Abstract
We propose an improved quantitative measure of EEG during brain injury and recovery after cardiac arrest. In our previous studies, we proposed a measure, information quantity (IQ), to detect the early effects of temperature manipulation on the EEG signals recorded from the scalp. IQ incorporates the wavelet transform and the Shannon entropy in full bands from delta to gamma. Unlike IQ, here we separately calculate IQ in each subband, i.e., the new measure is IQ in each subband. We will call it subband IQ (SIQ). We demonstrate the performance of the proposed method by comparing SIQ with IQ in terms of how well the meausres predict actual neurological outcomes. Thirteen rats, based on 7-min cardiac arrest were used. The experimental results show that the proposed measure was more highly correlated to neurological outcome than IQ.
Index Terms: Brain injury, cardiac arrest, EEG, entropy, hypothermia, subband, wavelet
I. INTRODUCTION
Cardiac arrest remains the major cause of death in the United States [1]. In general, only 17% of patients resuscitated from cardiac arrest survive to hospital discharge [2]–[4]. In survivors of cardiac arrest, neurological complications, which arise either during arrest by blood flow shortage or during recovery by blood flow restoration, are the leading cause of morbidity and disability among survivors [2], [5]–[7].
We hypothesize that information about brain injury and recovery is embedded in neuroelectrical activity, namely the EEG signal. Although visual inspection of the EEG provides limited insights, we expect that computational methods can be used to uncover useful details relating to neurological state. From the perspective of information theory, the information contained in a signal can be physically quantified by calculating its entropy [8]. Entropy-based EEG analysis methods that have recently been developed show promising results [9]–[11]. For example, Tong and coworkers used the time-dependent entropy (TDE) measure to study EEG during recovery from asphyxic cardiac arrest injury. They also compared Tsallis and Renyi entropy methods for hypoxic ischemic injury.
In a recent study of asphyxic cardiac arrest of graded duration, we introduced a quantitative metric called information quantity (IQ) [12]–[14], which we used to measure the early effects of temperature manipulation on electrical signals recorded from the scalp. IQ incorporates the discrete wavelet transform (DWT) to decompose the EEG signal. The components have frequency contents that correspond approximately with the standard clinical bands of interest: gamma, beta, alpha, theta, and delta (in accordance with a sampling rate of 250 Hz) [15], [16]. IQ then calculates Shannon entropy based on a distribution of all wavelet coefficients. Thus, IQ characterizes the recovery patterns of the gross EEG signal only.
In this paper, we propose an alternative quantitative measure of brain injury and recovery after cardiac arrest that is based on IQ, but accounts for the behavior of the clinical EEG subbands. To emphasize this difference, we will call the new measure subband IQ (SIQ). In the sections to follow, we describe the calculation of SIQ in further detail and compare the abilities of IQ and SIQ to predict actual neurological outcomes. Unlike IQ, we separately calculate the Shannon entropy in each subband after DWT, i.e., the new measure is the IQ in each subband. We will call it SIQ. We demonstrated the performance of SIQ by comparing the EEG measures in terms of how well the EEG measures predict actual neurological outcomes.
II. SUBBAND INFORMATION QUANTITY
Let {s(i) : i = 1, ⋯, N} denote the raw sampled EEG signal. To analyze nonstationary EEG signals, we need to get a temporal evolution of a quantitative measure. To do this, a time-dependent measure based on sliding temporal window technique is applied. Now, we define a sliding temporal window size w ≤ N and the sliding step Δ ≤ w. Sliding blocks of the raw sampled EEG signal are defined by
| (1) |
where n = 0, 1, …, [N/Δ] − w + 1, and [x] denotes the integer part of x.
Before calculating Shannon entropy, we apply DWT as in the IQ method for two reasons. First, DWT effectively removes redundancy. Second, DWT decomposes the signal into subbands that are good matches to the standard clinical bands of interest. The computation of the wavelet transform is formulated as a filtering operation with two related finite-impulse response (FIR) filters. The filtering operation is a simple linear digital filtering, followed by down-sampling. For example, assuming we use two-level DWT decomposition, the DWT coefficients are
| (2) |
where are the wavelet coefficients corresponding to three different subbands. Without loss of generality, we represent the DWT coefficients after r-level decomposition
| (3) |
Since the Shannon entropy is based on a probabilistic distribution, we estimate the probability functions, , respectively. To do this, we introduce intervals such that
| (4) |
The conventional IQ method used the probability function for all subbands, . The probability pn (m) that the sampled signal belongs to the interval Im is the ratio between the number of the samples found within interval Im and the total number of samples in all subbands. Then, IQ is defined as [12]
| (5) |
Equation (5) has little dependency on parameters such as the number of samples and intervals Im. More details on this have been studied in [9], [10], and [12].
Unlike IQ, SIQ uses the probability which is separately estimated in each subband. The probability in kth subband that the sampled signal belongs to the interval Im is the ratio between the number of samples found within interval Im and the total number of samples in kth subband. Using , SIQk (n) in the kth scale is defined as
| (6) |
where k = 1, ⋯, r + 1 and is the probability of finding the system in the mth microstate with . Thus, we can explore the SIQ evolution of the whole data {s(i) : i = 1, ⋯, N} in each subband. Finally, SIQs in each subband are averaged over all subbands
| (7) |
III. EXPERIMENTAL METHODS
We obtained experimental EEG recordings from rats during experiments designated to study the information evolution in brain rhythms following asphyxic cardiac arrest condition. EEG data are sampled at 1 kHz with antialiasing filter (<500 Hz). This brain injury model has been approved by the Animal Care and Use Committee, Johns Hopkins Medical Institutions. Asphyxic cardiac arrest and resuscitation protocol was performed as modified from Katz et al. [17].
Thirteen Wistar rats (300±25 g) were used. Anesthesia was induced with 4% halothane and 50:50% nitrous oxide:oxygen. Baseline recording of 10 min was followed by 5 min washout to ensure that halothane did not have a significant effect on EEG. After washout, asphyxia was induced by stopping and disconnecting the ventilator and clamping the tracheal tube. CA was confirmed by observing pulselessness with mean arterial blood pressure (MABP) < 10 mmHg. Asphyxia of 7 min followed. Resuscitation was done by chest compression until return of spontaneous circulation (ROCS) (i.e., achieving spontaneous MABP > 60 mmHg). For 6 of the 13 rats, clinical hypothermia was applied using cool mist, as described in [12]–[14].
All surviving rats underwent comprehensive psychological testing by an independent observer 72 h from the start of the recovery period. Neurological deficit score (NDS) was chosen as the basis for these studies because it accounts for several behavioral parameters, including motor and sensory function [18]. Furthermore, because NDS assesses performance quantitatively, on a scale of 0 (worst) to 80 (best), it is an appropriate means of mapping SIQ or IQ to neurological outcome. Rats were numbered from 1 to 13 based on increasing NDS, with rat #1 having the lowest NDS.
Two channels of EEG using subdermal needle electrodes (Plastics One, Roanoke, VA) were inserted in right and left parietal areas. Two channels of ECG and one channel of arterial pressure were recorded continuously before the insult, during the insult, and through 3 h of recovery. The signals were digitized using the data acquisition package CODAS (DATAQ Instruments, Inc., Akron, OH). Sampling frequency of 250 Hz and 12-bit A/D conversion were used.
IV. RESULTS
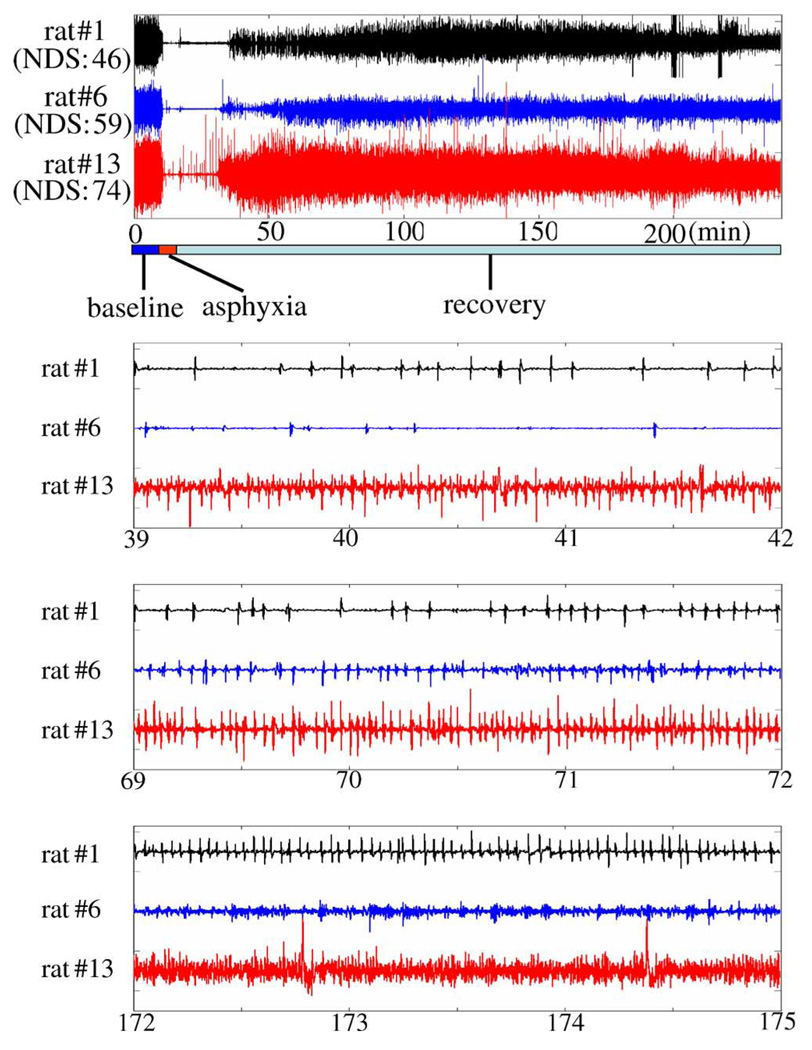
Fig. 1 presents typical EEG signals from three rats having low, middle, and high NDS. The EEG signal can be subdivided into three dynamically distinct periods relative to the time elapsed from the beginning of the experiment. The first period, comprising the 10-min baseline (BL) recording and 5 min washout, is characterized by high amplitude and frequency. During the second period, corresponding to the duration of hypoxic asphyxia, the signal becomes significantly damped, though occasional spiking persists. After the insult is relieved near the 22 min point, the EEG enters a final period where spiking frequency gradually increases, coinciding with early and late brain recovery stages.
Fig. 1.
Raw EEG data of three rats with low, middle, and high NDS (in minutes).
Microscopic views of EEGs during recovery clarify some of the major challenges of interpreting them in the time domain. EEG from rat #1 or #2 with low NDS shows slow progress of activity but EEG from rat #13 is very active and progresses fast. This is coincident with NDS, which is obtained after 72 h. This may imply that early EEG can predict neurological outcomes. But a meaningful and objective comparison of postinjury and baseline electrical activity, and thus, a measure of recovery specific to an individual subject, is very difficult in the time domain.
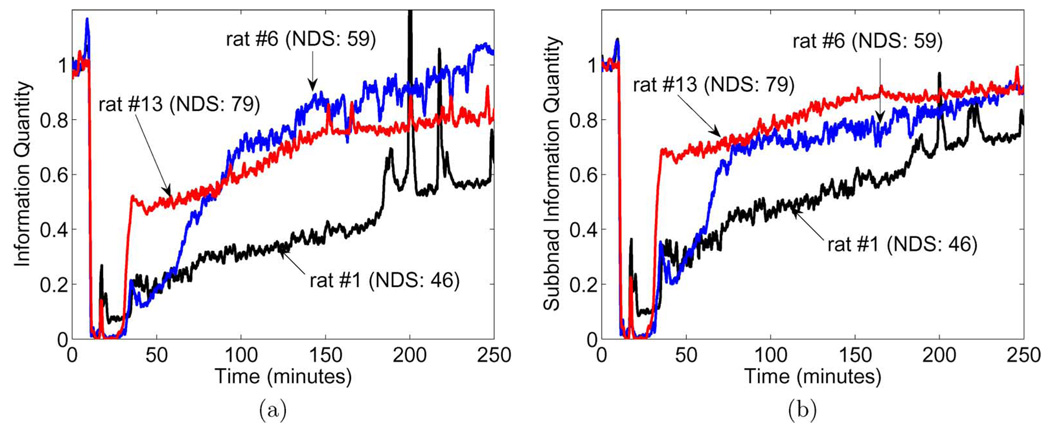
IQ and SIQ were determined for all EEG signals. For IQ, the following parameters were used: sliding window length ω = 8 s, sliding step Δ = 8 s, and M = 20, and decomposition scale r = 5. Resultant IQ functions were then averaged across left and right cortices to yield mean for each rat. Example plots of IQ as a function of time for rats 1, 6, and 13 are reproduced here [Fig. 2(a)]. Following IQ analysis, SIQ was computed from the same raw data. When applied to an EEG signal at these settings, the SIQ operation generated six signals corresponding to separate wavelet decomposition levels of the EEG. Of these, the middle four levels, which correspond roughly to EEG bands of conventional interest, were averaged together to produce mean SIQ for each hemisphere of the parietal cortex. Hemispheric SIQ pairs were then averaged, yielding an average SIQ for each rat in the cohort. Averages for rats 1, 6, and 13 are plotted [Fig. 2(b)] for comparison with the IQs previously determined. IQ and SIQ curves are normalized here to their average values over the baseline period.
Fig. 2.
Time evolution of quantitative EEG measures of three selected rats. (a) IQ. (b) SIQ.
Based on our previous study demonstrating a positive correlation between IQ and neurological function [12], we expect rise in IQ corresponding to improvement in neural function. As seen in Fig. 2, both IQ and SIQ are at initially high levels during the baseline period, then rapidly fall to approximately zero after washout, around 15-min ab initio. This trough coincides with the 7-min asphyxia period observed in raw EEG signals, when neuronal firing is suppressed. At 22 min, a singular spike indicates manual resuscitation and the beginning of the recovery period.
Nearly 13 min into recovery (35 min from the start of experiment), differing behavior is observed among the IQ and SIQ functions. Both IQ and SIQ of rat 13 escalate suddenly and reach a level (0.5 in IQ, 0.7 in SIQ) that is appreciably higher than those of rats 1 and 6. At approximately the 64-min point, however, the IQ of rat 6 begins to gradually elevate until permanently overtaking rat 13’s IQ at roughly the 92-min point. This behavior suggests that rat 6 recuperates better than rat 13; however, the higher NDS score of rat 13 contradicts this trend. The IQ of rat 1 tends consistently lower than that of rat 6 and rat 13, which is expected as its NDS value is the minimum of the three rats. In contrast, SIQ curves of the same rats are distinctly stratified in a manner that is compliant with NDS performance, with SIQ for rat 13 persistently holding the highest values [Fig. 2(b)].
To determine whether the improvements suggested by Fig. 2(b) held over a larger sample, IQ and SIQ calculations were extended to an additional ten rats (such that n = 13). Once computed for each parietal lobe and averaged as explained earlier, IQ and SIQ functions for each rat were time averaged over a selection of intervals, arbitrarily decided beforehand, spanning early and late posttrauma recovery stages. Subsequently, a comprehensive time average of the recovery period (30–240 min from the start of experiment) was calculated. Sets of IQ and SIQ averages were matched with NDS scores of corresponding rats, as shown in Table I. Here, aggregate data for each rat is organized into rows, arranged, and numbered in order of increasing NDS. IQ and SIQ data are presented together, with IQ results enclosed in parentheses.
TABLE 1.
Statistical results of IQ and SIQ (IQ results enclosed in parentheses)
| SIQ (IQ) | ||||||
|---|---|---|---|---|---|---|
| Rat # | 30–50 min | 50–100 min | 100–150 min | 150–250 min | Average | NDS |
| 1 | 0.25 (0.17) | 0.40 (0.27) | 0.51 (0.34) | 0.69 (0.55) | 0.55 (0.40) | 46 |
| 2 | 0.44 (0.36) | 0.65 (0.51) | 0.68 (0.54) | 0.83 (0.70) | 0.72 (0.59) | 49 |
| 3 | 0.42 (0.27) | 0.56 (0.38) | 0.65 (0.47) | 0.75 (0.57) | 0.65 (0.48) | 49 |
| 4 | 0.32 (0.21) | 0.56 (0.39) | 0.75 (0.58) | 0.78 (0.60) | 0.68 (0.51) | 49 |
| 5 | 0.41 (0.27) | 0.64 (0.45) | 0.72 (0.52) | 0.87 (0.72) | 0.74 (0.57) | 51 |
| 6 | 0.22 (0.13) | 0.58 (0.44) | 0.74 (0.77) | 0.83 (0.92) | 0.70 (0.71) | 59 |
| 7 | 0.41 (0.27) | 0.72 (0.64) | 0.90 (1.10) | 0.92 (1.03) | 0.82 (0.89) | 60 |
| 8 | 0.39 (0.26) | 0.76 (0.59) | 0.86 (0.71) | 0.96 (0.92) | 0.84 (0.73) | 62 |
| 9 | 0.50 (0.35) | 0.81 (0.75) | 0.90 (1.00) | 0.89 (1.00) | 0.84 (0.88) | 63 |
| 10 | 0.60 (0.43) | 0.85 (0.77) | 0.91 (0.94) | 0.90 (0.87) | 0.86 (0.82) | 63 |
| 11 | 0.58 (0.41) | 0.85 (0.77) | 0.88 (0.85) | 0.89 (0.91) | 0.85 (0.82) | 67 |
| 12 | 0.53 (0.37) | 0.70 (0.50) | 0.82 (0.64) | 0.88 (0.75) | 0.80 (0.63) | 72 |
| 13 | 0.60 (0.43) | 0.72 (0.53) | 0.83 (0.68) | 0.90 (0.79) | 0.81 (0.67) | 74 |
| correlation | 0.69 (0.64) | 0.72 (0.61) | 0.76 (0.56) | 0.73 (0.61) | 0.78 (0.65) | |
| P-value | 0.009 (0.02) | 0.006 (0.03) | 0.003 (0.04) | 0.005 (0.03) | 0.002 (0.02) | |
Note: Pearson correlation coefficients were used to assess the joint relationship between IQ or SIQ and NDS. NDS is a neurological deficit score (NDS) after 72 hours, ranging from 80 (best) to 0 (brain dead).
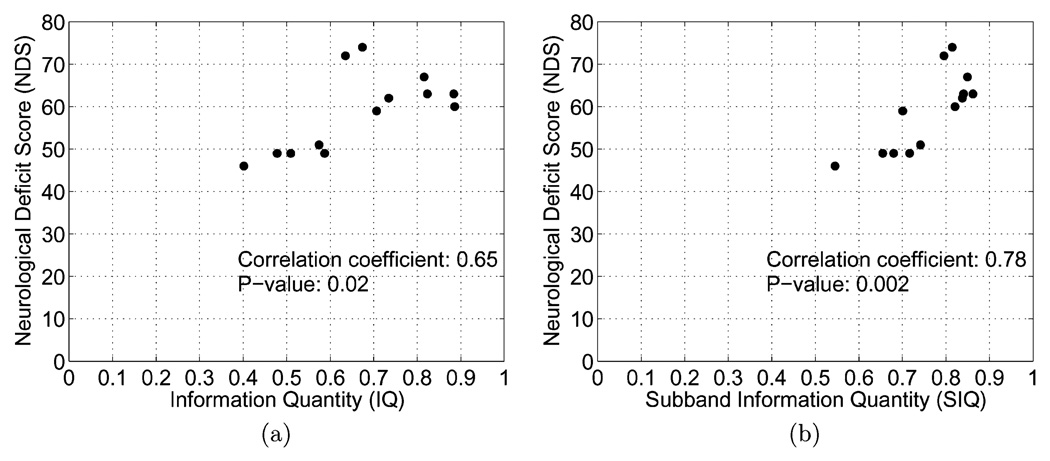
IQ and SIQ were analyzed with respect to NDS through two bivariate statistical methods. Pearson correlation coefficients were used to assess the joint relationship between IQ or SIQ and NDS. Over all given time intervals, a more significant correlation was observed between SIQ and NDS than between IQ and NDS. Hypothesis testing was then conducted to determine the significance of either IQ or SIQ as a predictor of NDS, using a student-t distribution (n = 13). Our findings from this test reveal that SIQ is more correlated to NDS than IQ. (9) Differences in IQ and SIQ correspondence to NDS are apparent when either metric is plotted against NDS, as shown in Fig. 3, where there is a more discernable linear relation between SIQ and NDS.
Fig. 3.
C(10) NDS for 13 rats. (a) NDS versus IQ (b) NDS versus SIQ. IQ and SIQ are averaged over the entire recovery period (30–240 min). (a) IQ versus NDS. (b) SIQ versus NDS.
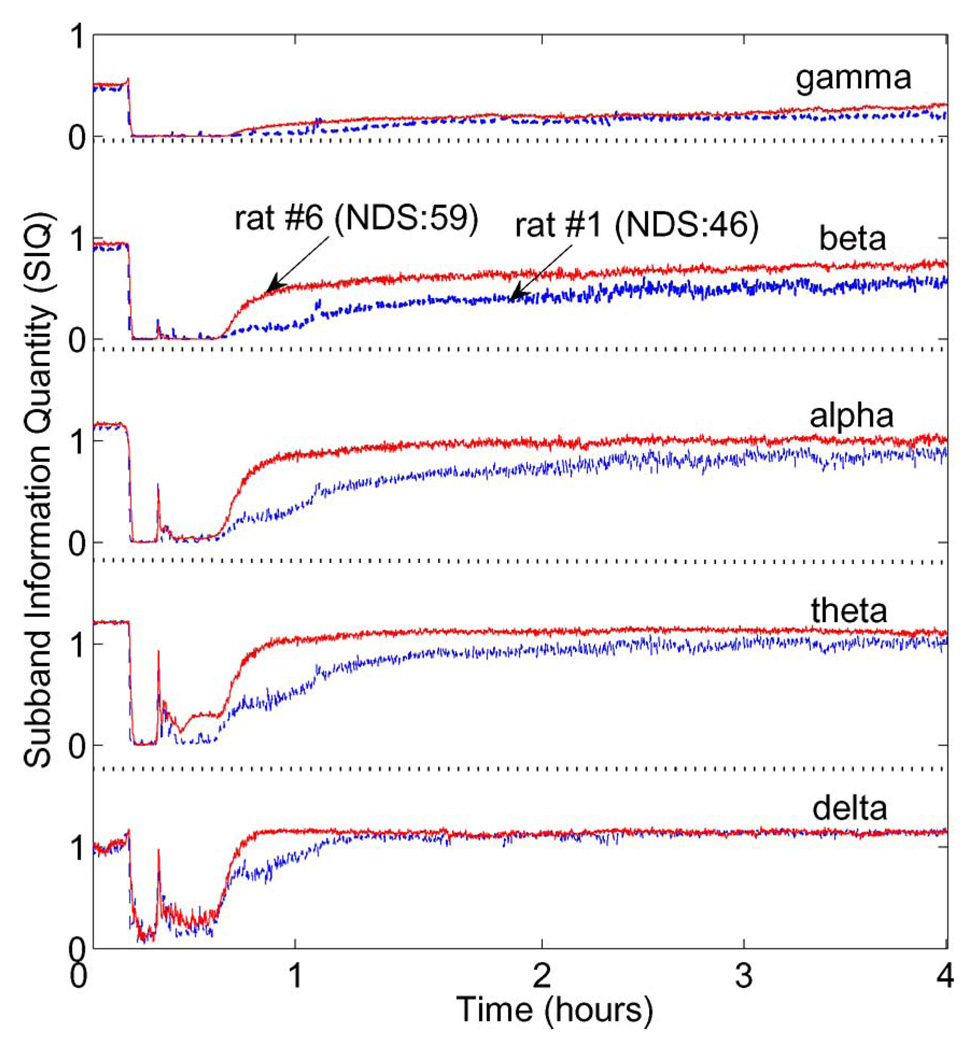
A distinguishing feature of SIQ is its ability to provide insights on the information content of individual bands. This is because SIQ analyzes wavelet coefficient entropy at each scale level of decomposition, rather than at the gross scale of the signal. Specifically, a five-level decomposition can be used to approximate the five standard EEG bands of clinical interest: gamma (above 30 Hz), beta (16–30 Hz), alpha (8–15 Hz), theta (4–8 Hz), and delta (below 4 Hz). Fig. 4 depicts the time evolution of mean SIQ of rat #1 (NDS:46) and #6 (NDS:59) for each of five EEG subbands attained from five-level resolution. While SIQ is higher in rat #6 showing better NDS than in rat #1 in most bands, the mean information contents of the gamma (31–62 Hz) and delta (below 4 Hz) bands are approximately equal. Conversely, the SIQ curves for the beta (16–30 Hz), alpha (8–15 Hz), and theta (4–8 Hz) bands show a distinct separation over the entire measurement, with SIQ being consistently higher in rat #6 than in rat #1.
Fig. 4.
SIQ of rat #1 (NDS:46) and rat #6 (NDS:59) for five clinical bands: gamma (above 30 Hz), beta (16–30 Hz), alpha (8–15 Hz), theta (4–8 Hz), and delta (below 4 Hz).
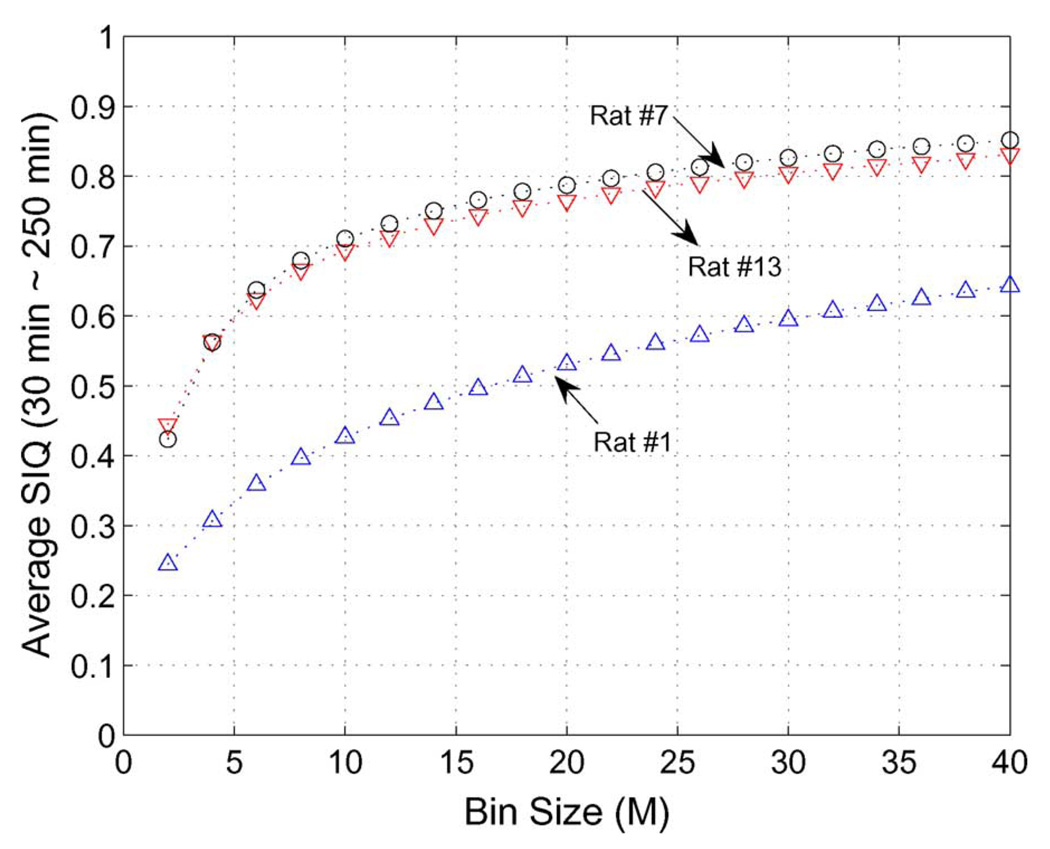
Fig. 5 shows the dependency of the bin size, M in (5) on SIQ. To illustrate this, we selected three rats showing low NDS (rat #1), middle (rat #7), and high NDS (rat #13). Definitely SIQ depends on the bin size. But the difference of SIQ among rats for a given bin size keeps almost the same and as M increases, the SIQ variation becomes smaller. In Table I and Fig. 3, we selected the bin size (M) as 20.
Fig. 5.
SIQ dependency on bin size (M) in (5) for rat #1 (NDS:46) and rat #7 (NDS:60), and rat #13 (NDS:74).
V. DISCUSSION AND CONCLUSION
We proposed a new quantitative EEG (qEEG) real-time indicator of brain injury called SIQ and evaluated its performance by way of a comparison with our previously reported measure of IQ. Both metrics analyze EEG in terms of its entropy, and were developed as quantitative alternatives to conventional methods for evaluating recovery of hypoxic–ischemic brain injury victims, which are often empirical. Such approaches, which are based on interpretation of temporal EEG, typically rely on the skill and judgment of a trained specialist to detect often subtle variations in brain rhythms, and thus, are highly subjective.
Using a rat model, IQ had previously been shown to successfully predict improvements in neurological outcome gained by lowering core body temperature to mild hypothermia following asphyxic cardiac arrest [12]. However, examinations of IQ trends during later periods of recovery (240 min from the baseline) suggest that the predictive accuracy of this model degrades over time. We present evidence that it is possible for IQ to overestimate the quality of recovery of rats subjected to cardiac arrest, and verify this by comparison to NDS scores. This likely suggests that IQ per se does not track all EEG phenomena that are important indicators of behavioral state. This shortcoming impairs the viability of IQ as a real-time indicator of patient recovery.
Whereas IQ evaluates information of a gross signal only, SIQ facilitates individual subband analysis. Differences in recovery among subjects are borne out even more clearly at the subband level, where theta, beta, and alpha bands showed the most significant variations in SIQ. Based on the nature of the SIQ algorithm, these results may imply that neurologic recovery from hypoxia–ischemia is linked to the activity of individual EEG subbands. Future studies in this direction may potentially uncover additional details about the recovery mechanism.
Although the scope of this study was to predict neurological outcomes after resuscitation from asphyxiation, we will further study hypothermia neuroprotection quantitatively. Hypothermia is widespread in clinical practice, yet the absence of a real-time direct bedside indicator of brain injury and recovery remains one of the biggest hindrances in the development of hypothermic therapies. We will use SIQ to directly titrate the efficacy of hypothermia treatment in response to variations in settings such as time of initiation and temperature range.
SIQ offers a prospective step forward in the ongoing search for strategies to noninvasively monitor critical brain injury. While further experiments encompassing more subjects and a broader assortment of insult conditions are necessary, qEEG approaches such as SIQ may hold an intriguing potential, both to serve as an accurate status monitor and to give real-time insights into neurological recovery.
Acknowledgments
This research was supported in part by the National Institutes of Health under Grants R01 HL071568 and R21 NS054146 and in part by the MKE (Ministry of Knowledge and Economy), Korea, under the ITRC (Information Technology Research Center) support program supervised by the IITA (Institute for Information Technology Advancement) (IITA-2008-C1090-0803-0006) and the Soongsil University Research Fund.
Biographies

Hyun-Chool Shin (M’00) received the B.Sc., M.Sc., and Ph.D. degrees in electronic and electrical engineering from Pohang University of Science and Technology (POSTECH), Pohang, Korea, in 1997, 1999, and 2004, respectively.
From 2004 to 2007, he was a Postdoctoral Researcher in the Department of Biomedical Engineering, Johns Hopkins University School of Medicine. He is currently with Soongsil University, Seoul, Korea. His current research interests include adaptive filtering theory, brain–computer interface, laser speckle imaging for brain microvessel, and bio/neurological signal processing, such as electroencephalogram, evoked potential, and neural spike analysis.

Xiaofeng Jia received the M.D. degree in clinical medicine from Zhejiang Medical University, Zhejiang, China, in 1994, the M.S. degree in surgery from Shanghai Medical University, Shanghai, China, in 1997, and the Ph.D. degree in surgery (orthopedics) from Fudan University, Shanghai, in 2003.
He has been a Faculty Member in the Department of Biomedical Engineering, Johns Hopkins University School of Medicine, Baltimore, MD, since 2007 after 3 years’ Postdoctoral Fellowship. He completed his surgery residency in the Huashan Hospital and Orthopedic Surgery fellowship in the Shanghai 6th People’s Hospital, Shanghai. Later, he was an Attending Surgeon in the Department of Orthopedics, Zhongshan Hospital, Shanghai. His current research interests include basic and clinical investigations in acute neurological injuries after global cerebral ischemia, novel application of neuroelectrophysiology for detection and restoration of spinal cord and peripheral nerve injury, therapeutic hypothermia of brain and spinal cord after asphyxial cardiac arrest.
Dr. Jia is a member of the American Association for Hand Surgery, Society of Critical Care Medicine, Society for Neuroscience, American Heart Association, American Stroke Association, International Society for Heart Research. He is a recipient of the Annual Research Awards from American Association for Hand Surgery, the Cardiopulmonary, Perioperative and Critical Care Junior Investigator Travel Award from American Heart Association, the Finalist of Best Overall Poster Award of American Academy of Orthopaedic Surgeons, the Finalist of Research Citation Award from Society of Critical Care Medicine, the Guanghua Scholarship, Government Public Scholarship, the Outstanding Moral–Intellectual–Physical Student and the Outstanding Graduate from Fudan University, and National Educational Ministry Outstanding Postgraduate Scholarship from Shanghai Medical University.
Robert Nickl, photograph and biography not available at the time of publication.

Romergryko G. Geocadin received the Graduate degree from the UERM College of Medicine, University of the Philippines, Quezon City, Philippines.
He is the Director of the Neurosciences Critical Care Unit (NCCU), Johns Hopkins Bayview Medical Center, and an Attending Neuro-Intensivist at the NCCU, The Johns Hopkins Hospital, Baltimore, MD. He is also an Assistant Professor of Neurology, Neurosurgery and Anesthesiology—Critical Care Medicine, Johns Hopkins University School of Medicine. He completed his Neurology residency, New York University Medical Center, and the Neurocritical Care Fellowship, The Johns Hopkins Hospital. His current research interests include basic and clinical investigations in acute neurological injuries after global cerebral ischemia, novel application of neuroelectrophysiology for early detection of acute neurological injuries, disorders of intracranial pressure, coma, cerebrovascular disorders, and neurologic disorders in critical illness.

Nitish V. Thakor (F’97) received the B.Tech. degree in electrical engineering from Indian Institute of Technology-Bombay, Mumbai, India, in 1974 and the Ph.D. degree in electrical and computer engineering from the University of Wisconsin, Madison, in 1981.
During 1981–1983, he joined the Faculty of Electrical Engineering and Computer Science, Northwestern University, Evanston, IL. Since 1983, he has been with Johns Hopkins University School of Medicine, Baltimore, MD, where he is currently a Professor of Biomedical Engineering and involved in teaching and research of cardiovascular and neurological instrumentation, biomedical signal processing, and micro- and nanotechnologies. He is engaged in developing international scientific programs, collaborative exchanges, tutorials, and conferences on neuroengineering and medical microsystems. He is the author or coauthor of more than 160 articles published in peer-reviewed journals.
Dr. Thakor is a Fellow of the American Institute of Medical and Biological Engineering and Founding Fellow of the Biomedical Engineering Society. He was a member of the editorial board for several journals and Editor-in-Chief of the IEEE TRANSACTIONS ON NEURAL AND REHABILITATION ENGINEERING. He was the recipient of the Research Career Development Award from the National Institutes of Health and the Presidential Young Investigator Award from the National Science Foundation, the Centennial Medal from the University of Wisconsin School of Engineering, Honorary Membership from the Alpha Eta Mu Beta Biomedical Engineering, the Student Honor Society, and Distinguished Service Award from IIT-Bombay.
Footnotes
Color versions of one or more of the figures in this paper are available online at http://ieeexplore.ieee.org.
Contributor Information
Hyun-Chool Shin, Department of Biomedical Engineering, Johns Hopkins School of Medicine, Baltimore, MD 21205 USA. He is now with Soongsil University, Seoul 156-743, Korea (shinhc@ssu.ac.kr)..
Xiaofeng Jia, Department of Biomedical Engineering, Johns Hopkins School of Medicine, Baltimore, MD 21205 USA (xjia1@jhmi.edu)..
Robert Nickl, Department of Biomedical Engineering, Johns Hopkins School of Medicine, Baltimore, MD 21205 USA (rnickl@jhu.edu)..
Romergryko G. Geocadin, Department of Neurology, Johns Hopkins School of Medicine, Baltimore, MD 21205 USA (rgeocadi@jhmi.edu).
Nitish V. Thakor, Department of Biomedical Engineering, Johns Hopkins School of Medicine, Baltimore, MD 21205 USA (nitish@jhu.edu)..
REFERENCES
- 1.American Heart Association. 2002 Heart and Stroke Statistical Update. Dallas, TX: American Heart Association; 2001. [Google Scholar]
- 2.Safar P. Cerebral resuscitation after cardiac arrest: A review. Circulation. 1986;vol. 74:IV138–IV153. [PubMed] [Google Scholar]
- 3.Morimoto Y, Kemmotsu O, Kitami K, Matsubara I, Tedo I. Acute brain swelling after out of hospital cardiac arrest: Pathogenesis and outcome. Crit. Care Med. 1993;vol. 21:104–110. doi: 10.1097/00003246-199301000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Cummins R, Chamberlain D, et al. Recommended Guidelines for Reviewing, Reporting, and Conducting Research on In-Hospital Resuscitation: The In-Hospital ‘Utstein Style. 1997 doi: 10.1016/s0196-0644(97)70256-7. [DOI] [PubMed] [Google Scholar]
- 5.Bedell SE, Delbanco TL, Cook EF, Epstein FH. Survival after cardiopulmonary resuscitation in the hospital. N. Engl. J. Med. 1983;vol. 309:569–576. doi: 10.1056/NEJM198309083091001. [DOI] [PubMed] [Google Scholar]
- 6.Berek K, Jeschow M, Aichner F. The prognostication of cerebral hypoxia after out of hospital cardiac arrest in adults. Eur. Neurol. 1997;vol. 37:135–145. doi: 10.1159/000117426. [DOI] [PubMed] [Google Scholar]
- 7.Vaagenes P, Ginsberg M, Ebmeyer U, Ernster L, Fischer M, Gisvold SE, Gurvitch A, Hossmann KA, Nemoto EM, Radovsky A, Severinghaus JW, Safar P, Schlichtig R, Sterz F, Tonnessen T, White RJ, Xiao F, Zhou Y. Cerebral resuscitation from cardiac arrest: Pathophysiologic mechanisms. Crit. Care Med. 1996;vol. 24:S57–S68. [PubMed] [Google Scholar]
- 8.E C, Shannon A mathematical theory of communication. Bell Syst. Technol. J. 1948;vol. 27:623–656. [Google Scholar]
- 9.Tong S, Bezerianos A, Malhotra A, Zhu Y, Thakor NV. Parameterized entropy analysis of EEG following hypoxic–ischemic brain injury. Phys. Lett. A. 2003;vol. 314:354–361. [Google Scholar]
- 10.Bezerianos A, Tong S, Thakor NV. Time-dependent entropy estimation of EEG rhythm changes following brain ischemia. Ann. Biomed. Eng. 2003;vol. 31:221–232. doi: 10.1114/1.1541013. [DOI] [PubMed] [Google Scholar]
- 11.Thakor NV, Tong S. Advances in quantitative electroencephalogram analysis methods. Annu. Rev. Biomed. Eng. 2004;vol. 6:453–495. doi: 10.1146/annurev.bioeng.5.040202.121601. [DOI] [PubMed] [Google Scholar]
- 12.Shin HC, Tong S, Yamashita S, Jia X, Geocadin R, Thakor NV. Quantitative EEG and effect of hypothermia on brain recovery after cardiac arrest. IEEE Trans. Biomed. Eng. 2006 Jun;vol. 53(no. 6):1016–1023. doi: 10.1109/TBME.2006.873394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thakor NV, Shin HC, Tong S, Geocadin R. Quantitative EEG assessment: Hypothermic neural protection after ischemic brain injury. IEEE Eng. Med. Biol. Mag. 2006 Jul;vol. 25(no. 4):20–25. doi: 10.1109/memb.2006.1657783. [DOI] [PubMed] [Google Scholar]
- 14.Jia X, Koenig MA, Shin HC, Zhen G, Yamashita S, Thakor NV, Geocadin RG. Quantitative EEG and neurological recovery after therapeutic hypothermia of asphyxial cardiac arrest in rats. Brain Res. 2006 Sep;vol. 1111(no. 1):166–175. doi: 10.1016/j.brainres.2006.04.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosso OA, Blanco S, Yordanova J, Kolev V, Figliola A, Schürmann M, Başar E. Wavelet entropy: A new tool for analysis of short duration brain electrical signals. J. Neurosci. Methods. 2001;vol. 105:65–75. doi: 10.1016/s0165-0270(00)00356-3. [DOI] [PubMed] [Google Scholar]
- 16.Yordanova J, Kolev V, Rosso OA, Schürmann M, Sakowitz OW, Özgö ren M, Başar E. Wavelet entropy analysis of event-related potentials indicates modality-independent theta dominance. J. Neurosci. Methods. 2002;vol. 117:99–109. doi: 10.1016/s0165-0270(02)00095-x. [DOI] [PubMed] [Google Scholar]
- 17.Katz L, Ebmeyer U, Safar P, Radovsky A. Outcome model of asphyxial cardiac arrest in rats. J. Cerebral Blood Flow Metab. 1995;vol. 15:1032–1039. doi: 10.1038/jcbfm.1995.129. [DOI] [PubMed] [Google Scholar]
- 18.Geocardin RG, Ghodadra R, Kimura T, Lei H, Sherman DL, Hanley DF, Thakor NV. A novel quantitative EEG injury measure of global cerebral ischemia. Clin. Neurophysiol. 2000;vol. 111:1779–1787. doi: 10.1016/s1388-2457(00)00379-5. [DOI] [PubMed] [Google Scholar]