Abstract
Neurological complications after cardiac arrest (CA) can be fatal. Although hypothermia has been shown to be beneficial, understanding the mechanism and establishing neurological outcomes remains challenging because effects of CA and hypothermia are not well characterized. This paper aims to analyze EEG (and the α-rhythms) using multiscale entropy (MSE) to demonstrate the ability of MSE in tracking changes due to hypothermia and compare MSE during early recovery with long-term neurological examinations. Ten Wistar rats, upon post-CA resuscitation, were randomly subjected to hypothermia (32 °C–34 °C, N = 5) or normothermia (36.5 °C–37.5 °C, N = 5). EEG was recorded and analyzed using MSE during seven recovery phases for each experiment: baseline, CA, and five early recovery phases (R1–R5). Postresuscitation neurological examination was performed at 6, 24, 48, and 72 h to obtain neurological deficit scores (NDSs). Results showed MSE to be a sensitive marker of changes in α-rhythms. Significant difference (p < 0.05) was found between the MSE for two groups during recovery, suggesting that MSE can successfully reflect temperature modulation. A comparison of short-term MSE and long-term NDS suggested that MSE could be used for predicting favorability of long-term outcome. These experiments point to the role of cortical rhythms in reporting early neurological response to ischemia and therapeutic hypothermia.
Index Terms: Cardiac arrest (CA), entropy, neurological injury, quantitative EEG
I. INTRODUCTION
Cardiac arrest (CA) is the leading cause of deaths in the United States [1]. In United States, about 460 000 sudden cardiac deaths were reported in 1999 [2]. The survival rate after CA is generally low: only 2%–9% following out-of-hospital CA [3]. Poor functional outcomes, such as coma or persistent vegetative state, are prevalent among survivors, with only 3%–7% survivors resuming normal functioning [4]. Devastating neurological complications induced by CA and early reperfusion are recognized as the main causes of short-term and long-term mortality and morbidity [5]. While various neuroprotective strategies have failed to improve the outcome statistics for CA [6]–[10], the neuroprotective effect of mild hypothermia was confirmed in animal models of global ischemia [11], [12] and human clinical trials [13], [14]. In 2005, the International Liaison Committee on Resuscitation and the American Heart Association recommended the use of therapeutic hypothermia in comatose survivors from CA [15]. Yet, therapeutic hypothermia is still underutilized, partly because there is no established method to track and verify the benefits of hypothermia during post-CA recovery [16].
Recent studies have suggested that different brain regions have different sensitivity to hypothermia [11], [17]. Previous pathological studies revealed that hypoxic-ischemic insult predominantly affects the cerebral cortex, basal ganglia, thalamus, hippocampus, and brain stem. The thalamus plays an important role in regulating states of arousal and the level of awareness. Damage to the thalamus may lead to permanent coma [18]. However, there is no definite conclusion about the effects of hypothermia on the thalamus [17], [19]. Therefore, we are interested in investigating the effect of therapeutic hypothermia on the thalamus, as well as the relationship between the status of the thalamus and post-CA recovery outcomes. Recent research reported in several animal models and human clinical trials suggests that the α-rhythm is strongly influenced by the thalamus [20], [21]. The thalamic lesions can lead to pronounced disorganization or even complete suppression of α-rhythms [22], [23]. Therefore, we may infer the evidence of thalamic lesions through the monitoring and analysis of α-rhythms.
EEG is a noninvasive global measure of electrical activity in the brain, and is commonly employed for neuromonitoring [24]. It is influenced by various interacting mechanisms in the brain. Living organs, including the brain, can be seen as a system with high complexity, allowing for adaptive responses to a broad range of stimuli [25]. A reduction in complexity is often interpreted as an unhealthy state for a biological system [26]–[28]. Given that the α-rhythm (a pattern of 8–12 Hz oscillations in EEG) is attributed to synchronous activity in thalamic pacemaker cells [18], the changes in complexity of α-rhythms may reveal different degrees of function. These changes in complexity can be tracked using an appropriate entropy analysis.
Our first objective is to analyze the changes in the complexity of EEG (and the component α-rhythms) before, during, and after CA using MSE; our second objective is to demonstrate the ability of MSE in tracking changes in EEG due to temperature modulation (normothermia and hypothermia); our third objective is to compare MSE during early recovery (within 3 h) with long-term (72 h) NDSs to examine the relationship between these two numerical measures.
II. MATERIALS AND EXPERIMENTS
Ten male Wistar rats (325 ± 25 g, Charles River, Wilmington, MA) were used in our experiments. The animals were divided into two groups of five each. All rats in both groups were subjected to asphyxia-induced CA for 7 min, resuscitated, and subjected to therapeutic hypothermia (32 °C–34 °C) or normothermia (36.5 °C–37.5 °C). The protocol described shortly [41], [42] was approved by the Institutional Animal Care and Use Committee of the Johns Hopkins Medical Institutions.
A. Globally Ischemic Rat Model of CA
Rats were ventilated with 1.5% halothane and N2/O2 (1:1). The femoral artery and vein were cannulated for sampling arterial blood gas (ABG) and monitoring arterial blood pressure. EEG was recorded for the first 5 min as baseline (BL) with halothane and the following 5 min without halothane to wash out the possible residual effects of halothane on EEG [42]. Seven minute CA was initiated with cessation of mechanical ventilation. The cardiopulmonary resuscitation (CPR) was performed by chest compression until return of spontaneous circulation (ROSC), which was defined as mean arterial blood pressure (MABP) higher than 60 mmHg [41]–[46]. During the experiments, ABG was sampled during BL, 10, 20, and 40 min after resuscitation.
B. Temperature Modulation Immediately After ROSC
Core temperature of the animals was monitored by an intraperitoneal sensor (G2 E-mitter 870-0010-01, Mini Mitter, Sun River, OR) implanted one week before experiments [41]–[46]. Therapeutic hypothermia (32 °C–34 °C) was induced immediately after ROSC through surface cooling with misted water in hypothermia group. The temperature transition duration was approximately 16 min. Therapeutic hypothermia was maintained for 6 h, and then, the rats were rewarmed to 37 °C over another 2 h [41]–[46]. In the normothermia group, normothermia (36.5 °C–37.5 °C) was maintained for 8 h after ROSC. All rats in two groups were kept inside a neonatal incubator (Isolette infant incubator model C-86, Air-Shields, Hatboro, PA) for the first day after temperature modulation in case of temperature fluctuation.
C. EEG Recording
Two channels of EEG using epidural screw electrodes (Plastics One, Roanoke, VA) were recorded continuously for 3 h from the beginning of the experiments in the right and left parietal areas of the rats. The sampling rate was 250 Hz and the cutoff frequency was 30 Hz for low-pass filter. Serial 30-min EEG recordings were conducted at 6, 24, 48, and 72 h for all rats.
D. Neurological Evaluation
NDS was evaluated at 6, 24, 48, and 72 h for all rats in order to test post-CA functional recovery, such as the level of arousal, respiration, brain-stem function, and motor behavior [41]. The NDS, similar to normal procedures for human neurological examination, is established for functional recovery examination on animal models. NDS ranges from 0 (worst outcome) to 80 (best outcome). The evaluation was performed by an independent trained observer blind to the experiments. Here, good neurological states were defined as 72-h NDS ≥ 60, while poor neurological states were defined as 72-h NDS < 60 based on our previous experience and observation [42]–[46].
III. METHODS AND QUANTITATIVE ANALYSIS
A. Preprocessing of EEG Signals
EEG was first checked for artifact contamination, such as mechanical artifacts induced by CPR or AC power 60 Hz noise. Analysis was performed in both time domain with WinDaq software (Data Instruments, Akron, OH) and in frequency domain with MATLAB (MathWorks, Natick, MA). The contaminated channels of EEG were excluded from further analysis.
B. Sample Entropy (SampEn)
SampEn is defined as the negative natural logarithm of the conditional probability that two sequences similar to each other for the first m points remain similar at the next m + 1 points, while self-matches are excluded [33]–[35]. It measures the complexity in a time series on a single time scale. There are two specified SampEn parameters: pattern length m(m ≥ 1) and tolerance level r for similarity comparison. Given a 1-D time series X = {x(1), x(2),…, x(N)}, SampEn is calculated as follows [33]–[35]: first, construct N − m + 1 vectors
| (1) |
and the distance between two vectors is defined as absolute maximum difference between the corresponding scalar components
| (2) |
where 0 ≤ k ≤ m − 1. Given r, is defined as 1/(N − m) times the number of vectors Xm(j) falling within vector distance r of Xm(i), where 1 ≤ j ≤ N − m(j ≠ i)
| (3) |
Similarly, is defined as 1/(N − m − 1) times the number of vectors Xm+1(j) falling within vector distance r of Xm+1(i), where 1 ≤ j ≤ N − m − 1
| (4) |
SampEn is defined as
| (5) |
C. Multiscale Entropy (MSE)
MSE is designed to measure time-domain complexity in a signal over multiple time scales. A time scale factor (λ) is set as the width of nonoverlapping time windows. The mean of all the samples within each time window of the original time series Y = {y1, y2, …, yN } is calculated and used to form a new coarse-grained time series. The coarse-grained time series obtained for a given λ is denoted by
| (6) |
where ⌊⌋ denotes the integer part. Scalar entropy is calculated for each coarse-grain time series. Since SampEn is used in the first introduction of MSE [29] and most commonly applied in previous MSE analysis [36]–[40], SampEn was applied in our MSE analysis of the complexity in EEG. We have tried different combinations of m (m = 1, 2) and r values (0.1 ≤ r ≤ 0.25), and they gave similar results in the MSE analysis. Here, we used m = 2 and r = 0.1 σY. SampEn is plotted against time scale factors to form MSE curves (Figs. 1 and 2).
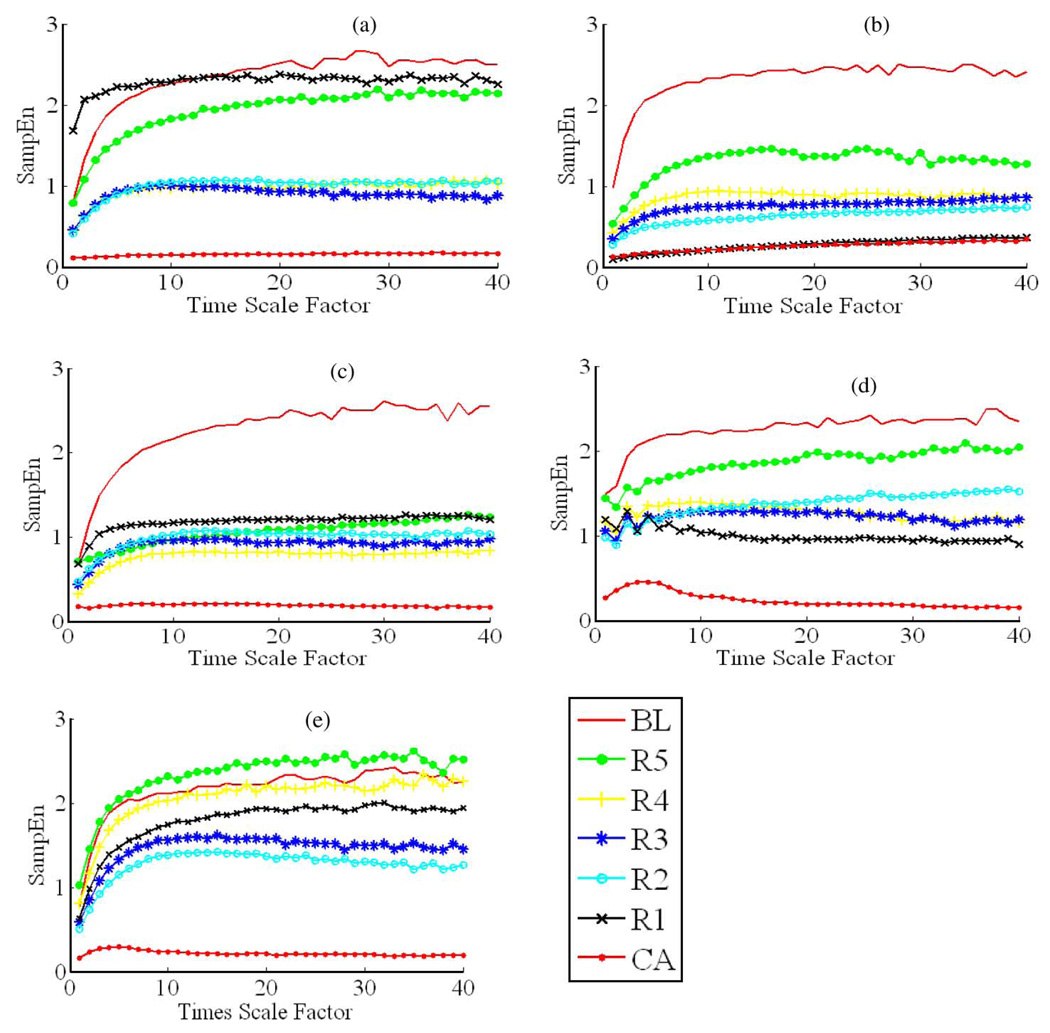
Fig. 1.
MSE curves for the seven different recording phases in all five normothermia experiments. (a)–(e), respectively, represent the results from MSE analysis for a normothermia experiment. Take (a) for example, there are seven MSE curves within (a), and each MSE curve corresponds to a recording phase in an experiment from BL to R5 defined in Fig. 3. In each MSE curve, SampEn increases monotonically with time scale factor ranging from 1 to 19, and reaches a “plateau” or “saturation” when time scale factor ranges from 20 to 40. For each MSE curve in CA, SampEn stays significantly low compared to those in other recording phases. The results show that the saturation value of MSE curves is the highest in BL and the lowest in CA except in (c), where the saturation value in R5 is the highest and the saturation value in BL is the second highest. In (a) and (b)–(e), the saturation value in R5 is always lower than that in BL, along with poor recovery outcomes (72-h NDS < 60).
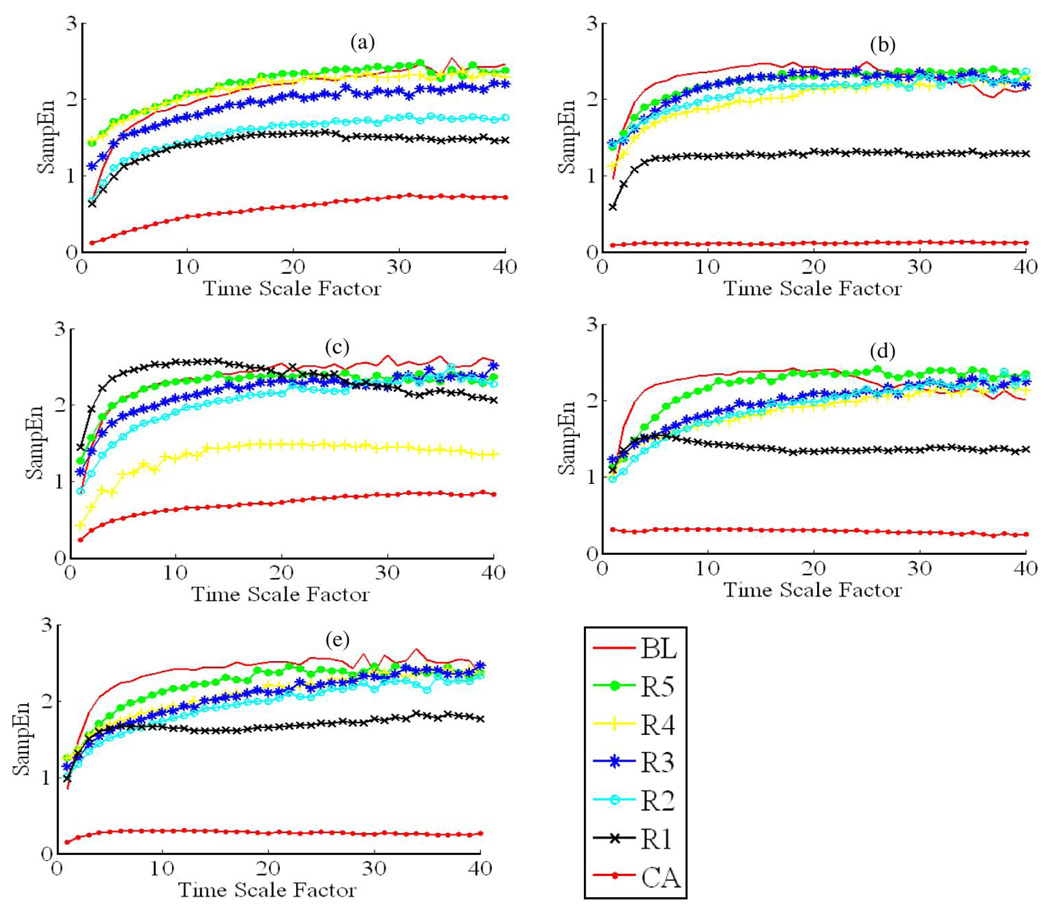
Fig. 2.
MSE curves for the seven different recording phases in all five hypothermia experiments. (a)–(e), respectively, represent the results from MSE analysis for a hypothermia experiment. Take (a) for example, there are seven MSE curves within (a), and each MSE curve corresponds to a recording phase in an experiment from BL to R5 defined in Fig. 3. In each MSE curve, SampEn increases monotonically with time scale factors ranging from 1 to 19, and reaches a “plateau” or “saturation” when time scale factor ranges from 20 to 40. Unlike the conditions in the normothermia group, most of the MSE curves in R5 are very close to or above the MSE curves for BL, along with good recovery outcomes (72-h NDS > 60).
We divided EEG obtained from each experiment in both groups into seven recording phases: BL, CA, and five recording phases R1–R5 during postresuscitation recovery according to our experimental protocol (Fig. 3). The recording phase CA is followed by electric silence for approximately 15 min before the reappearance of continuous EEG bursting, and thus, the first recovery period starts at 32 min. For a series of consecutive time scale factors λ ranging from 1 to 40, 40 corresponding coarse-grained time series are generated for seven recording phase, respectively.
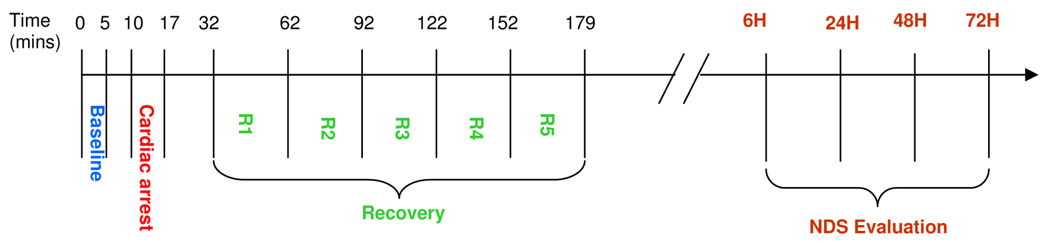
Fig. 3.
Timeline for EEG recording in ischemia CA rodent experiments and the following neurological evaluation. BL lasts from the start of the experiment to 5 min, while ischemia CA lasts from 10 to 17 min. The recovery period includes five recording phases R1–R5, lasting from 32 to 179 min relative to the beginning of the experiment. NDS evaluation follows the EEG recording at 6, 24, 48, and 72 h after CA.
Here, a typical MSE curve can be seen to saturate after a monotonic increase with the first 20 time scale factors λ (Figs. 1 and 2).We define the saturation value of each MSE curve, which is the average of SampEn corresponding to time scale factors λ ranging from 20 to 30, as MSEα here. Given the sampling rate of 250 Hz in our EEG recording system, the time scale factors λ = 20 to λ = 30 approximately correspond to the α-rhythm (8–12 Hz) since
| (7) |
| (8) |
Therefore, MSEα mainly reflects the changes in the complexity in the α-rhythms.
D. Statistical Methods
In order to uncover the relationship between MSEα and 72-h neurological performance of the rats, the Pearson correlation coefficients and the corresponding p-values out of bivariate analysis between MSEα and NDS are calculated for the two groups. Because different SDs are observed in the NDS and the MSEα between the normothermia and hypothermia groups, data in the normothermia and hypothermia groups are compared to each other using two sample t-test under the assumption of unequal population variances. p < 0.05 was treated as significant. All the data are expressed as the mean ± SD.
IV. RESULTS
A. Recovery Outcomes Summary
The evaluation of NDS at 6, 24, 48, and 72 h in the normothermia and hypothermia groups showed that the NDS of the hypothermia group was significantly higher than that of the normothermia group at 6 and 24 h, indicating better functional recovery in the hypothermia group during the acute stages of recovery (Table I). Moreover, the number of hypothermic rats with better neurological recovery was greater than that of normothermic animals in every NDS examination (Table II).
TABLE I.
NDS (mean ± SD) for normothermia and hypothermia groups at different stages of post-CA recovery
| NDS(6H) | NDS(24H) | NDS(48H) | NDS(72H) | |
|---|---|---|---|---|
| Normothermia (N = 5) | 43.2±5.31 | 55.8±11.69 | 57.75±11.56 | 55.8±11.69 |
| Hypothermia (N = 5) | 55.8±6.38 | 71.6±3.58 | 73.2±1.10 | 71.6±3.58 |
| p-value | <0.01 | 0.03 | 0.09 | 0.07 |
TABLE II.
Number of animals with favorable outcomes at different stages of post-CA recovery in normothermia and hypothermia groups
| 6H | 24H | 48H | 72H | |
|---|---|---|---|---|
| Normothermia (N = 5) | 0 | 2 | 1 | 1 |
| Hypothermia (N = 5) | 2 | 5 | 5 | 5 |
B. MSE Curve
A single MSE curve was generated for each recording phase for every rat in the normothermia and hypothermia groups (Figs. 1 and 2). Through the observation of MSE curves in Figs. 1 and 2, we can see that good experimental outcomes (72-h NDS ≥ 60) would appear when the MSE curve for R5 is either very close to or above the MSE curve for BL over most of the time scales; and poor experimental outcomes (72-h NDS < 60)would turn up when the MSE curve for R5 is far below the BL MSE curve over the same time scales. In order to quantify the relationship, MSEα for each recording phase was calculated and compared between the two groups (Table III). There was significant difference in MSEα between the normothermia and hypothermia groups in R2–R4. We found that good recovery outcomes was always associated with MSEα (R5/BL) greater than 0.85 (Table IV).
TABLE III.
MSEα (mean ± SD) between normothermia and hypothermia groups
| BL | CA | R1 | R2 | R3 | R4 | R5 | |
|---|---|---|---|---|---|---|---|
| Normothermia (N = 5) | 2.39±0.10 | 0.24±0.06 | 1.39±0.78 | 1.13±0.29 | 1.11±0.29 | 1.22±0.54 | 1.79±0.57 |
| Hypothermia (N = 5) | 2.36±0.13 | 0.47±0.30 | 1.65±0.42 | 2.07±0.22 | 2.20±0.10 | 2.02±0.34 | 2.33±0.07 |
| p-value | 0.75 | 0.17 | 0.53 | <0.01 | <0.01 | 0.02 | 0.07 |
TABLE IV.
MSEα (BL/R5) and corresponding 72-h NDS for all rats
| ID | MSEα(BL/R5) | 72-hr NDS | Condition |
|---|---|---|---|
| rat #1 | 0.81 | 0 | Normothermia |
| rat #2 | 0.60 | 46 | Normothermia |
| rat #3 | 0.48 | 50 | Normothermia |
| rat #4 | 0.82 | 59 | Normothermia |
| rat #5 | 1.04 | 72 | Hypothermia |
| rat #6 | 1.07 | 74 | Normothermia |
| rat #7 | 1.01 | 74 | Hypothermia |
| rat #8 | 0.96 | 75 | Hypothermia |
| rat #9 | 1.07 | 78 | Hypothermia |
| rat #10 | 0.88 | 80 | Hypothermia |
C. Correlation Between MSEα and NDS
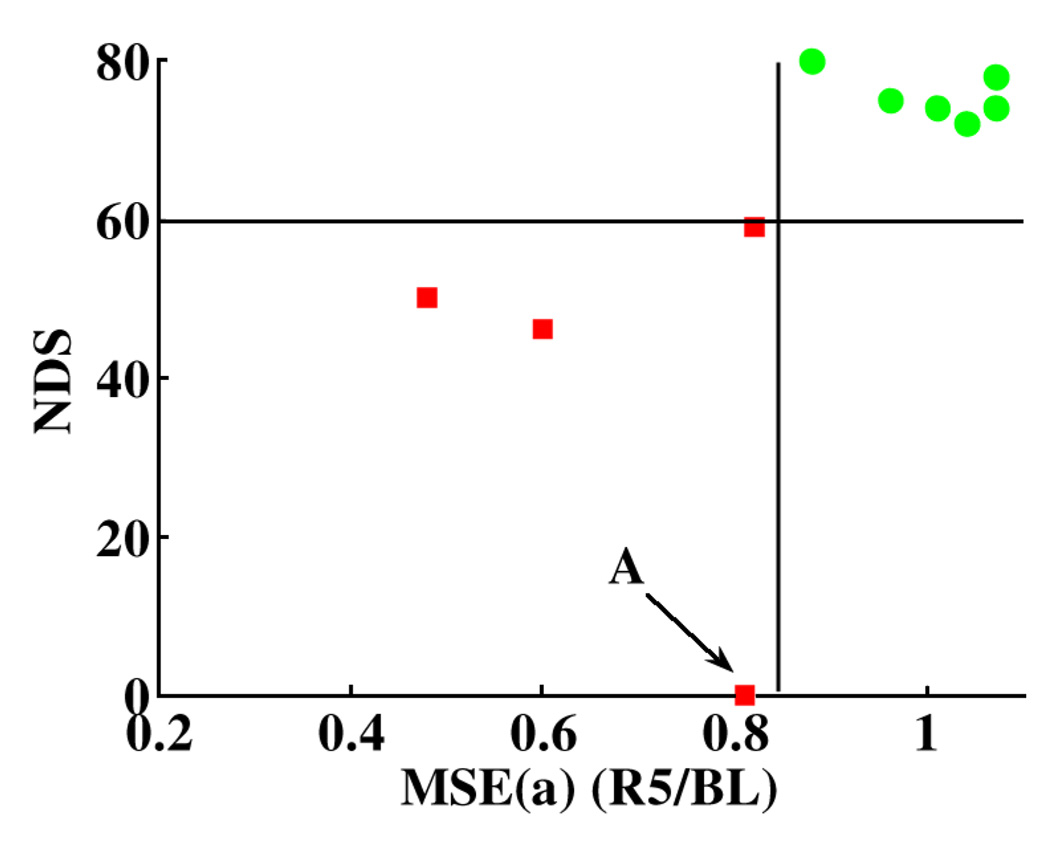
In order to uncover the relationship between α-rhythms during the acute stages of recovery and the recovery outcomes within three days, the Pearson correlation coefficients and the corresponding p-values between NDS and MSEα were calculated for the two groups, respectively. MSEα of the normothermia group in R3–R5 had high correlation with NDS at 24, 48, and 72 h. On the other hand, the correlation between MSEα of the hypothermia group and NDS at four examination times (Table V) was low. Fig. 4 shows that the binary classification of the 72-h recovery outcomes as good or poor with the threshold of 0.85 for MSEα (R5/BL) and the threshold of 60 for NDS [42]–[46]. The good and poor recovery outcomes are well separated into two distinct clusters, indicating that MSEα (R5/BL) can classify the 72-h recovery outcomes as favorable and unfavorable.
TABLE V.
Pearson correlation coefficients (p-value) between NDS and MSEα in normothermia and hypothermia groups
| MSEα | ||||||
|---|---|---|---|---|---|---|
| BL | R1 | R2 | R3 | R4 | R5 | |
| Normothermia | ||||||
| NDS(6H) | 0.21 (0.07) | 0.73 (0.01) | 0.19 (0.07) | 0.49 (0.04) | 0.60 (0.02) | 0.80 (0.01) |
| NDS(24H) | −0.16 (0.08) | 0.57 (0.03) | 0.35 (0.05) | 0.75 (0.01) | 0.87 (0.05) | 0.89 (0.04) |
| NDS(48H) | −0.85 (0.06) | 0.59 (0.05) | 0.65 (0.06) | 0.97 (0.03) | 0.99 (0.03) | 0.97 (0.07) |
| NDS(72H) | −0.84 (0.07) | 0.63 (0.05) | 0.74 (0.05) | 0.99 (0.03) | 0.97 (0.03) | 0.96 (0.06) |
| Hypothermia | ||||||
| NDS(6H) | 0.79 (0.01) | 0.33 (0.05) | 0.73 (0.01) | 0.68 (0.02) | −0.36 (0.05) | −0.63 (0.02) |
| NDS(24H) | 0.59 (0.02) | 0.23 (0.07) | 0.88 (0.04) | 0.86 (0.06) | −0.44 (0.04) | −0.47 (0.04) |
| NDS(48H) | 0.74 (0.01) | 0.24 (0.07) | 0.68 (0.02) | 0.85 (0.07) | −0.36 (0.05) | −0.46 (0.04) |
| NDS(72H) | 0.33 (0.05) | 0.06 (0.09) | 0.40 (0.05) | −0.01 (0.09) | 0.04 (0.09) | −0.72 (0.01) |
Fig. 4.
Binary classification of 72-h recovery outcome represented by NDS as good (circles) or poor (squares) with MSEα (R5/BL). Good recovery outcome is defined as 72-h NDS ≥ 60 (horizontal line corresponding to NDS = 60) and vice versa [41]–[45]. The result indicates that with the threshold of 0.85 for MSEα (R5/BL) (vertical line corresponding to MSEα x(R5/BL) = 0.85), 72-h recovery outcomes can be well classified into two clusters without any overlap. Point A is an outlier, representing a rat with MSEα (R5/BL) = 0.81 died before 72-h NDS examination.
V. DISCUSSION
EEG as a tool for neuromonitoring is easily accessible in clinics. Neurologists usually inspect EEG to obtain the information about the patients’ neurological status. However, the determination of neurological status of the brain from simple EEG inspection does not provide enough information for early prediction of long-term outcomes. The first few hours during the postresuscitation period constitutes the most critical time for therapeutic interventions to minimize potential brain injury induced by CA and early reperfusion. Our results suggest that 72-h recovery outcomes from global ischemia CA may be predicted early and divided into two classes as favorable and unfavorable during the first 3-h postresuscitation period using the MSE analysis. Neurologists may further adopt therapies such as therapeutic hypothermia to improve recovery outcomes. The limitation is that MSEα (R5/BL) needs the BL information, which may not be available in real clinical situations, especially for patients suffering from out-of-hospital CA. Yet, according to our results in rats, MSEα (BL) for both groups showed little variation: 2.37 ± 0.11. In real clinical occasions, neurologists may define MSEα (BL)s from normal subjects in different age groups and genders.
The beneficial effects of therapeutic hypothermia on post-CA recovery have been proved in various animal models [47], [48] and human clinical trials [49], [50]. However, several challenges and uncertainties persist in its application, including and not limited to the effects of hypothermia on different EEG subbands as well as related brain structures, and the understanding of basic mechanism. While MSE can show the change in the complexity of each EEG subband over multiple time scales, MSEα can be considered as a measure of complexity level within the α-rhythms. The significant difference in MSEα between the normothermia and hypothermia groups in R2–R4 (Table III) indicates the significant difference in the complexity of the α-rhythms under temperature modulation. Table III also shows that in the normothermia group, MSEα (R5) is about 25% less than MSEα (BL), and it may indicate the decreased complexity level in the α-rhythms due to the ischemia in CA and early reperfusion. In the hypothermia group, MSEα (R5) is 1% less than MSEα (BL), and it shows that there is little decrease in the complexity of the α-rhythms under hypothermia. We have previously shown that it is not the temperature (32 °C–34 °C) itself that causes the change in EEG, but the response of the injured brain to hypothermia as manifested in the quantitative EEG analysis [51]. The loss of complexity in the α-rhythms in the normothermia group is in accordance with the “complexity-loss” theory in the unhealthy organs [30], [31]. It may suggest that the neurological injuries induced by CA in the thalamus reduce the thalamus’s capability to respond to various stimuli, and therapeutic hypothermia has significant neuroprotective effect on the thalamus. Yet, further detailed investigation is needed to confirm the projection.
The significant differences in NDS at 6 and 24 h between the normothermia and hypothermia groups indicate that temperature modulation has significant effects on early recovery outcomes (Table I). On the other hand, the loss of significant differences between the normothermia and hypothermia groups in NDS at 48 and 72 h may indicate that 6-h mild hypothermia has limited neuroprotective effects on the eventual recovery outcomes, and the effects of mild hypothermia with longer duration on the neurological recovery outcomes may be worth of further investigation.
We hypothesizes that the decreased complexity associated with the α-rhythms serves as potential evidence of neurological injuries in the thalamus, and the results in Table IV indicate that the degeneration in the thalamus may be a factor in poor recovery outcomes during the acute stages of postresuscitation period under normothermia. The poor correlation between MSEα and NDS in the hypothermia group (Table V), we hypothesize, may indicate that recovery outcomes from CA may be the results of neurological injuries under hypothermia in brain regions other than the thalamus under hypothermia. Histological examination as well as experimentation with multichannel recording in the thalamus and other related brain structures may be needed to confirm our hypotheses.
Limitations of this study include a small number of animals. The results of EEG analysis are quite consistent and statistically significant with this cohort. However, mechanistic insight on the cortical injury as well as reaching conclusions on hypothermia efficacy would require larger cohorts. Another potential limitation of our study is the lack of corresponding histopathological data to support our hypothesis. Though our group has previously demonstrated that the histopathological markers for ischemic neuron death in various brain regions correlate with quantitative EEG and NDS measures [41], we also realize that postmortem histologic markers in rats are poor indicators of clinical significance in human trials of the same therapies if used as the only method [52], and neurobehavioral studies would be more predictive [53]. Consequently, in this paper, more attention is put on biological complexity measures (MSE) and neurological recovery outcomes (NDS). Our observation and analysis are based on EEG recorded during the first 3 h after resuscitation. Additional experiments under different degrees and durations of hypothermia, followed by signal analysis with a longer observation window for EEG, would be done to demonstrate the utility of EEG analysis in long-term monitoring and prognosis.
VI. CONCLUSION
Our results suggest that the MSE analysis can be used to track and differentiate the changes in complexity of each EEG subband over multiple time scales under hypothermia and normothermia. Specifically, MSEα can characterize the complexity in α-rhythms in different recording phases of the experiments, and is reflective of the benefit of hypothermia during the acute stages of recovery. The ratio MSEα(R5/BL) may be used as a numeric index for early prediction and classification of late recovery outcomes (good/poor). Given that decreased complexity of α-rhythms partially represents neurological injuries in the thalamus, we hypothesized that therapeutic hypothermia may produce significant neuroprotective effect on the thalamus early after resuscitation. These experiments have the potential to help uncover the mechanism of early neurological response to therapeutic hypothermia and produce major shift in brain recovery monitoring with clinical translation.
Acknowledgments
This work was supported in part by the National Institute of Health (NIH) under Grant R01 HL071568 andGrant R21 NS054146.
Biographies

Xiaoxu Kang (M’08) was born in Harbin, China, on October 7, 1984. She received the B.S. degree in biomedical engineering from Beijing University of Aeronautics and Astronautics, Beijing, China, in 2007. She is currently working toward the M.S. degree in the Department of Biomedical Engineering, Johns Hopkins University, Baltimore, MD.
Her current research interests include neural signal processing and neurophysiology of brain injury.
Ms. Kang is a student member of the Society of Neuroscience and the Biomedical Engineering Society. She was the recipient of a National Chiang Chen Industry Charity Fellowship from Chiang Chen Industrial Charity Foundation, China, in 2007.

Xiaofeng Jia received the M.D. degree in clinical medicine from Zhejiang Medical University, Zhejiang, China, in 1994, the M.S. degree in surgery from Shanghai Medical University, Shanghai, China, in 1997, and the Ph.D. degree in surgery (orthopedics) from Fudan University, Shanghai, in 2003.
Since 2007, he has been a Faculty member in the Department of Biomedical Engineering, Johns Hopkins University School of Medicine, Baltimore, MD, after three years of Postdoctoral Fellowship. He completed his surgery residency in the Huashan Hospital and Orthopedic Surgery Fellowship, Shanghai 6th People’s Hospital, Shanghai. He was an Attending Surgeon in the Department of Orthopedics, Zhongshan Hospital, Shanghai. His current research interests include novel application of neuroelectrophysiology for detection and restoration of spinal cord and peripheral nerve injury, therapeutic hypothermia of brain and spinal cord after asphyxial cardiac arrest, and basic and clinical investigations in acute neurological injuries after global cerebral ischemia.
Dr. Jia is a member of the American Academy of Orthopaedic Surgeons, the American Association for Hand Surgery, the Society of Critical Care Medicine, the Society for Neuroscience, the American Heart Association, the American Stroke Association, and the International Society for Heart Research. He was the recipient of the Annual Research Awards from the American Association for Hand Surgery, the Cardiopulmonary, Perioperative, and Critical Care Junior Investigator Travel Award from the American Heart Association, the Finalist of Best Overall Poster Award of the American Academy of Orthopaedic Surgeons, the Finalist of Research Citation Award from the Society of Critical Care Medicine, the Guanghua Scholarship, the Government Public Scholarship, the Outstanding Moral-Intellectual-Physical Student and the Outstanding Graduate from Fudan University, and the National Educational Ministry Outstanding Postgraduate Scholarship from Shanghai Medical University.

Romergryko G. Geocadin received the B.Sc. degree from the University of the Philippines-Diliman, Quezon City, Philippines, and the Medical degree from the University of the East Ramon Magsaysay College of Medicine, Quezon City.
He completed his Neurology residency at New York University Medical Center, and the Neurocritical Care Fellowship at Johns Hopkins Hospital. He is currently an Associate Professor of Neurology, Neurosurgery and Anesthesiology—Critical Care Medicine in the Department of Nuurology, Johns Hopkins University School of Medicine, Baltimore, MD. He is also the Director of the Neurosciences Critical Care Unit (NCCU), Johns Hopkins Bayview Medical Center, and the Associate Director of the Neurosciences Critical Care Division, Johns Hopkins Medical Institutions, Baltimore. His current research includes basic and clinical investigations in acute neurological injuries after global cerebral ischemia (with special focus on cardiac arrest and therapeutic hypothermia), novel application of neuroelectrophysiology for early detection of acute neurological injuries, coma, disorders of intracranial pressure, cerebrovascular disorders, and neurologic disorders in critical illness.
Dr. Geocadin has been the Guest Editor of the Seminars in Neurology, Neurologic Clinics of North America, Critical Care Clinics of North America, and Emergency Medicine Clinics of North America. He is active in advancing the clinical practice related to brain injury and cardiac resuscitation, working on the development of guidelines and practice parameters with the American Heart Association, the International Liaison Committee on Resuscitation (ILCOR), and the American Academy of Neurology. He is the Director in the Board of the Neurocritical Care Society, and the Founding Editor of Currents, the society’s newsletter.

Nitish V. Thakor (S’78–M’81–SM’89–F’97) received the B.Tech. degree from Indian Institute of Technology, India, in 1974, the M.S. degree in biomedical engineering from the University of Wisconsin, Madison, WI, in 1978, and the Ph.D. degree in electrical and computer engineering from the University of Wisconsin, Madison, WI, in 1981.
He is currently a Professor of biomedical engineering at Johns Hopkins University, Baltimore, MD, where he directs the Laboratory for Neuroengineering. His research was supported by the National Institute of Health (NIH), the National Science Foundation (NSF), and the Defense Advanced Research Projects Agency (DARPA). He is the Director of a Neuroengineering Training Program funded by the National Institute of Biomedical Imaging and Bioengineering, a multidisciplinary and collaborative training program for doctoral students. His current research interests include neural diagnostic sensors and instrumentation for clinical applications, signal processing, optical and MRI imaging, and microsystems for basic neuroscience research. He is the author or coauthor of more than 180 refereed journal articles. He is the holder of six patents.
Prof. Thakor is a Fellow of the American Institute of Medical and Biological Engineering, and a Founding Fellow of the Biomedical Engineering Society. He is the Editor-in-Chief of the IEEE Transactions on Neural Systems and Rehabilitation Engineering, and contributes to many IEEE publication and conference activities. He was the recipient of the Research Career Development Award from the NIH, the Presidential Young Investigator Award from the NSF, the Centennial Medal from the University of Wisconsin School of Engineering, the Honorary Membership from Alpha Eta Mu Beta Biomedical Engineering Student Honor Society, and the Distinguished Service Award from the Indian Institute of Technology, Mumbai, India.

Anil Maybhate (M’08) received the Ph.D. degree in physics from the University of Pune, Pune, India, in 2003.
He is currently a Postdoctoral Fellow in the Biomedical Engineering Department, Johns Hopkins University, Baltimore, MD. He was a Postdoctoral Fellow at Cornell University and Pennsylvania State University, and has previously developed novel methods of parameter estimation as well as methods to detect repolarization alternans from an intracardiac electrogram. His current research interests include translational biomedical research, especially advanced cardiac [EKG, heart rate variability (HRV)] and neurophysiological (EEG, SEP) signal processing for monitoring progress of disease and recovery in animal models as well as patients, nonlinear time series analysis, nonlinear dynamics, and its applications to physiological signals.
Footnotes
Color versions of one or more of the figures in this paper are available online at http://ieeexplore.ieee.org.
Contributor Information
Xiaoxu Kang, Email: xkang2@jhu.edu, Department of Biomedical Engineering, Johns Hopkins University, Baltimore, MD 21205 USA.
Xiaofeng Jia, Email: xjia1@jhmi.edu, Department of Biomedical Engineering, Johns Hopkins University, Baltimore, MD 21205 USA.
Romergryko G. Geocadin, Email: rgeocadi@jhmi.edu, Department of Neurology, Johns Hopkins School of Medicine, Baltimore, MD 21287 USA.
Nitish V. Thakor, Email: nitish@jhu.edu, Department of Biomedical Engineering, Johns Hopkins University, Baltimore, MD 21205 USA.
Anil Maybhate, Email: anil@jhmi.edu, Department of Biomedical Engineering, Johns Hopkins University, Baltimore, MD 21205 USA.
REFERENCES
- 1.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics—2007 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;vol. 115:169–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) State-specific mortality from sudden cardiac death—United States, 1999. MMWR Morb. Mortal Wkly. Rep. 2002;vol. 51:123–126. [PubMed] [Google Scholar]
- 3.Herlitz J, Andersson E, Bång A, Engdahl J, Holmberg M, Lindqvist J, Karlson BW, Waagstein L. Experiences from treatment of out-of-hospital cardiac arrest during 17 years in Goteborg. Eur. Heart J. 2000;vol. 21:1251–1258. doi: 10.1053/euhj.2000.2150. [DOI] [PubMed] [Google Scholar]
- 4.Geocadin RG, Koenig MA, Jia X, Stevens RD, Peberdy MA. Management of brain injury after resuscitation from cardiac arrest. Neurol. Clin. 2008;vol. 26:487–506. doi: 10.1016/j.ncl.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaagenes P, Ginsberg M, Ebmeyer U, Ernster L, Fischer M, Gisvold SE, Gurvitch A, Hossmann KA, Nemoto EM, Radovsky A, Severinghaus JW, Safar P, Schlichtig R, Sterz F, Tonnessen T, White RJ, Xiao F, Zhou Y. Cerebral resuscitation from cardiac arrest: Pathophysiologic mechanisms. Crit. Care Med. 1996;vol. 24:57–68. [PubMed] [Google Scholar]
- 6.Brain Resuscitation Clinical Trial I Study Group. Randomized clinical study of thiopental loading in comatose survivors of cardiac arrest. N. Engl. J. Med. 1986;vol. 314:397–403. doi: 10.1056/NEJM198602133140701. [DOI] [PubMed] [Google Scholar]
- 7.Jastremski M, Sutton-Tyrrell K, Vaagenes P, Abramson N, Heiselman D, Safar P. Glucocorticoid treatment does not improve neurological recovery following cardiac arrest. J. Amer. Med. Assoc. 1989;vol. 262:3427–3430. [PubMed] [Google Scholar]
- 8.Brain Resuscitation Clinical Trial II Study Group. A randomized clinical study of a calcium-entry blocker (lidoflazine) in the treatment of comatose survivors of cardiac arrest. N. Engl. J. Med. 1991;vol. 324:1225–1231. doi: 10.1056/NEJM199105023241801. [DOI] [PubMed] [Google Scholar]
- 9.Roine RO, Kaste M, Kinnunen A, Nikki P, Sarna S, Kajaste S. Nimodipine after resuscitation from out-of-hospital ventricular fibrillation. Aplacebo-controlled, double-blind, randomized trial. J. Amer.Med. Assoc. 1990;vol. 264:3171–3177. [PubMed] [Google Scholar]
- 10.Allen GS, Ahn HS, Preziosi TJ, Battye R, Boone SC, Chou SN, Kelly DL, Weir BK, Crabbe RA, Lavik PJ, Rosenbloom SB, Dorsey FC, Ingram CR, Mellits DE, Bertsch LA, Boisvert DP, Hundley MB, Johnson RK, Strom JA, Transou CR. Cerebral arterial spasm—A controlled trial of nimodipine in patients with subarachnoid hemorrhage. N. Engl. J. Med. 1983;vol. 308:619–624. doi: 10.1056/NEJM198303173081103. [DOI] [PubMed] [Google Scholar]
- 11.Minamisawa H, Nordstrom C-H, Smith M-L, Siesjo BK. The influence of mild body and brain hypothermia on ischemic brain damage. J. Cereb. Blood Flow Metab. 1990;vol. 10:365–374. doi: 10.1038/jcbfm.1990.66. [DOI] [PubMed] [Google Scholar]
- 12.Welsh FA, Sims RE, Harris VA. Mild hypothermia prevents ischemic injury in gerbil hippocampus. J. Cereb. Blood Flow Metab. 1990;vol. 10:557–563. doi: 10.1038/jcbfm.1990.98. [DOI] [PubMed] [Google Scholar]
- 13.The Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med. 2002;vol. 346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 14.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 2002;vol. 346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 15.Nolan JP, Morley PT, VandenHoek TL, Hickey RW, Kloeck WGJ, Billi J, Bötiger BW, Morley PT, Nolan JP, Okada K, Reyes C, Shuster M, Steen PA, Weil MH, Wenzel V. Therapeutic hypothermia after cardiac arrest: An advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation. 2003;vol. 108:118–121. doi: 10.1161/01.CIR.0000079019.02601.90. [DOI] [PubMed] [Google Scholar]
- 16.Abella BS, Rhee JW, Huang KN, Hoek TLV, Becker LB. Induced hypothermia is underused after resuscitation from cardiac arrest: A current practice survey. Resuscitation. 2005;vol. 64:181–186. doi: 10.1016/j.resuscitation.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Minamisawa H, Smith ML, Siesjo BK. The effect of mild hyperthermia and hypothermia on brain damage following 5, 10, and 15 minutes of forebrain ischemia. Ann. Neurol. 1990;vol. 28:26–33. doi: 10.1002/ana.410280107. [DOI] [PubMed] [Google Scholar]
- 18.Steriade M, Llinas R. The functional states of the thalamus and the associated neuronal interplay. Physiol. Rev. 1988;vol. 68:699–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- 19.Ross DT, Duhaime AC. Degeneration of neurons in the thalamic reticular nucleus following transient ischemia due to raised intracranial pressure: Excitotoxic degeneration mediated via non-NMDA receptors? Brain Res. 1989;vol. 501:129–143. doi: 10.1016/0006-8993(89)91034-2. [DOI] [PubMed] [Google Scholar]
- 20.Hughes SW, Crunelli V. Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neuroscientist. 2005;vol. 11(no. 4):357–372. doi: 10.1177/1073858405277450. [DOI] [PubMed] [Google Scholar]
- 21.Buzsaki G. The thalamic clock: Emergent network properties. Neuroscience. 1991;vol. 41:351–364. doi: 10.1016/0306-4522(91)90332-i. [DOI] [PubMed] [Google Scholar]
- 22.Ohmoto T, Mimura Y, Baba Y, Miyamoto T, Matsumoto Y, Nishimoto A, Matsumoto K. Thalamic control of spontaneous alpharhythms and evoked responses. Appl. Neurophysiol. 1973;vol. 41:188–192. doi: 10.1159/000102415. [DOI] [PubMed] [Google Scholar]
- 23.Lindgrena KA, Larsona CL, Schaefera SM, Abercrombiea HC, Warda RT, Oakesa TR, Holdene JE, Perlmanbd SB, Bencac RM, Davidsonac RJ. Thalamic metabolic rate predicts EEG alpha power in healthy control subjects but not in depressed patients. Biol. Psychiatry. 1999;vol. 8(no. 45):943–952. doi: 10.1016/s0006-3223(98)00350-3. [DOI] [PubMed] [Google Scholar]
- 24.Jorgensen EO, Malchow-Moller A. Natural history of global and critical brain ischaemia. Part II: EEG and neurological signs in patients remaining unconscious after cardiopulmonary resuscitation. Resuscitation. 1981;vol. 9:155–174. doi: 10.1016/0300-9572(81)90024-1. [DOI] [PubMed] [Google Scholar]
- 25.Guasello SJ. Progress in applied nonlinear dynamics: Welcome to NDPLS. Nonlinear Dyn., Psychol., Life Sci. 2004;vol. 8:1–15. [Google Scholar]
- 26.Bhattacharya J. Complexity analysis of spontaneous EEG. Acta Neurobiol. Exp. (Wars) 2000;vol. 60:495–501. doi: 10.55782/ane-2000-1369. [DOI] [PubMed] [Google Scholar]
- 27.Goldberger AL, Amaral LA, Hausdorff JM, Ivanov P, Peng CK, Stanley HE. Fractal dynamics in physiology: Alterations with disease and aging. Proc. Nat. Acad. Sci. USA. 2002;vol. 99:2466–2472. doi: 10.1073/pnas.012579499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vigo DE, Siri LN, De Guevara MSL, Martinez-Martinez JA, Fahrer RD, Cardinali DP, Masoli O, Guinjoan SM. Relation of depression to heart rate nonlinear dynamics in patients > or = 60 years of age with recent unstable angina pectoris or acute myocardial infarction. Amer. J. Cardiol. 2004;vol. 93:756–760. doi: 10.1016/j.amjcard.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 29.Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of complex physiologic time series. Phys. Rev. Lett. 2002;vol. 89:068 102-1–068 102-4. doi: 10.1103/PhysRevLett.89.068102. [DOI] [PubMed] [Google Scholar]
- 30.Vaillancourt DE, Newell KM. Aging and the time and frequency structure of force output variability. J. Appl. Physiol. 2003;vol. 94:903–912. doi: 10.1152/japplphysiol.00166.2002. [DOI] [PubMed] [Google Scholar]
- 31.Goldberger AL, Peng C-K, Lipsitz LA. What is physiological complexity and how does it change with aging and disease? Neurobiol. Aging. 2002;vol. 23:23–26. doi: 10.1016/s0197-4580(01)00266-4. [DOI] [PubMed] [Google Scholar]
- 32.Pincus SM, Gladstone IM, Ehrenkranz RA. A regularity statistic for medical data analysis. J. Clin. Monit. 1991;vol. 7:335–345. doi: 10.1007/BF01619355. [DOI] [PubMed] [Google Scholar]
- 33.Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Amer. J. Physiol. Heart. Circ. Physiol. 2000;vol. 278:H2039–H2049. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- 34.Lake DE, Richman JS, Griffin MP, Moorman JR. Sample entropy analysis of neonatal heart rate variability. Amer. J. Physiol. Regul. Integr. Comp. Physiol. 2002;vol. 283:R789–R797. doi: 10.1152/ajpregu.00069.2002. [DOI] [PubMed] [Google Scholar]
- 35.Ferenets R, Lipping T, Anier A, Jantti V, Melto S, Hovilehto S. Comparison of entropy and complexity measures for the assessment of depth of sedation. IEEE Trans. Biomed. Eng. 2006 Jun;vol. 53(no. 6):1067–1077. doi: 10.1109/TBME.2006.873543. [DOI] [PubMed] [Google Scholar]
- 36.Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of biological signals. Phys. Rev. E. Statist. Nonlinear Soft Matter Phys. 2005;vol. 71:021 906-1–021 906-18. doi: 10.1103/PhysRevE.71.021906. [DOI] [PubMed] [Google Scholar]
- 37.Bornas X, Llabres J, Noguera M, Lopez AM, Gelabert JM, Vila I. Fear induced complexity loss in the electrocardiogram of flight phobics: A multiscale entropy analysis. Biol. Psychol. 2006;vol. 73:272–279. doi: 10.1016/j.biopsycho.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Escudero J, Abasolo D, Hornero R, Espino P, Lopez M. Analysis of electroencephalograms in Alzheimer’s disease patients with multiscale entropy. Physiol. Meas. 2006;vol. 27:1091–1106. doi: 10.1088/0967-3334/27/11/004. [DOI] [PubMed] [Google Scholar]
- 39.Angelini L, Maestri R, Marinazzo D, Nitti L, Pellicoro M, Pinna GD, et al. Multiscale analysis of short term heart beat interval, arterial blood pressure, and instantaneous lung volume time series. Artif. Intell. Med. 2007;vol. 41:237–250. doi: 10.1016/j.artmed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Bhattacharya J, Edwards J, Mamelak AN, Schuman EM. Long-range temporal correlations in the spontaneous spiking of neurons in the hippocampal-amygdala complex of humans. Neuroscience. 2005;vol. 131:547–555. doi: 10.1016/j.neuroscience.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Geocadin RG, Muthuswamy J, Sherman DL, Thakor NV, Hanley DF. Early electrophysiological and histologic changes after global cerebral ischemia in rats. Movement Disord. 2000;vol. 15(no. 1):14–21. doi: 10.1002/mds.870150704. [DOI] [PubMed] [Google Scholar]
- 42.Geocadin RG, Ghodadra R, Kimura T, Lei H, Sherman DL, Hanley DF, et al. A novel quantitative EEG injury measure of global cerebral ischemia. Clin. Neurophysiol. 2000;vol. 111:1779–1787. doi: 10.1016/s1388-2457(00)00379-5. [DOI] [PubMed] [Google Scholar]
- 43.Geocadin RG, Koenig MA, Jia X, Stevens RD, Peberdy MA. Management of brain injury after resuscitation from cardiac arrest. Neurol. Clin. 2008;vol. 26(no. 2):487–506. doi: 10.1016/j.ncl.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jia X, Koenig MA, Nickl R, Zhen G, Thakor NV, Geocadin RG. Early electrophysiologic markers predict functional outcome associated with temperature manipulation after cardiac arrest in rats. Crit. Care Med. 2008;vol. 36(no. 6):1909–1916. doi: 10.1097/CCM.0b013e3181760eb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jia X, Koenig MA, Shin HC, Zhen G, Pardo CA, Hanley DH, et al. Improving neurological outcomes post-cardiac arrest in a rat model: Immediate hypothermia and quantitative EEG monitoring. Resuscitation. 2008;vol. 76(no. 3):431–442. doi: 10.1016/j.resuscitation.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jia X, Koenig MA, Venkatraman A, Thakor NV, Geocadin RG. Post-cardiac arrest temperature manipulation alters early EEG bursting in rats. Resuscitation. 2008;vol. 78(no. 3):367–373. doi: 10.1016/j.resuscitation.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minamisawa H, Nordstrom CH, Smith ML, Siesjö BK. The influence of mild body and brain hypothermia on ischemic brain damage. J. Cereb. Blood Flow Metab. 1990;vol. 10(no. 3):365–374. doi: 10.1038/jcbfm.1990.66. [DOI] [PubMed] [Google Scholar]
- 48.Welsh FA, Sims RE, Harris VA. Mild hypothermia prevents ischemic injury in gerbil hippocampus. J. Cereb. Blood Flow Metab. 1990;vol. 10(no. 4):557–563. doi: 10.1038/jcbfm.1990.98. [DOI] [PubMed] [Google Scholar]
- 49.The Hypothermia After Cardiac Arrest Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med. 2002;vol. 346(no. 8):549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 50.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 2002;vol. 346(no. 8):557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 51.Jia X, Koenig MA, Shin HC, Thakor NV, Geocadin RG. Quantitative EEG and neurological recovery with therapeutic hypothermia after asphyxial cardiac arrest in rats. Brain Res. 2006;vol. 1111:166–175. doi: 10.1016/j.brainres.2006.04.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corbett D, Nurse S. The problem of assessing effective neuroprotection in experimental cerebral ischemia. Prog. Neurobiol. 1998 Apr;vol. 54:531–548. doi: 10.1016/s0301-0082(97)00078-6. [DOI] [PubMed] [Google Scholar]
- 53.Hunter AJ, Mackay KB, Rogers DC. To what extent have functional studies of ischemia in animals been useful in the assessment of potential neuroprotective agents? Trends Pharmacol. Sci. 1998 Feb;vol. 19:59–66. doi: 10.1016/s0165-6147(97)01157-7. [DOI] [PubMed] [Google Scholar]