Abstract
In this article, we report that in visual search, desaturated reddish targets are much easier to find than other desaturated targets, even when perceptual differences between targets and distractors are carefully equated. Observers searched for desaturated targets among mixtures of white and saturated distractors. Reaction times were hundreds of milliseconds faster for the most effective (reddish) targets than for the least effective (purplish) targets. The advantage for desaturated reds did not reflect an advantage for the lexical category “pink,” because reaction times did not follow named color categories. Many pink stimuli were not found quickly, and many quickly found stimuli were not labeled “pink.” Other possible explanations (e.g., linear-separability effects) also failed. Instead, we propose that guidance of visual search for desaturated colors is based on a combination of low-level color-opponent signals that is different from the combinations that produce perceived color. We speculate that this guidance might reflect a specialization for human skin.
Keywords: visual attention, visual search, color perception, visual perception, basic color terms
In visual search tasks, observers search for target stimuli among distractors. Some of the easiest, most efficient searches are those for targets of one color among homogeneous distractors of another color (Carter, 1982; Treisman & Gormican, 1988). However, although color is a salient aspect of visual experience and a powerful guide to visual attention, not all searches for colored targets are easy. For example, when the target color is collinear in color space with its distractors (e.g., orange among reddish-orange and yellowish-orange), search can be very inefficient (Bauer, Jolicoeur, & Cowan, 1996; D'Zmura, 1991).
In this article, we report a study examining search for a desaturated target (e.g., pink) among saturated (e.g., red) and unsaturated (white) distractors. We found that hue makes a massive difference to the efficiency of such a search, and we tested the hypothesis that visual search is influenced by the lexical categories to which target and distractor colors belong. Although some authors have been skeptical of lexical effects (Smallman & Boynton, 1990), a number of recent reaction time (RT) studies have reported color categorical effects (visual search—Daoutis, Pilling, & Davies, 2006; Yokoi & Uchikawa, 2005; speeded color discrimination—Winawer et al., 2007). Categorical effects have also been seen in search for features other than color (e.g., orientation—Hodsoll & Humphreys, 2007; Wolfe, Friedman-Hill, Stewart, & O'Connell, 1992).
Our examination of this question was based on the fact that pink is the only basic color term in English that names a category of desaturated hues. Furthermore, “pink” is a comparatively universal color category. Basic color lexicons around the world, though diverse in many ways, often contain a word for “pink” (Lindsey & Brown, 2006), whereas basic color terms for other desaturated colors are rare. Famous exceptions are Russian (Paramei, 2005) and Turkish (Ozgen & Davies, 1998), which have basic color terms for “pale blue.” Nonetheless, “lavender,” “peach,” “pale yellow,” and “pale green” do not have basic color terms in English, or in any other language that has been studied in this way.
Method
Visual search
Two groups of 12 native-English-speaking observers searched for desaturated targets among saturated and white distractors (Fig. 1a). The two groups were tested with different stimulus sets of 6 or 7 hues, with the red hue being common to both stimulus sets, for a total of 12 hues. Salience models (e.g., Itti & Koch, 2000; Nothdurft, 2000) might predict that the ability to find one target color among other distractor colors would be related to the magnitude of differences in color appearance between the target and distractor colors. Each of our 30-cd/m2 desaturated targets lay at the midpoint of a line segment in CIELAB color space (Wyszecki & Stiles, 1982) that connected a 12-cd/m2 saturated distractor color to the 60-cd/m2 white distractor (metameric to CIE Illuminant C; see section I.A.1 of the Supplemental Material available online for further description). This particular luminance configuration gave compelling examples of saturated and desaturated red, green, and blue. This would not have been possible had we restricted the stimuli to be isoluminant, and our pink target in particular would have been a poor example of that color. All targets were the same distance in CIELAB ΔE units (ΔE ∼ 40) from the white point and from their respective saturated distractors (Fig. 1b). In each trial, a desaturated target was presented in a field (∼20° × ∼20°) of distractors that were evenly divided between white patches and saturated color patches of the target hue (Fig. 1a). Each target or distractor item subtended ∼1° × ∼1° at the eye. Total set size was either 20 or 40 items. A target was present on 50% of trials, and observers gave speeded “target present” versus “target absent” responses (see section I.A.3 of the Supplemental Material for further details on the procedure).
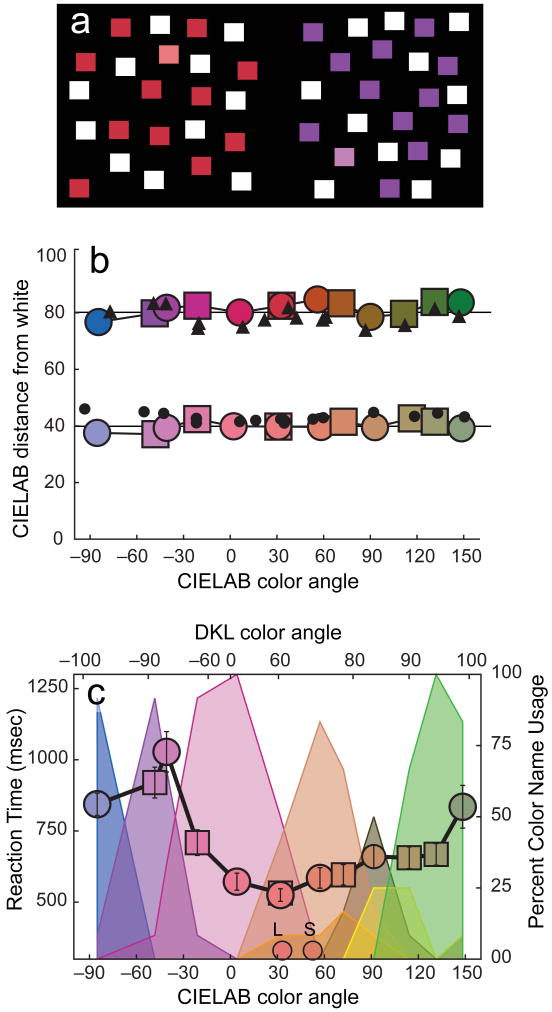
Figure 1.
Search for a desaturated target among white and saturated distractors. (a) Examples of desaturated red (left) and purple targets (right), along with their distractors; these colors are only illustrative, and will not be correctly displayed in print or on an uncalibrated video monitor. (b) The colors used in these experiments. Target colors and distractor colors were at constant separation from white in CIELAB color space (horizontal lines). The colors of the symbols show the approximate colors of the stimuli. Disks and squares represent data from two different stimulus sets and different observers; black symbols: constant-saturation settings of stimuli for observer DTL. (c) Reaction times (left ordinate, ± 1 s.e.m.) for correct responses, target-present searches at set size = 40. Small dots on the x-axis show the chromaticities of lips (L) and skin (S) from Gozalo-Diaz et al., 2007. Observers named each target color as blue, purple, pink, peach, orange, brown, yellow or green. Pastel areas plot the frequency with which observers used each color term (percentages on right ordinate).
Search for a desaturated target among white and saturated distractors. Panel (a) shows examples of desaturated red (left) and purple (right) targets, along with their saturated and white distractors. Panel (b) shows the colors used in these experiments (colored symbols) as distance from the white point (ordinate in ΔE units), as a function of hue angle in CIELAB color space. The squares and circles are data for two different stimulus sets, tested with two different groups of observers; notice that the stimuli at color angle 30° were common to the two stimulus sets. The horizontal lines in (b) indicate the intended colors, which are constant relative to the white point at ΔE = 40 for the targets (lower row) and at ΔE = 80 for the distractors (upper row). The black dots are the heterochromatic-matching data for observer D.T.L. Panel (c) shows reaction times (± 1 SEM) for correct responses on target-present trials with a set size of 40 (large colored symbols, left ordinate), as a function of hue angle in CIELAB color space (bottom abscissa) and in DKL color space (Derrington, Krauskopf, & Lennie, 1984; upper abscissa). As in (b), the squares and circles are data for the two different stimulus sets. The small colored circles near the x-axis show the chromaticities of lips (L) and skin (S) from Gozalo-Diaz, Lindsey, Johnston, and Wee (2007). The area graphs in (c) present color-naming data. The pastel areas plot the frequency with which observers used each color term (percentages on the right ordinate) to name each target color. The pastel colors are keyed to the color terms blue, purple, pink, peach, orange, brown, yellow, and green, which were chosen from a menu of 12 possible color terms. The colors of the stimuli in (a) and the symbols in (b) and (c) show the approximate colors of the stimuli, but all the colors in this figure are only illustrative and are not correctly displayed in print or on an uncalibrated video monitor.
Determining target-distractor perceptual differences
Color differences specified in CIELAB are only approximate. Therefore, we ran two control experiments to verify the perceived color differences among our stimuli. The first control experiment was a heterochromatic-matching experiment (see section I.A.2.a in the Supplemental Material). Three observers adjusted saturation of each of the target stimuli from the main experiment so that its saturation appeared equal to that of the desaturated orange target from the main experiment. Then they adjusted each saturated distractor's saturation so that the color difference between the adjusted saturated distractor and the corresponding desaturated target stimulus (defined in the first part of this experiment) was subjectively equal to the color difference between the desaturated target and white. In our second control experiment, we used maximum likelihood difference scaling (MLDS; Knoblauch & Maloney, 2008; Maloney & Yang, 2003) on 5 observers to determine the perceptual intervals between the target and distractor stimuli. In MLDS, perceptual intervals between stimuli are estimated from forced-choice judgments of differences between pairs of stimuli (see section I.A.2.b in the Supplemental Material).
Color naming
We collected color-naming data on another group of 12 native-English-speaking observers, who named the target and distractor colors using a fixed set of 12 color names: the 11 basic color terms plus peach. The stimuli (0.5°) were presented one at a time, and the observers provided the color term that best fit each stimulus (see section I.C in the Supplemental Material for further methodological details).
Results
Figure 1c shows the main result for the 40-item displays. RTs were fastest for warm colors—notably, desaturated reddish and orangish targets—and slowest for cool colors, such as desaturated blues and purples (see Fig. S7 in section I.A.4 of the Supplemental Material for data from the 20-item displays). Strikingly, the fastest RTs were approximately 550 ms faster than the slowest RTs (see also Table S1 in section I.A.4 of the Supplemental Material). This is a large effect for a simple color search task like this one, and it is all the more remarkable considering how carefully the colors were equated. For target-present trials, RT × Set Size slopes, computed from RTs for the 20- and 40-item displays, were near zero for warm colors and ranged from 5 to 15 ms/item for the cooler colors. Target-absent slopes were approximately twice as steep as target-present slopes (see Fig. S7 in section I.a.4 of the Supplemental Material).
Figure 1b shows that the results for a typical observer (D.T.L.) in our heterochromatic-matching control experiment corresponded well to the actual values for the target and the saturated distractor stimuli used in the main experiment, and both the target and the distractor matches were close to the corresponding colors in CIELAB (horizontal lines). The MLDS control experiment also confirmed this result (see Fig. S6 in section I.A.2.b of the Supplemental Material). Thus, we can reject the hypothesis that search performance was controlled by variations in the perceptual distinctiveness of the various targets with respect to their respective saturated distractors and white. Consequently, a salience model based on perceptual differences would not predict these results. The signal guiding attention to the target must have been a different combination of early color signals (see the Discussion section).
Color-Naming Results
The results for the targets in the color-naming experiment are presented in the area graphs in Figure 1c (see Fig. S9 in section I.C.3 of the Supplemental Material for the results for distractors). Comparison of the RTs from our main experiment with our color-naming results did not show any strong effect of named color categories. The range of target stimuli called “pink” was systematically different from the range of target colors that produced fast, efficient search. There were categorically “pink” targets that were not particularly easy to find in our search experiment, as well as targets that were not called “pink” but that were among the easiest search targets. Thus, we found no evidence that pink things are called “pink” because they are easy to find, nor that easy-to-find things tend to be called “pink,” and the lexical explanation for our results fails.
Additional Control Experiments
Choosing the right color space
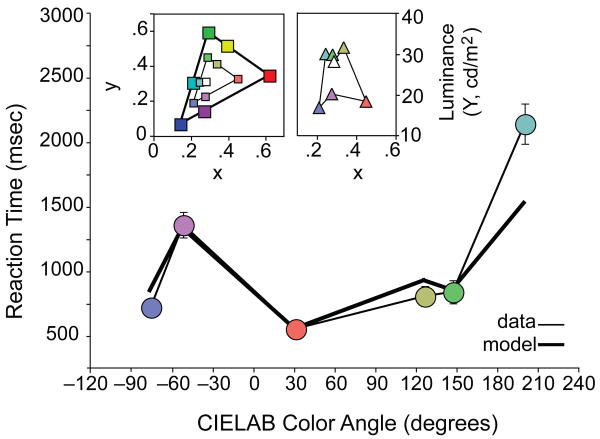
Could our results be an unintended consequence of the use of the CIELAB color space? To investigate this possibility, we replicated our main RT experiment with colors chosen under several different rules, expressed in different color spaces. For example, Figure 2 shows the results we obtained for 11 observers when the saturated hues were the “best” blue, purple, red, yellow, green, and cyan that our monitor could produce (chosen by inspection), and each target was chosen to be roughly halfway between the white and the corresponding saturated distractor hue in the Hue, Saturation, Value (HSV) space of our software (defined by the color gamut of our monitor). These stimuli did not generally fall on lines of constant saturation or luminance in CIELAB space (see section I.B.2.a in the Supplemental Material). Nevertheless, in this and every other replication we could devise, the basic pattern of results was replicated, with search being fastest for desaturated red stimuli.
Figure 2.
Replication of the main result. Symbol colors are the RGB values of the desaturated targets (circles, triangles, and small squares) and distractors (big squares) These colors will not be chromatically correct on the reader's monitor or printout. Circles: reaction time data, +/- 1 s.e.m. Bold line, model described in text and Supplement. Inset, stimulus chromaticities in xyY coordinates. Squares, y (left ordinate) vs. x (abscissa); Triangles, luminance (Y, right ordinate) vs. x (abscissa).
Replication of the main result. The colored circles in the main graph show mean reaction times (± 1 SEM) for six desaturated target colors in a control experiment. The bold line is the model described in the text (Equation 1) and section II.A of the Supplemental Material (Equation S4) available online. The insets show the stimulus chromaticities in xyY coordinates. The left panel shows the x and y coordinates of targets (small colored squares), the white distractor (white square), and saturated distractors (big squares), and the right panel shows the x and Y (luminance) coordinates of targets (triangles). The colors of the symbols approximate the colors used in the experiment, but they are not chromatically correct in print or on readers' monitors.
Examining linear separability
Previous investigators have shown that a target pops out and search is efficient when the target is linearly separable from its distractors (Bauer et al., 1996; D'Zmura, 1991)—that is, when the target can be separated from all the distractors by a plane in CIE xyY space (or linear transformations of this space) or, more generally, when the target falls outside the convex hull enclosing the distractors (Bauer, Jolicoeur, & Cowan, 1999). Otherwise, search is slow and difficult. We tested the hypothesis that the variations in RT in our experiments could be explained by variations in the degree to which our target-distractor combinations violated this linear-separability constraint. Rather than attempting to manipulate linear separability by parametrically varying individual target chromaticities, we created a convex hull with a diverse set of saturated distractor chromaticities, which were present on every trial. The convex hull of the distractors contained all the target stimuli, so in this experiment, none of the targets were linearly separable from the distractors.
Eleven observers searched for targets of different colors presented among a diverse set of saturated distractors. The set of distractor colors was similar to the saturated hues in Figure 2, but all distractor colors were present on every trial. The set of distractors was the same for all target colors. Thus, every desaturated target color was contained within the convex hull defined by the distractors in any plausible color space on every trial. The same result was obtained—a strong advantage for desaturated red and orange targets (see section I.B.2 in the Supplemental Material).
Discussion
The large differences in RT and slope in our search data cannot be explained by perceptual differences between the target and distractor colors. The color-naming data show that these differences in RT do not depend on the color categories of our target and distractor colors. Nor are the results an artifact of our choice of CIELAB color space. Even when we used the best-looking colors possible with our computer graphics software, we replicated the main result. Finally, the results cannot be explained by differences in linear separability. So, what can explain this dramatic advantage for desaturated reds relative to other desaturated hues?
We propose that attentional guidance, and thus RT, depends on a straightforward combination of the outputs of early-stage chromatic mechanisms (Fig. 3). Interestingly, this signal differs from the signals that give rise to perceived differences between attended colors. The RT data in Figure 1c are well fit by a simple model (Figs. 2 and 3) expressed in the following equation:
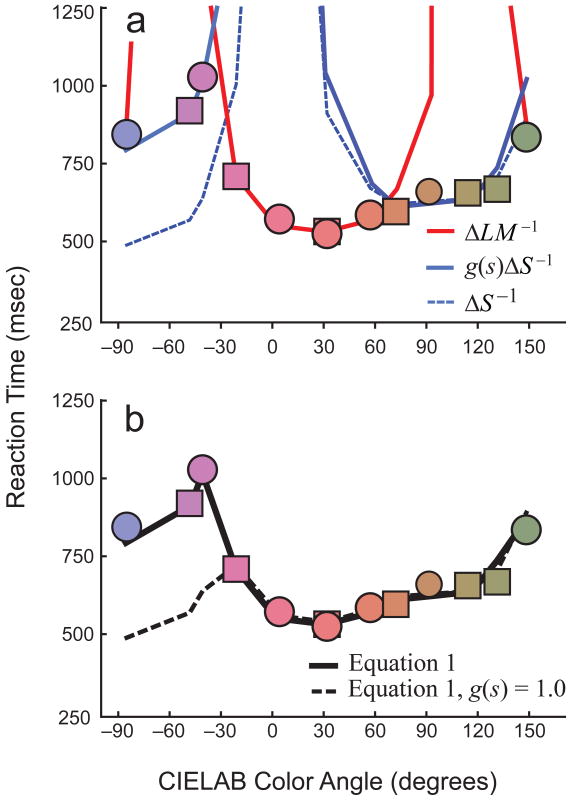
Figure 3.
Fits of a quantitative model described by Equ.1 in the text to the results of the main experiment. Symbols, the reaction time data from Figure 1c. (a) The reciprocals of the responses of the LM- (red line) or S-cone (blue line) components of the model. (b) Fit of the model (solid black line) to the reaction time data, based on selection of “fastest” channel response. Dotted lines in each panel indicate fits based on Equ. 1, but without g(s).
| (1) |
The ΔLM and ΔS terms are derived from early linear transformations of photoreceptor signals into color-opponent channels—specifically, the cardinal channels postulated to define second-stage color vision (Boynton & Kambe, 1980; Derrington, Krauskopf, & Lennie, 1984; Krauskopf, Williams, & Heeley, 1982; MacLeod & Boynton, 1979). For each search condition, the target stimulus and the white and saturated distractors each generate LM and S signals. The difference signals, ΔLM and ΔS, are the average target-distractor differences in low-level chromatic channels. The model proposes that RT varies inversely with the larger of these two differences for each hue. The model includes a minimum RT term (RTmin) and scale factors k1 and k2. This model does not incorporate any term for the luminance differences among target and distractor items, as those were constant across conditions in the main experiment. Furthermore, luminance differences between targets and distractors are a poor guide to visual search (Bauer et al., 1996), and have little effect on search times (Nagy, 1999), when the target luminance is midway between the distractor luminances. In Figure 2, the fit is less good for the cyan target than for the other targets, perhaps because the CIELAB chromaticity of the cyan stimulus was very close to that of its distractors.
Best fits of the quantitative model described by Equation1 (see the text) to the results of the main experiment. The circles and squares are the reaction time data from Figure 1c. The solid lines in (a) show the ΔLM−1 (red lines) and g(s)ΔS−1 (blue lines) components of Equation 1, and the dashed lines show the ΔS−1 component (with g(s) held to a constant value of 1.0). The solid line in (b) shows the overall best fit of Equation 1. The dashed line in (b) is Equation 1 with g(s) held constant at 1.0. See sections II.A and II.B of the Supplemental Material available online for more details.
Our model also incorporates a factor, g(s), that depends on the S-channel responses to the distractors and scales the values of ΔS as principal hue is varied. This factor is derived from classical studies of S-cone contributions to wavelength discrimination (Boynton & Kambe, 1980). These studies showed that S-channel-mediated discrimination thresholds (ΔS) are an increasing function of S-channel responses to the standard colors (see Equation S5 in section II.B in the Supplemental Material). Hence, S-channel discrimination is much better for yellowish colors, which excite the S cones less, than for bluish and purplish colors, which excite the S cones more (cf. the solid and dotted lines in Fig. 3). We confirmed the plausibility of the g(s) factor in separate control experiments (see Fig. S13 in section II.B of the Supplemental Material). A similar, Weber-like function is not needed to scale LM-channel-mediated RTs because so much of LM excitation is due to luminance, which is typically held constant in a discrimination experiment (as it was in our own main experiment).
Desaturated stimuli have rarely been used to study visual search. In the few experiments in which they were used, desaturated targets were usually (Nagy & Cone, 1996), but not always (Santhi & Reeves, 2004), harder to find among saturated distractors than the other way around, at least on an achromatic background (Rosenholtz, 2001). Because humans have only L, M, and S cones, both color appearance and the guidance of attention by color must be based on combinations of L, M, and S signals arising in the early visual system. However, the present results show that different combinations of these signals support color appearance and categorical naming, on one hand, and guidance of visual search, on the other. All our target stimuli had equal perceived saturation, and each desaturated target was perceptually equidistant from its saturated and white distractors. However, equating the stimuli in color appearance in this way produced large, systematic target-distractor differences in the ΔLM and ΔS channel responses. It was these differences that predicted the large variation in RTs. Our model also does a reasonably good job of fitting the data from the best-examples (bold line in Fig. 2) and linear-separability (not shown) control experiments, especially considering the simplicity of the model.
Our model is not intended to rigorously account for all aspects of visual search for chromatic targets. For example, our assumption that RT goes down as color-channel response goes up, though plausible, is not based on any well-established theory of color vision or visual search. Also, the model is designed to account for conditions in which target and saturated distractor items differ principally in saturation, and therefore excite the cardinal chromatic channels in approximately the same ratios. The results of some (Bauer et al., 1996; D'Zmura, 1991) but not all (Nagy, Neriani, & Young, 2004) studies of color and visual search suggest that visual search may engage more than two independent chromatic channels when target and distractors differ in hue. Nonetheless, our main experiment shows that RT can vary greatly across hues, even when target-distractor differences in color appearance are matched. The model makes some interesting, testable predictions. For example, if we equated two sets of targets and distractors for chromatic difference as defined by Equation 1, RT should be constant, even if the targets were quite different in perceptual salience.
Returning to our original hypothesis, we found no evidence that variations in visual search RT are related to the lexical color categories to which the stimulus colors belong. Search for a target defined by a basic color term, such as “pink,” is not necessarily faster than search for a target defined by a nonbasic term, like “pale green.” Changizi, Zhang, and Shimojo (2006) proposed that trichromatic color vision is specialized for perception of blood-related modulations of skin appearance (blushing, blanching, etc.). It is interesting to note that, in our experiments, the fastest RTs were located near the chromatic loci of lips and skin (Gozalo-Diaz, Lindsey, Johnston, & Wee, 2007). The coincidence of these loci is worth further study. For the present, we can say that a quantitative model based on the well-understood low-level features of human color vision can describe the data well. Our results suggest that speeded visual search for colors is based on combinations of early color signals that are not the same as the combinations that give rise to perceptual judgments of color differences, and that are not the same as those underpinning linguistic color categories.
Supplementary Material
Acknowledgments
Funding: This work was supported by grants from the National Eye Institute (R21-EY018321 to A.M.B. and D.T.L., R01-EY017001 to J.M.W.) and the National Institute of Mental Health (R01-MH56020 to J.M.W.); E.R. was supported by the Swiss National Science Foundation. A.N.R. was supported by the National Health and Medical Research Council and the Menzies Foundation (Australia; 359244).
Footnotes
Declaration of Conflicting Interests: The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Supplemental Material: Additional supporting information may be found at http://pss.sagepub.com/content/by/supplemental-data
References
- Bauer B, Jolicoeur P, Cowan WB. Visual search for colour targets that are or are not linearly separable from distractors. Vision Research. 1996;36:1439–1466. doi: 10.1016/0042-6989(95)00207-3. [DOI] [PubMed] [Google Scholar]
- Bauer B, Jolicoeur P, Cowan WB. Convex hull test of the linear separability hypothesis in visual search. Vision Research. 1999;39:2681–2695. doi: 10.1016/s0042-6989(98)00302-2. [DOI] [PubMed] [Google Scholar]
- Boynton RM, Kambe N. Chromatic difference steps of moderate size measured along theoretically critical axes. Color Research and Application. 1980;5:13–23. [Google Scholar]
- Carter RC. Visual search with color. Journal of Experimental Psychology: Human Perception and Performance. 1982;8:127–136. doi: 10.1037//0096-1523.8.1.127. [DOI] [PubMed] [Google Scholar]
- Changizi MA, Zhang Q, Shimojo S. Bare skin, blood and the evolution of primate colour vision. Biological Letters. 2006;2:217–221. doi: 10.1098/rsbl.2006.0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoutis CA, Pilling M, Davies IRL. Categorical effects in visual search for colour. Visual Cognition. 2006;14:217–240. [Google Scholar]
- Derrington AM, Krauskopf J, Lennie P. Chromatic mechanisms in lateral geniculate-nucleus of macaque. Journal of Physiology (London) 1984;357:241–265. doi: 10.1113/jphysiol.1984.sp015499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Zmura M. Color in visual search. Vision Research. 1991;31:951–966. doi: 10.1016/0042-6989(91)90203-h. [DOI] [PubMed] [Google Scholar]
- Gozalo-Diaz DJ, Lindsey DT, Johnston WM, Wee AG. Measurement of color for craniofacial structures using a 45/0-degree optical configuration. Journal of Prosthetic Dentistry. 2007;97:45–53. doi: 10.1016/j.prosdent.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodsoll JP, Humphreys GW. No previews are good news: Using preview search to probe categorical grouping for orientation. Vision Research. 2007;47:1464–1478. doi: 10.1016/j.visres.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Itti L, Koch C. A saliency-based search mechanism for overt and covert shifts of visual attention. Vision Research. 2000;40:1489–1506. doi: 10.1016/s0042-6989(99)00163-7. [DOI] [PubMed] [Google Scholar]
- Knoblauch K, Maloney LT. MLDS: Maximum likelihood difference scaling in R. Journal of Statistical Software. 2008;25(2):1–26. [Google Scholar]
- Krauskopf J, Williams DR, Heeley DW. Cardinal directions of color space. Vision Research. 1982;22:1123–1131. doi: 10.1016/0042-6989(82)90077-3. [DOI] [PubMed] [Google Scholar]
- Lindsey DT, Brown AM. Universality of color names. Proceedings of the National Academy of Sciences, USA. 2006;103:16608–16613. doi: 10.1073/pnas.0607708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod DIA, Boynton RM. Chromaticity diagram showing cone excitation by stimuli of equal luminance. Journal of the Optical Society of America. 1979;69:1183–1186. doi: 10.1364/josa.69.001183. [DOI] [PubMed] [Google Scholar]
- Maloney LT, Yang JN. Maximum likelihood difference scaling. Journal of Vision. 2003;3:573–585. doi: 10.1167/3.8.5. [DOI] [PubMed] [Google Scholar]
- Nagy AL. Interactions between achromatic and chromatic mechanisms in visual search. Vision Research. 1999;39:3253–3266. doi: 10.1016/s0042-6989(99)00009-7. [DOI] [PubMed] [Google Scholar]
- Nagy AL, Cone SM. Asymmetries in simple feature searches for color. Vision Research. 1996;36:2837–2847. doi: 10.1016/0042-6989(96)00046-6. [DOI] [PubMed] [Google Scholar]
- Nagy AL, Neriani KE, Young TL. Color mechanisms used in selecting stimuli for attention and making discriminations. Visual Neuroscience. 2004;21:295–299. doi: 10.1017/s0952523804213190. [DOI] [PubMed] [Google Scholar]
- Nothdurft HC. Salience from feature contrast: Additivity across dimensions. Vision Research. 2000;40:1183–1201. doi: 10.1016/s0042-6989(00)00031-6. [DOI] [PubMed] [Google Scholar]
- Ozgen E, Davies IRL. Turkish color terms: Tests of Berlin and Kay's theory of color universals and linguistic relativity. Linguistics. 1998;36:919–956. [Google Scholar]
- Paramei GV. Singing the Russian blues: An argument for culturally basic color terms. Cross-Cultural Research. 2005;39:10–38. [Google Scholar]
- Rosenholtz R. Search asymmetries? What search asymmetries? Perception & Psychophysics. 2001;63:476–489. doi: 10.3758/bf03194414. [DOI] [PubMed] [Google Scholar]
- Santhi N, Reeves A. The roles of distractor noise and target certainty in search: A signal detection model. Vision Research. 2004;44:1235–1256. doi: 10.1016/j.visres.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Smallman HS, Boynton RM. Segregation of basic colors in an information display. Journal of the Optical Society of America A. 1990;7:1985–1994. doi: 10.1364/josaa.7.001985. [DOI] [PubMed] [Google Scholar]
- Treisman A, Gormican S. Feature analysis in early vision: Evidence from search asymmetries. Psychological Review. 1988;95:15–48. doi: 10.1037/0033-295x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Winawer J, Witthoft N, Frank MC, Wu L, Wade AR, Boroditsky L. Russian blues reveal effects of language on color discrimination. Proceedings of the National Academy of Sciences, USA. 2007;104:7780–7785. doi: 10.1073/pnas.0701644104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe JM, Friedman-Hill SR, Stewart MI, O'Connell KM. The role of categorization in visual search for orientation. Journal of Experimental Psychology: Human Perception and Performance. 1992;18:34–49. doi: 10.1037//0096-1523.18.1.34. [DOI] [PubMed] [Google Scholar]
- Wyszecki G, Stiles WS. Color science: Concepts and methods, quantitative data and formulae. 2nd. New York: Wiley; 1982. [Google Scholar]
- Yokoi K, Uchikawa K. Color category influences heterogeneous visual search for color. Journal of the Optical Society of America A. 2005;22:2309–2317. doi: 10.1364/josaa.22.002309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.