanol’s locomotor-activating effects have been considered an index of the appetitive, rewarding effects of the drug (Pautassi et al., 2009). In the infant rat, ethanol’s locomotor-stimulating and appetitive rewarding effects appear to share similar temporal dynamics. Ethanol (0.5–2.0 g/kg) has been observed to induce conditioned reinforcement when training occurs soon after ethanol administration (approximately 5–20 min), a period that coincides with the ascending limb of the blood ethanol curve (Molina et al., 2007). Additionally, similar to ethanol-induced activity, ethanol reinforcement is inhibited by nonspecific and specific opioid antagonists (Nizhnikov et al., 2009).
The present study suggests an ontogenetic continuity between infant and adolescent rats. Similar to the infants tested by Arias et al. (2008), ethanol induced greater locomotor activation in the present adolescents when testing occurred during the ascending limb of the blood ethanol curve than when testing occurred at a later (30–36 min) time-point. In humans, the acute psychomotor stimulant effects of ethanol also emerge shortly after the onset of intoxication and are dependent on the integrity of the endogenous opioid system (Peterson et al., 1996). Opioid involvement in ethanol-induced activity in preweanling rats (Arias et al., 2009b) also suggests that the psychomotor effects of ethanol in adolescent rats are associated with activation of the endogenous opioid system. In heterogeneous adult rats, systemic or intracerebral ethanol induced locomotor-stimulating effects and also facilitated the release of dopamine in the nucleus accumbens (Löf et al., 2007; Yim and Gonzales, 2000; Yim et al., 1998). Similar results have been found in preweanling rats (Arias et al., 2009a). Therefore, the direct or indirect involvement of the dopaminergic system in ethanol’s stimulating effects during adolescence appears likely.
Consistent with recent work (Varlinskaya and Spear, 2008), adolescents readily learn ethanol’s aversive consequences tested by CTA. This finding was similar across initiated and non-initiated males and females in the present study. Moreover, the development of CTA was not related to sensitivity to ethanol-induced locomotor stimulation on PD28 and did not affect later ethanol consumption. The CTA design of Experiment 2, however, lacks groups given ethanol administration only or unpaired exposure to ethanol and saccharin, respectively. The absence of these controls precludes more definitive conclusions about the apparent insensitivity of CTA to the previous ethanol experience.
Ethanol intake was greater in Phase 4 compared to Phase 1. Ethanol deprivation for several days increases subsequent ethanol intake, a phenomenon called alcohol deprivation effect (Füllgrabe et al., 2007). Although lacking necessary controls (e.g., animals given continuous access to ethanol across phases) this result indicates that adolescent rats may be sensitive to the facilitative effects of ethanol deprivation (García-Burgos et al., 2009).
In Phase 2 of the intake protocol female rats drank more ethanol than males. Ethanol intake in females may be influenced by the cyclical variations associated with the estrous cycle and the release of hormones. Ford et al., (2002) reported alterations in the microstructure of ethanol intake during the estrous cycle and another study (Lancaster et al., 1996) observed a sharp increase in ethanol intake by females by PD52, when the estrous cycle is achieving maturity. Estrogen is released by female rats from PD36, approximately. Therefore, the possible involvement of estrogen in the sex-related difference observed in our study at ~ PD41 should not be dismissed. Sex-related differences in the breakdown of ethanol could also account for the results. Several experiments, however, indicate similar blood or brain ethanol levels in female and male adolescent rats when using a wide range of doses and sampling intervals (Pautassi et al., 2008; Silveri and Spear, 2000).
Single, binge-like exposure on PD28 in the present study was associated with greater ethanol consumption later in adolescence. When assessed in sequential two-bottle choice tests (Phases 1 and 4), the facilitating effect of early initiation was relatively small but significant. Under conditions of continuous homecage access to ethanol (Phase 2), a significant, yet relatively transient, effect of early initiation was observed, particularly in females. These females, incidentally, ingested almost 8 g/kg of ethanol in their first 24 h cycle of ethanol access, and similar to a previous study in female adolescents (Doremus et al., 2005; Truxell et al., 2007) had higher intake scores than males.
Intriguingly, ethanol exposure on PD28 increased ethanol intake during late adolescence, but exposure on PD31 apparently did not. Perhaps, in the rat, ethanol initiation facilitates later ethanol intake when initiation occurs during a specific stage, such as during the earlier stages of adolescence. Interestingly, the mesolimbic dopaminergic system of the rat undergoes marked changes during adolescence, notably a substantial pruning of receptors between postnatal days 28 to 35 (Tarazi and Baldesarini, 2000). The number of mesolimbic dopamine receptors rises steadily beginning about PD7 to a peak at PD28, and then declines significantly (Tarazi and Baldesarini, 2000). The dopaminergic system modulates motor activation and motivational effects induced by ethanol (Arias et al., 2009a). Therefore, ethanol dosing at PD28 may have affected ethanol intake in Exp. 2 by altering the normal process of dopamine receptor pruning. This is just a hypothesis and further studies are needed to test it, although it is intriguing that Pascual et al. (2009) found that ethanol administration during adolescence altered the mesolimbic dopaminergic system, a change that was associated with heightened ethanol consumption at adulthood.
These findings should be discussed within the framework of previous attempts to model the “early debut effect.” Slawecki and Betancourt (2002) found that extensive ethanol initiation during adolescence (12 h per day, PD30–40, via vapor inhalation) did not affect ethanol affinity in adulthood. A 3 or 10 day exposure to oral ethanol also failed to affect later operant self-administration of ethanol (Tolliver and Samson, 1991). Engagement in and relapse of ethanol self-administration, however, was facilitated in adult ethanol-preferring rats that had been exposed to ethanol during adolescence (Rodd-Henricks et al., 2002). Tambour et al. (2008) found that early drinking transiently increased ethanol consumption in adolescent mice, although ethanol consumption after or during stress exposure was not altered by early experience with the drug. Another study (Siegmund et al., 2005) found similar levels of ethanol intake in animals initiated with the drug during either adolescence or adulthood. However, adolescents drank more than adults if given swim or footshock stress exposure, suggesting that, similar to humans (Dawson et al., 2007), early ethanol initiation may facilitate stress-reactive ethanol consumption. Recently, Truxell et al. (2007) found that prior adolescent exposure to ethanol by an apparently voluntary intake preparation (consumption-off-the-floor) heightened ethanol intake on PD36. Pascual et al. (2009) found that chronic and intermittent ethanol treatment (3 g/kg, i.p.) during adolescence enhanced ethanol intake when tested during adulthood, but only after repeated testing.
Altogether, these studies suggest that the conditions under which alcohol initiation affects later ethanol intake are still unclear, and more work is needed. A common denominator, however, among Pascual et al. (2009), Siegmund et al. (2005), and the present study is that expression of an initiation effect was not apparent when intake was first assessed but emerged after animals underwent substantial ethanol exposure or were exposed to a source of stress. In the present study, water deprivation, which may have induced some degree of stress, was used to facilitate postweaning ethanol drinking.
An apparent association was found between sensitivity to ethanol’s locomotor-stimulating effects and ethanol intake. Female rats selected for their high sensitivity to the activating effects of ethanol ingested, during the first 24 h of forced-access to ethanol, significantly more ethanol than non-initiated females and significantly more than counterparts initiated to ethanol but classified as low-responders. The level of ethanol intake found in low-responders was not significantly different from non-initiated animals. Greater intake in HR than LR animals was also found when animals were offered 6% ethanol during Phases 1 and 4. These findings are consistent with several studies, indicating that rat strains genetically selected for heightened ethanol intake are more sensitive to the locomotor-stimulating effects of ethanol than strains selected for low ethanol consumption (Bell et al., 2006; Colombo et al., 2006; Quintanilla et al., 2006). Similarly, mice selectively bred for sensitivity to ethanol-induced locomotor stimulation drank more ethanol and showed less ethanol-mediated CTA than counterparts selected for low ethanol affinity (Risinger et al., 1994).
In conclusion, the present study introduces a simple model for detecting locomotor-stimulating effects of ethanol in adolescent rats and supports the hypothesis that even brief ethanol exposure during adolescence can promote later ethanol intake. Further work is needed to better identify the conditions that promote high-affinity ethanol intake during adolescence as well as the factors mediating the link between early ethanol initiation and vulnerability to ethanol abuse and dependence.
Acknowledgments
This work was supported by National Institute on Alcohol Abuse and Alcoholism grants AA011960 and AA01309 to NES, PICT 05-14024 from the Agencia Nacional de Promocion Cientifica y Tecnologica (Argentina) to JCM, and grant PRH-UNC (FONCyT-SPU) (Argentina) to RMP. We would like to thank Beatriz Haymal for her technical assistance in the blood ethanol measurements and Carlos Arias for his thoughtful and insightful comments throughout the data analyses.
References
- Acevedo MB, Molina JC, Pautassi RM. Ethanol-induced activation in adolescent rats. Paper presented at the 12th Meeting of the Argentinean Association of Behavioral Sciences, Catholic University; August 27–29; Buenos Aires, Argentina. 2009. [Google Scholar]
- Anderson RI, Varlinskaya EI, Spear LP. Isolation stress and ethanol-induced conditioned taste aversion in adolescent and adult male rats. Paper presented at the 41st Annual Meeting of the International Society for Developmental Psychobiology; Nov. 12–15; Washington DC. 2008. [Google Scholar]
- Anthony JC, Petronis KR. Early-onset drug use and risk of later drug problems. Drug and Alcohol Dependence. 1995;40:9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- Arias C, Mlewsky C, Hansen C, Molina JC, Paglini MG, Spear NE. Dopamine receptors modulate ethanol’s locomotor-activating effects in preweanling rats. Developmental Psychobiology. 2009a doi: 10.1002/dev.20407. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Molina JC, Spear NE. Naloxone and baclofen attenuate ethanol's locomotor-activating effects in preweanling Sprague-Dawley rats. Behavioral Neuroscience. 2009b;123:172–180. doi: 10.1037/a0014049. [DOI] [PubMed] [Google Scholar]
- Arias C, Molina JC, Mlewski EC, Pautassi RM, Spear NE. Acute sensitivity and acute tolerance to ethanol in preweanling rats with or without prenatal experience with the drug. Pharmacology Biochemistry and Behavior. 2008;89:608–622. doi: 10.1016/j.pbb.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Molina JC, Spear NE. Ethanol-mediated aversive learning as a function of locomotor activity in a novel environment in infant Sprague-Dawley rats. Pharmacology Biochemistry and Behavior. 2009c;92:621–628. doi: 10.1016/j.pbb.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Sable HJK, Schultz JA, Hsu CC, Lumeng L, Murphy JM, McBride WJ. Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacology Biochemistry and Behavior. 2006;83:35–46. doi: 10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Schultz JA, Peper CL, Lumeng L, Murphy JM, McBride WJ. Effects of short deprivation and re-exposure intervals on the ethanol drinking behavior of selectively bred high alcohol-consuming rats. Alcohol. 2008;42:407–416. doi: 10.1016/j.alcohol.2008.03.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuck TL, McLaughlin PJ, Arizzi-Lafrance MN, Salamone JD, Correa M. Comparison between multiple behavioral effects of peripheral ethanol administration in rats: sedation, ataxia, and bradykinesia. Life Sciences. 2006;79:154–161. doi: 10.1016/j.lfs.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Colombo G, Lobina C, Carai MA, Gessa GL. Phenotypic characterization of genetically selected Sardinian alcohol-preferring (sP) and -non-preferring (sNP) rats. Addiction Biology. 2006;11:324–338. doi: 10.1111/j.1369-1600.2006.00031.x. [DOI] [PubMed] [Google Scholar]
- Cools AR, Gingras MA. Nijmegen high and low responders to novelty: a new tool in the search after the neurobiology of drug abuse liability. Pharmacology Biochemistry and Behavior. 1998;60:151–159. doi: 10.1016/s0091-3057(97)00586-8. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus JS, Noble D. Species difference in sensitivity to ethanol’s hedonic effects. Alcohol. 1993;10:97–102. doi: 10.1016/0741-8329(93)90087-5. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Li TK. Impact of age at first drink on stress-reactive drinking. Alcoholism: Clinical and Experimental Research. 2007;31:69–77. doi: 10.1111/j.1530-0277.2006.00265.x. [DOI] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. American Journal of Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcoholism: Clinical and Experimental Research. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Faria RR, Lima Rueda AV, Sayuri C, Soares SL, Malta MB, Carrara-Nascimento PF, da Silva Alves A, Marcourakis T, Yonamine M, Scavone C, Giorgetti Britto LR, Camarini R. Environmental modulation of ethanol-induced locomotor activity: correlation with neuronal activity in distinct brain regions of adolescent and adult Swiss mice. Brain Research. 2008;1239:127–140. doi: 10.1016/j.brainres.2008.08.056. [DOI] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, Samson HH. Microanalysis of ethanol self-administration: estrous cycle phase-related changes in consumption patterns. Alcohol Clinical and Experimental Research. 2002;26:635–643. [PubMed] [Google Scholar]
- Füllgrabe MW, Vengeliene V, Spanagel R. Influence of age at drinking onset on the alcohol deprivation effect and stress-induced drinking in female rats'. Pharmacology Biochemistry and Behavior. 2007;86:320–326. doi: 10.1016/j.pbb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- García-Burgos D, González F, Manrique T, Gallo M. Patterns of ethanol intake in preadolescent, adolescent, and adult Wistar rats under acquisition, maintenance, and relapse-like conditions. Alcoholism: Clinical and Experimental Research. 2009;33:722–728. doi: 10.1111/j.1530-0277.2008.00889.x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of Substance Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology (Berl) 2007;191:311–322. doi: 10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicology and Teratology. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Klebaur JE, Bardo MT. Individual differences in novelty seeking on the playground maze predict amphetamine conditioned place preference. Pharmacology Biochemistry and Behavior. 1999;63:131–136. doi: 10.1016/s0091-3057(98)00258-5. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcoholism: Clinical and Experimental Research. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Löf E, Ericsson M, Strömberg R, Söderpalm B. Characterization of ethanol-induced dopamine elevation in the rat nucleus accumbens. European Journal of Pharmacology. 2007;555:148–155. doi: 10.1016/j.ejphar.2006.10.055. [DOI] [PubMed] [Google Scholar]
- Molina JC, Pautassi RM, Truxell E, Spear N. Differential motivational properties of ethanol during early ontogeny as a function of dose and postadministration time. Alcohol. 2007;41:41–55. doi: 10.1016/j.alcohol.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal R, Armario A, Janak PH. Positive relationship between activity in a novel environment and operant ethanol self-administration in rats. Psychopharmacology (Berl) 2002;162:333–338. doi: 10.1007/s00213-002-1091-5. [DOI] [PubMed] [Google Scholar]
- Nadal R, Rotllant D, Armario A. Perseverance of exploration in novel environments predicts morphine place conditioning in rats. Behavioural Brain Research. 2005;165:72–79. doi: 10.1016/j.bbr.2005.06.039. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington DC: National Academy Press; 1996. [Google Scholar]
- Nizhnikov ME, Pautassi RM, Truxell E, Spear NE. Opioid antagonists block the acquisition of ethanol-mediated conditioned tactile preference in infant rats. Alcohol. 2009;43:347–358. doi: 10.1016/j.alcohol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocjar C, Middaugh LD, Tavernetti M. Ethanol consumption and place-preference conditioning in the alcohol-preferring C57BL/6 mouse: relationship with motor activity patterns. Alcoholism: Clinical and Experimental Research. 1999;23:683–692. [PubMed] [Google Scholar]
- Orsini C, Buchini F, Piazza PV, Puglisi-Allegra S, Cabib S. Susceptibility to amphetamine-induced place preference is predicted by locomotor response to novelty and amphetamine in the mouse. Psychopharmacology (Berl) 2004;172:264–270. doi: 10.1007/s00213-003-1647-z. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. Journal of Neurochemistry. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Adolescent but not adult rats exhibit ethanol-mediated appetitive second-order conditioning. Alcoholism: Clinical and Experimental Research. 2008;32:2016–2027. doi: 10.1111/j.1530-0277.2008.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Nizhnikov ME, Spear NE. Assessing appetitive, aversive, and negative ethanol-mediated reinforcement through an immature rat model. Neuroscience and Biobehavioral Reviews. 2009;33:953–974. doi: 10.1016/j.neubiorev.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepino MY, Abate P, Spear NE, Molina JC. Heightened ethanol intake in infant and adolescent rats after nursing experiences with an ethanol-intoxicated dam. Alcoholism: Clinical and Experimental Research. 2004;28:895–905. doi: 10.1097/01.alc.0000128223.95184.c9. [DOI] [PubMed] [Google Scholar]
- Peterson JB, Pihl RO, Gianoulakis C, Conrod P, Finn PR, Stewart SH, LeMarquand DG, Bruce KR. Ethanol-induced change in cardiac and endogenous opiate function and risk for alcoholism. Alcoholism: Clinical and Experimental Research. 1996;20:1542–1552. doi: 10.1111/j.1530-0277.1996.tb01697.x. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Badanich KA, Kirstein CL. Place conditioning: age-related changes in the rewarding and aversive effects of alcohol. Alcoholism: Clinical and Experimental Research. 2003;27:593–599. doi: 10.1097/01.ALC.0000060530.71596.D1. [DOI] [PubMed] [Google Scholar]
- Ponce LF, Pautassi RM, Spear NE, Molina JC. Nursing from an ethanol intoxicated dam induces short- and long-term disruptions in motor performance and enhances later self-administration of the drug. Alcoholism: Clinical and Experimental Research. 2004;28:1039–1050. doi: 10.1097/01.alc.0000131298.32045.96. [DOI] [PubMed] [Google Scholar]
- Ponce LF, Pautassi RM, Spear NE, Molina JC. Ethanol-mediated operant learning in the infant rat leads to increased ethanol intake during adolescence. Pharmacology Biochemistry and Behavior. 2008;90:640–650. doi: 10.1016/j.pbb.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla ME, Israel Y, Sapag A, Tampier L. The UChA and UChB rat lines: metabolic and genetic differences influencing ethanol intake. Addiction Biology. 2006;11:310–323. doi: 10.1111/j.1369-1600.2006.00030.x. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Malott DH, Prather LK, Niehus DR, Cunningham CL. Motivational properties of ethanol in mice selectively bred for ethanol-induced locomotor differences. Psychopharmacology (Berl) 1994;116:207–216. doi: 10.1007/BF02245064. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of concurrent access to multiple ethanol concentrations and repeated deprivations on alcohol intake of alcohol-preferring rats. Alcoholism: Clinical and Experimental Research. 2001;25:1140–1150. [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: I. Periadolescent exposure. Alcoholism: Clinical and Experimental Research. 2002;26:1632–1641. doi: 10.1097/01.ALC.0000036301.36192.BC. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M. Effects of adolescent ethanol exposure on ethanol consumption in adult rats. Alcohol. 2002;26:23–30. doi: 10.1016/s0741-8329(01)00192-6. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Kingsley MA, Rezvani AH, Propst K, Swartzwelder HS, Kuhn CM. Early ethanol consumption predicts relapse-like behavior in adolescent male rats. Alcoholism: Clinical and Experimental Research. 2008;32:754–762. doi: 10.1111/j.1530-0277.2008.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Morris RW, Kuhn CM. Adolescent rats are protected from the conditioned aversive properties of cocaine and lithium chloride. Pharmacology Biochemistry and Behavior. 2006;84:344–352. doi: 10.1016/j.pbb.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcoholism: Clinical and Experimental Research. 2005;29:1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Silveri M, Spear L. Ontogeny of ethanol elimination and ethanol-induced hypothermia. Alcohol. 2000;20:45–53. doi: 10.1016/s0741-8329(99)00055-5. [DOI] [PubMed] [Google Scholar]
- Stevenson RA, Besheer J, Hodge CW. Comparison of ethanol locomotor sensitization in adolescent and adult DBA/2J mice. Psychopharmacology (Berl) 2008;197:361–370. doi: 10.1007/s00213-007-1038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence: alcohol sensitivity, tolerance, and intake. Recent Developments in Alcoholism. 2005;17:143–159. [PubMed] [Google Scholar]
- Tambour S, Brown LL, Crabbe JC. Gender and age at drinking onset affect voluntary alcohol consumption but neither the alcohol deprivation effect nor the response to stress in mice. Alcoholism: Clinical and Experimental Research. 2008;32:2100–2106. doi: 10.1111/j.1530-0277.2008.00798.x. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D1, D2 and D4 receptors in rat forebrain. International Journal of Developmental Neuroscience. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Tolliver GA, Samson HH. The influence of early postweaning ethanol exposure on oral self-administration behavior in the rat. Pharmacology Biochemistry and Behavior. 1991;38:575–580. doi: 10.1016/0091-3057(91)90016-u. [DOI] [PubMed] [Google Scholar]
- Truxell EM, Molina JC, Spear NE. Ethanol intake in the juvenile, adolescent, and adult rat: effects of age and prior exposure to ethanol. Alcoholism: Clinical and Experimental Research. 2007;31:755–765. doi: 10.1111/j.1530-0277.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Attenuated aversive effects of ethanol among adolescent rats are diminished further in adolescent males by the presence of a social partner. Alcoholism: Clinical and Experimental Research. 2008;32 Suppl 1:94A. [Google Scholar]
- Walker BM, Ehlers CL. Age-related Differences in the Blood Alcohol Levels of Wistar Rats. Pharmacology Biochemistry and Behavior. 2009;91:560–565. doi: 10.1016/j.pbb.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacology Biochemistry and Behavior. 2002;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design. 2rd ed. New York: McGraw-Hill; 1991. [Google Scholar]
- Yim HJ, Schallert T, Randall PK, Gonzales RA. Comparison of local and systemic ethanol effects on extracellular dopamine concentration in rat nucleus accumbens by microdialysis. Alcoholism: Clinical and Experimental Research. 1998;22:367–374. [PubMed] [Google Scholar]
- Yim HJ, Gonzales RA. Ethanol-induced increases in dopamine extracellular concentration in rat nucleus accumbens are accounted for by increased release and not uptake inhibition. Alcohol. 2000;22:107–115. doi: 10.1016/s0741-8329(00)00121-x. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Kent PF, Sheehe PR, Molina JC, Spear NE, Youngentob LM. Experience-induced fetal plasticity: the effect of gestational ethanol exposure on the behavioral and neurophysiologic olfactory response to ethanol odor in early postnatal and adult rats. Behavioral Neuroscience. 2007;121:1293–1305. doi: 10.1037/0735-7044.121.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]