Abstract
Objective
High incidence of poor neurologic sequelae after resuscitation from cardiac arrest underscores the need for objective electrophysiological markers for assessment and prognosis. This study aims to develop a novel marker based on somatosensory evoked potentials (SSEPs). Normal SSEPs involve thalamocortical circuits suggested to play a role in arousal. Due to the vulnerability of these circuits to hypoxic-ischemic insults, we hypothesize that quantitative SSEP markers may indicate future neurologic status.
Design
Laboratory investigation.
Setting
University Medical School and Animal Research Facility.
Subjects
Sixteen adult male Wistar rats.
Interventions
None.
Measurements and Main Results
SSEPs were recorded during baseline, during the first 4 hrs, and at 24, 48, and 72 hrs postasphyxia from animals subjected to asphyxia-induced cardiac arrest for 7 or 9 mins (n = 8/group). Functional evaluation was performed using the Neurologic Deficit Score (NDS). For quantitative analysis, the phase space representation of the SSEPs—a plot of the signal vs. its slope—was used to compute the phase space area bounded by the waveforms recorded after injury and recovery. Phase space areas during the first 85–190 mins postasphyxia were significantly different between rats with good (72 hr NDS ≥50) and poor (72 hr NDS <50) outcomes (p = .02). Phase space area not only had a high outcome prediction accuracy (80 –93%, p < .05) during 85–190 mins postasphyxia but also offered 78% sensitivity to good outcomes without compromising specificity (83–100%). A very early peak of SSEPs that precedes the primary somatosensory response was found to have a modest correlation with the 72 hr NDS subscores for thalamic and brainstem function (p = .066) and not with sensory-motor function (p = .30).
Conclusions
Phase space area, a quantitative measure of the entire SSEP morphology, was shown to robustly track neurologic recovery after cardiac arrest. SSEPs are among the most reliable predictors of poor outcome after cardiac arrest; however, phase space area values early after resuscitation can enhance the ability to prognosticate not only poor but also good long-term neurologic outcomes.
Keywords: cardiac arrest, prognosis, functional outcomes, somatosensory evoked potentials, eectrophysiology, quantitative neural monitoring
According to the Resuscitation Outcomes Consortium, 294,851 emergency medical services-treated cardiac arrests (CAs) occurred in the United States in 2008 (1). The overall survival after CA remains low, and only 3–7% of the survivors regain normal neurologic status (2). Among survivors, poor neurologic outcomes are the main causes for morbidity and mortality (3–5). In light of these facts, the American Heart Association has recently redefined traditional cardiopulmonary resuscitation as cardiopulmonary-cerebral resuscitation to emphasize the importance of neuroprotection after CA (6).
CA-induced hypoxic ischemic injury initiates a cascade of molecular events that lead to disruption of membrane potential, cellular depolarization, and swelling, ultimately causing secondary neuronal injury (7). Thalamocortical pathways are shown to be especially vulnerable to hypoxic-ischemic insults (8) with a higher degree of neuronal cell death and injury in regions of the cortex and thalamus observed both in animals (9–12) and in humans (13–16). A loss of scalp potentials in patients with thalamic lesions has also been reported (17). Injury to the cortical and subcortical structures has been suggested as the cause of comatose states after ischemia (18). Furthermore, deep-brain thalamic nuclei, such as the intralaminar (19) and central median (20) nuclei, have been found to play vital roles in arousal pathways. These findings, along with the knowledge of the thalamus as a sensory relay center, have led us to hypothesize that the thalamus may play a major role in recovery after CA-induced coma. The normal conduction of somatosensory evoked potentials (SSEPs) requires both an intact cortex and an intact thalamus and provides an avenue to assess the integrity of the somatosensory pathway, the restoration of normal thalamocortical coupling, and the onset of arousal.
While the prognostic value of SSEPs in comatose patients is well established, their clinical use is limited to studying the presence or absence of characteristic short and long latency peaks (N20 and N70 in humans). While the absence of cortical evoked potentials within 24 hrs after cardiopulmonary resuscitation has been shown to result in poor neurologic outcomes (13), the presence of these peaks does not necessarily correlate with better recovery. In addition to the lack of reliable quantitative indicators for SSEP monitoring, electrophysiological examination of patients is often done 8–24 hrs after CA. Since neural damage due to ischemia begins soon after CA, we hypothesize that studying neural activity as soon as possible after injury may be of value not only in prognostication but also in guiding the treatment regime by studying in real time the effect of therapeutic interventions.
SSEPs are only a few microvolts in amplitude and are often corrupted by biological (from nonspecific neural activity) and electrical noise. Thus, waveform averaging (typically 100 to 1000 sweeps in intraoperative monitoring) is required to enhance signal quality (21). SSEPs are traditionally evaluated using peak-to-peak amplitudes and their latencies relying on sophisticated peak detection techniques. Several time-frequency measures have been used to evaluate evoked responses including but not limited to peak power, peak frequency, and time-to-peak frequency (22, 23). An inherent drawback of all the above methods is that they ignore other signal characteristics, such as the rate of rise and fall, the width of the crests and troughs, and the overall morphology of the waveform, which might contain information about the functionality of the conduction pathway. Prior research suggests that the negative slope of the scalp evoked potential in humans is generated by thalamocortical radiation, whereas the subsequent positive wave reflects the cortex (24). Further, SSEPs after injury and during recovery are complex low-amplitude signals, often with indistinguishable peaks which motivated us to assess the shape of the evoked response.
We studied SSEPs using a dynamical systems perspective in the phase space. In order to obtain the phase space curve (PSC) for an SSEP waveform, we plotted its first time derivative against its magnitude. We propose to use the area bounded by the PSC, the phase space area (PSA), as a simple graphical and quantitative measure to characterize SSEPs and to assess the integrity of the somatosensory pathway after hypoxic-ischemic injury. Other than incorporating the entire SSEP waveform, this method does not rely on averaging hundreds of sweeps and bypasses peak detection. We evaluated the association of both the PSA and the amplitudes of the obligate potentials of the rat somatosensory response with neurologic outcomes. In addition, we used PSA to study the correlation between different parts of the SSEP with the thalamic, brainstem, and cortical function. Finally, we sought to identify the time frame most suited for electrophysiological examination after CA to optimize the use of PSA as a prognostic tool and as a quantitative aid in clinical decision-making.
MATERIALS AND METHODS
Sixteen adult male Wistar rats (361 ± 12 g; Charles River, Wilmington, MA) were randomly divided into two groups and subjected to a 7-min (n = 8) or a 9-min (n = 8) asphyxia. This experimental model of global cerebral ischemic injury after CA has been previously well established and validated (11, 25). The animal protocol used in this study was approved by the Institutional Animal Care and Use Committee at the Johns Hopkins University School of Medicine.
Electrode Implantation
One week before the experiments, the rats were implanted with five epidural screw electrodes (Plastics One, Roanoke, VA) to record bipolar SSEPs from the left and right cerebral hemispheres. Electrodes were placed over the somatosensory cortex near areas that topographically represent the fore- and hindlimbs. A ground electrode was placed over the parasagittal right frontal lobe. Care was taken to ensure that electrodes did not puncture the dura mater so as to not come into contact with the brain. The electrodes, lead wires, and the exposed portion of the skull were covered with dental cement. Finally, the skin was closed with sutures and the animal was allowed to recover for a week.
Experimental Procedures
On the day of the experiment, the rats were intubated and mechanically ventilated using a pressure-controlled ventilator. Ventilation was adjusted to maintain physiologic pH, pO2, and pCO2. The rats were anesthetized using 1.5% isoflurane in a 1:1 N2/O2 gas mixture. The femoral artery and vein distal to the inguinal ligament were cannulated in order to monitor blood pressure and arterial blood gas and to provide medications. Baseline SSEPs (15 mins) were recorded using the previously implanted electrodes, followed by a 5-min anesthesia washout. During the last 2 mins of anesthesia washout, 2 mg/kg of vecuronium, a muscle paralytic, was administered, and the gas mixture was switched to room air. At the end of washout, mechanical ventilation was stopped by clamping the endotracheal tube, and the time to CA was recorded as the time taken for the mean arterial pressure to fall below 10 mm Hg. The total duration of asphyxia for each rat was predetermined to be either 7 or 9 mins. Cardiopulmonary resuscitation was performed with external chest compressions and mechanical ventilation, along with the use of epinephrine. Sodium bicarbonate was used to counter acidosis. The time to return of spontaneous circulation (ROSC) was indicated by a rise in mean arterial pressure to a value greater than 60 mm Hg.
Isoflurane was restarted at 0.5% 45 mins post-ROSC to maintain animal comfort during SSEP recording. All animals were subjected to an identical and rigid protocol, and the isoflurane was maintained constant at 0.5%. If the rats showed any visible signs of discomfort, the anesthetic was turned up to 1 or 1.5% for short durations. Since all the signals analyzed in this study had similar levels of anesthetic in the baseline and recovery period, the effect of the anesthetic is the same across all animals.
SSEP Recording and Stimulation Protocol
SSEPs were recorded using the TDT System3 data acquisition system (Tucker-Davis Technologies, Alachua, FL). The median nerves were stimulated with subdermal needle electrodes placed in the distal forelimbs. The needle electrodes were connected to a stimulus generator and direct current stimulation was applied with 200-μsec-long 0.6-mA pulses. Instead of recording at 3 Hz, the typical stimulation frequency used in clinical studies, we selected a lower stimulus rate of 0.5 Hz to reduce animal discomfort, as minimal anesthetic was used during recovery. Further, since our experimental design required near-continuous stimulation for at least 4 hrs postresuscitation, there was no need for stimulation at higher frequencies, the purpose of which is to obtain a high number of sweeps to average over short bursts of time. The electrodes were connected to an amplifier and SSEP data were sampled at a frequency of 6.1 kHz using the TDT system. Baseline SSEPs were recorded for 15 mins followed by a 5-min anesthesia washout. Following CA, SSEPs were recorded continuously for 1 hr and then at 15-min intervals with a 15-min rest period in between each interval for the next 3 hrs. After the last 15-min interval, the rats were returned to their respective cages and allowed to recover naturally with access to food and water. Finally, SSEPs were recorded for 15 mins at 24, 48, and 72 hrs after injury.
Preprocessing of SSEP Waveforms
The forelimbs of the rats were stimulated in an alternating fashion at 0.5 Hz, and the SSEPs were recorded from the corresponding contralateral hemisphere. Sixty-sweep averages were mean corrected, and the short-latency response between 5 and 20 msec with reference to stimulation time was used for further processing. In order to test the efficacy of our metric and compare it with the amplitudes, an automated peak detection algorithm was used to find the local maxima and minima in user-defined windows of interest (8–12 msec and 13–20 msec for the negative and positive peaks, respectively). To measure peak-to-peak amplitudes, 180 sweeps were averaged in the middle of each of the experimental recovery durations at 15, 30, 45, 60, 90, 120, 150, 180, 210, and 240 mins postasphyxia. This required manual inspection in cases where one or more peaks were either not present or indistinguishable by the automated peak detection algorithm.
Neurologic Assessment
Neurologic function in rats was evaluated by an independent trained observer using Neurologic Deficit Score (NDS) at 24, 48, and 72 hrs postresuscitation (26, 27). This scale has been developed and previously validated by our group and seeks to assess arousal, brainstem function, motor and sensory inputs, behavior, and seizures (11, 27); it was adapted from several human and animal scales (28–31). The score ranges from 0 to 80, where 80 is the best possible outcome and 0 the worst (details described in the results). The 72-hr NDS score was the primary outcome measure of the experiment.
Quantitative SSEP Assessment: The PSA
The phase space is the set of all possible states of a system with each state occupying a distinct point. In order to extract morphologic information from the SSEP, we plot the slope against its magnitude. We call this transformation the PSC. Whereas the signal itself dictates the shape of the PSC, the area bounded by the curve is indicative of the power (energy) of the signal. In order to quantify the transformed waveform, we compute the area enclosed by the curve, termed the PSA, using numerical integration by first fitting a convex hull to it using the Quickhull algorithm described by Barber et al (32). Analogous to the manner in which a stretched rubber band takes the shape of the object it is placed around, the convex hull, a simple closed polygon chain, captures the extent of a nonempty set of points in a planar sense.
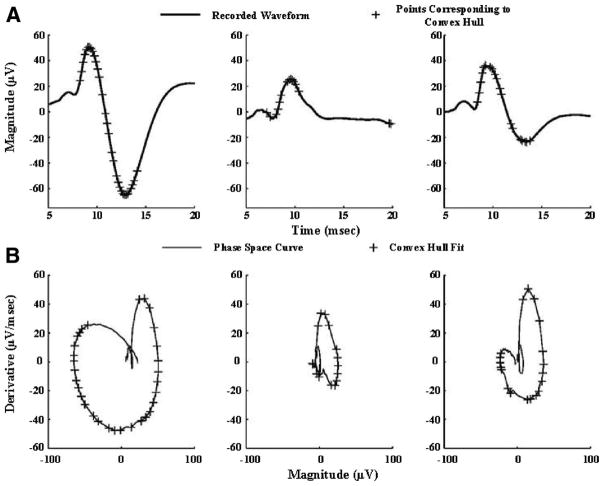
The PSCs of three different SSEPs and their corresponding PSAs are shown in Figure 1. The PSC stretches further out with larger amplitudes and sharper transitions (steep slopes). The points selected by the convex hull algorithm in order to capture the extent of the curve include the salient signal peaks and points that capture the slopes of transition. The other features of the signal, such as the smaller peaks, are within the PSC. Upon comparing the normalized peak-to-peak amplitudes with the PSA, it was seen that the PSA was able to better discriminate different morphologies. Whereas the ratio of the N10-P15 peak-to-peak amplitudes (N10-P15 pk-pk) of the SSEPs shown is 3.6:1:1.96 (normalized to the second waveform), the ratio of the PSAs is 6.25:1:2.7 (Fig. 1). This indicates that PSA is more sensitive to differences in SSEP shapes as it incorporates instantaneous slope information while indirectly incorporating information about peak amplitudes and interpeak latencies. A steeper high-amplitude evoked potential encloses a greater area in the phase space domain than does a low-amplitude slowly rising/falling response.
Figure 1.
Median nerve evoked short-latency somatosensory evoked potentials (SSEPs) and their representation in the phase space. A, Three SSEPs with varying morphology in terms of peak amplitudes, slopes, and interpeak intervals. The primary short-latency responses in rats are characterized by a negative peak around 10 msec (N10) and a positive peak around 15 msec (P15). B, Phase space curves (PSCs) corresponding to the SSEPs in Panel A. The points selected to numerically compute the area enclosed by the PSCs are indicated on both the PSCs and SSEPs with a “+.” It should be noted that the second SSEP is an example of an atypical waveform recorded during recovery from injury, where the peak at 15 msec is absent, necessitating manual inspection to approximate peak-to-peak amplitude.
Experimental Stages and PSA Calculation
The experimental monitoring for the evaluation of SSEPs was divided into three stages: immediate injury (0–60 mins), early recovery (60–240 mins), and late recovery (day 1–3), with all time points referenced to the onset of asphyxia. Each stage was further subdivided into smaller time intervals to study the temporal evolution of the SSEPs. Successive 60-sweep averages (2-min averages) were calculated, and the PSA was obtained for each averaged waveform. PSA values were normalized with respect to the baseline PSA for each rat.
Statistical Methods
Statistical analyses were performed using MedCalc for Windows, version 11.2.1.0 (Med-Calc Software, Mariakerke, Belgium). Parametric group values (arterial blood gas results, times to CA and ROSC) were reported as mean ± SD. Univariate analysis was performed for parametric data with the use of Student’s t test for continuous variables (two-tailed under the assumption of unequal variances). Nonparametric variables (NDS) were reported as median (25th–75th percentile). The nonparametric Mann-Whitney test was used to test if the NDS in one group was statistically greater than the other by replacing actual data points with a set of ordered ranks.
The Kolmogorov-Smirnov test was used to establish normality of the quantitative SSEP marker (PSA values) and the peak-to-peak amplitudes at each experimental phase. Repeated-measures analysis of variance was used to compare the PSA values between the functional groups across time. Further, to compare the overall trend of neurologic recovery between the two groups, the PSA data were fitted with a linear mixed (random) effects model and the slopes of recovery between the two groups were compared. Spearman’s rank-order correlation coefficient was used to study correlation between the PSA and nonparametric NDS components.
Sensitivity and specificity of the proposed quantitative marker and those of the amplitudes were assessed using receiver operating characteristic curves. Optimal thresholds for minimizing false-positive and false-negative results were determined, and the prognostic accuracy was quantified using the area under the receiver operating characteristic curve. p values less than .05 were considered statistically significant.
RESULTS
Animal Survival and Functional Outcomes
The rats were a posteriori grouped based on neurologic outcomes recorded at 72 hrs after CA. We prespecified the NDS cutoff into two functional outcome groups: G1, NDS 72 hr <50; and G2, NDS 72 hr ≥50. Rats with NDS <50 were immobile, had sluggish responses, and reacted minimally to environmental stimuli. Rats with NDS ≥50 generally retained some mobility and reacted briskly to stimuli. Table 1 shows the NDS breakdown of a few animals with different functional outcomes. The NDS scores for the groups at 24, 48, and 72 hrs postrecovery are shown in Figure 2A. As can be seen from the Kaplan-Meier curves in Figure 2B, 67% of the rats in G1 did not survive 72 hrs postasphyxia. Since SSEPs were recorded in an alternating fashion from both hemispheres, two bipolar SSEPs were analyzed for each rat, and the average PSA of the two signals at every time was used for the analysis. Data from one rat that did not have characteristic baseline SSEP waveforms, possibly due to loosened electrode connections, was excluded from the shape analysis.
Table 1.
Representative breakdown of Neurologic Deficit Score of three rats with poor, borderline, and good outcomes, respectively
| Brainstem Function (19) | Arousal (21) | Sensory Assessment (6) | Motor Assessment (6) | Motor Behavior (6) | Behavior (12) | Seizures (10) | |
|---|---|---|---|---|---|---|---|
| NDS: 30 | 11 | 9 | 0 | 0 | 0 | 0 | 10 |
| NDS: 49 | 16 | 12 | 2 | 2 | 1 | 3 | 10 |
| NDS: 72 | 19 | 18 | 6 | 6 | 4 | 9 | 10 |
NDS, Neurologic Deficit Score. The NDS ranges from 0 to 80 and the highest score possible under each assessment category is indicated in the brackets.
Figure 2.
Behavioral assessment of the outcome groups at 24, 48, and 72 hrs postasphyxia using the Neurologic Deficit Score (NDS) is summarized using a box-whisker plot in A, and the respective survival functions are shown in B. The box represents the interquartile range (25th–75th percentile), and the line inside represents the median of the NDS. Minimum and maximum values are represented by lines extending out from the boxes. A significant difference was noted between the NDS values of G1 and G2 at all three time points of evaluation (p = .045, p = .002, p = .001, respectively). G2 (n = 9) had a 100% survival rate compared to G1 (n = 6), which showed a high mortality rate, with only 33% of the rats alive at 72 hrs. The median survival for G2 was 3 days, and that for G1 was 2 days. The survival curves for the two outcome groups were significantly different from each other (p = .0046, log-rank test).
Animal Characteristics and Arterial Blood Gas Monitoring
In order to ensure that the longer duration of CA in half the cohort did not skew those rats to worse neurologic recovery, we compared the time to CA, time to ROSC, and arterial blood gas data between the two groups (Table 2) and found no significant differences in values during baseline and after CA.
Table 2.
Animal characteristics and arterial blood gas data (mean ± SD) before and after the cardiac arrest experiment among the two outcome groups
| NDS 72 <50 (n = 6) | NDS 72 ≥50 (n = 9) | p Value | |
|---|---|---|---|
| Weight (g) | 360 ± 12 | 361 ± 13.4 | .84 |
| Time to CA (sec) | 181 ± 36 | 184 ± 34 | .90 |
| Time to ROSC (sec) | 30 ± 0 | 29 ± 4 | .36 |
| Arterial Blood Gas Data | |||
| Baseline | |||
| pH | 7.47 ± 0.04 | 7.43 ± 0.02 | .09 |
| pCO2 (mm Hg) | 36 ± 7 | 42 ± 7 | .09 |
| pO2 (mm Hg) | 185 ± 51 | 195 ± 42 | .71 |
| HCO3 (mmol/L) | 26 ± 4 | 28 ± 4 | .27 |
| O2 Sat (%) | 100 ± 0 | 100 ± 0 | 1 |
| Post-CA | |||
| pH | 7.42 ± 0.05 | 7.40 ± 0.08 | .62 |
| pCO2 (mm Hg) | 42 ± 5 | 44 ± 11 | .65 |
| pO2 (mm Hg) | 347 ± 104 | 278 ± 165 | .42 |
| HCO3 (mmol/L) | 27 ± 5 | 27 ± 4 | .87 |
| O2 Sat (%) | 100 ± 0 | 100 ± 0 | 1 |
CA, Cardiac arrest; ROSC, time to return of spontaneous circulation; Post-CA, timeframe within 1 hour after return of spontaneous circulation; Sat, saturation. All measurements were taken at 37°C.
Temporal Evolution of SSEPs and PSCs After Hypoxic-Ischemic Injury
Representative SSEP waveforms at different experimental stages from a rat in each group are depicted in Figure 3. The onset of ischemia is accompanied by the sudden disappearance of evoked activity. During the first 60 mins, the SSEPs in all rats reappear slowly with the negative peak at around 10 msec (N10) appearing before the recovery of the positive peak at 15 msec (P15). Interestingly, a small negative peak at 7 msec (N7) was also observed in every rat. This peak gets submerged as the N10-P15 peaks recover. It can also be seen that the reappearance of SSEPs follows a pattern with sequential return of peaks: N7, N10, and P15 in that order, giving rise to signals of varying morphologies.
Figure 3.
Evolution of somatosensory evoked potentials (SSEPs) and the corresponding phase space curves during the course of the cardiac arrest experiment in rats with Neurologic Deficit Score (NDS) >50 (A, C) and NDS <50 (B, D), representative of the two outcome groups in the study. The signals are shown in baseline and then at 5, 15, 30, 45, 60, 90, 120, 150, 180, 210, and 240 mins postasphyxia. It is worthy to observe that the signals recover with atypical morphologies for as long as 120 mins into recovery, due to which amplitude latency determination would require trained inspection. The phase space transformation robustly tracked the recovery of the SSEPs in an unsupervised manner and showed a marked difference during the earlier stages of recovery. The area enclosed by the phase space curves was able to better characterize SSEPs and showed a better gradation of recovery based on functional outcomes.
The PSCs corresponding to the SSEPs in Figure 3, A and B, are shown in Figure 3, C and D, respectively. The typical PSC of a normal SSEP is a curve that forms a closed loop that incorporates not only the peak amplitudes but also the slopes, the interpeak latency, and other features of the response. The PSC diminishes in size at the onset of asphyxia in both groups but recovers faster and to a greater extent in G2 (Fig. 3A, C), most prominently during the early recovery phase (85–240 mins).
Recovery of PSAs in the Different Outcome Groups
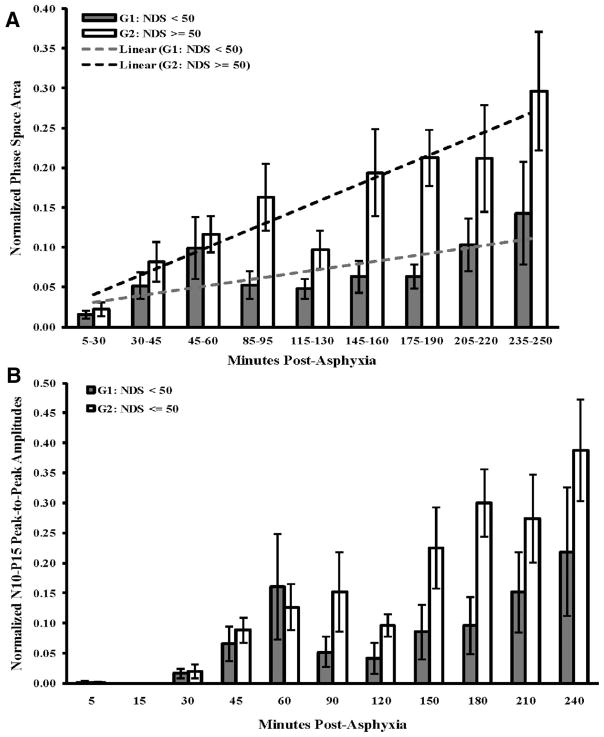
In all rats combined, the PSA increased significantly as time progressed (p = .003) highlighting the dynamic nature of SSEP recovery during the first 4 hrs postasphyxia. During the first 60 mins, the PSA was on average higher in G2 than G1, but no statistical difference was observed (Fig. 4A). The SSEPs in both groups reappeared around the same time. Analysis during the next 3 hrs of recovery—the early recovery duration—demonstrated a significant separation of PSAs between two groups, with G2 showing a greater recovery than G1 (85–190 min, p = .02; 85–250 min; p = .04) (Fig. 4A). G2 also had a significantly greater overall trend of recovery over time compared to G1, as estimated by fitting a linear mixed effects model to the data (slopes of G2 and G1 are 0.028 and 0.010, respectively, p = .05).
Figure 4.
Evolution of somatosensory evoked potentials (SSEPs) as assessed by the phase space area (PSA) and N10-P15 amplitudes for the two outcome groups during the course of the CA experiment. A, PSA recovery in both outcome groups follows an almost linear trend (R2 = 0.87 and 0.53 for G2 and G1, respectively), with G2 having a greater rate of recovery than G1, as estimated by the slope of the linear fit (slopes of G2 and G1 are 0.028 and 0.010, respectively, p = .05). While there is no statistical difference between the PSAs of SSEPs during the immediate injury duration (0–60 min postasphyxia), there is a greater recovery of the good outcome group during the early recovery stages (85–190 min, p = .02; 85–250 min, p = .04). B, Progression of N10-P15 peak-to-peak amplitudes among the two groups at distinct time points. PSA offered a better separation between the functional groups with lower standard errors. Further, the amplitude trends were found to have a lower statistical difference (90–180 min, p = .07; 90–240 min, p = .10). No statistical differences were found between the SSEPs recorded at 24, 48, and 72 hrs after cardiac arrest using both PSA and amplitudes. NDS, Neurologic Deficit Score.
For the sake of completeness and comparison, peaks were manually inspected, and the N10-P15 pk-pk results for both of the outcome groups were compared with each other. The N10-P15 pk-pk results are in close agreement with the results of PSA, but they offer lesser separation and a have a higher degree of variability. Further, the N10-P15 pk-pk trends across time are less statistically significant than PSA (Fig. 4B).
Comparison of the Predictive Abilities of PSA and N10-P15 pk-pk
The receiver operating characteristic curves were plotted for the early recovery stages to study the costs versus benefits of using PSA as a tool for diagnostic decision making. The binary neurologic outcome classification on the basis of PSAs was found to be 80–93% accurate during the period 85–190 mins after asphyxia. The sensitivity, specificity, accuracy, and corresponding optimal threshold (ThOptimal) for the normalized PSAs are summarized in Table 3.
Table 3.
Summary of the receiver operating characteristic analysis during the early recovery duration
| 85–90 mins | 115–130 mins | 145–160 mins | 175–190 mins | 205–220 mins | 235–250 mins | |
|---|---|---|---|---|---|---|
| AUC | ||||||
| PSA | 0.85 | 0.82 | 0.80 | 0.93 | 0.70 | 0.70 |
| N10-P15 | 0.67 | 0.73 | 0.75 | 0.85 | 0.68 | 0.72 |
| p Value | ||||||
| PSA | .0006 | .019 | .023 | .0001 | .17 | .16 |
| N10-P15 | .26 | .13 | .06 | .001 | .22 | .16 |
| Sensitivity | ||||||
| PSA | 0.78 | 0.78 | 0.78 | 0.78 | 0.78 | 0.67 |
| N10-P15 | 0.44 | 0.89 | 0.67 | 0.89 | 0.56 | 1.00 |
| Specificity | ||||||
| PSA | 0.83 | 0.83 | 0.83 | 1.00 | 0.67 | 0.83 |
| N10-P15 | 1.00 | 0.67 | 0.83 | 0.83 | 0.83 | 0.50 |
| ThOptimal (%) | ||||||
| PSA | 8.06 | 5.86 | 8.51 | 11.29 | 9.3 | 13.99 |
| N10-P15 | 13.3 | 0.0 | 10.4 | 11.9 | 21.0 | 0.0 |
AUC, Area under the receiver operating characteristic curve; PSA, phase space area. The AUC was used to evaluate the overall accuracy of the test. A p value of <.05 (italicized) indicates that the AUC is significantly different from 0.5, indicating that the quantitative marker has the ability to distinguish between the two outcome groups. Sensitivity was defined as the proportion of rats with good functional outcome classified correctly and specificity was defined as the proportion of rats with poor functional outcomes classified correctly using PSA as the classification criterion. ThOptimal gives the value of the marker (as a percentage of baseline) for best separation between the groups, i.e., lowest false-positive and false-negative rates. Receiver operating characteristic parameters derived using N10-P15 peak-to-peak amplitudes are indicated below the values derived using the PSA marker.
The absence of N20 predicts poor outcome in humans with excellent specificity. However, in our study, N10, the rat correlate of the human N20, was present in all rats at 24 hrs except for one that did not survive till then. Thus, our objective was to gauge eventual outcomes in subjects whose outcomes are uncertain, i.e., in which N10 was present. Since a direct comparison of our marker to the presence/absence of N10 could not be evaluated, we compared the prognostic abilities of normalized N10-P15 pk-pk at different time points (Table 3). Amplitudes were found to have a lower accuracy in classifying rats based on 72-hr functional outcomes than PSA. The sensitivity-specificity tradeoff using PSA was found to be superior. Interestingly, the PSA provided us with consistent thresholds for outcome classification across time (greater than 6–9% recovery with respect to baseline during 85–160 mins and >9–14% recovery during 175–250 mins), whereas the criteria provided by N10-P15 pk-pk fluctuated randomly between 0% and 20% of recovery during 1–4 hrs after CA.
The N7-Phase Space Subarea: Correlation With NDS Components at 72 hrs
The N7 peak appeared before the main N10-P15 signal during early stages of recovery (Fig. 5). In order to study whether this early response associates with the underlying functioning of deep-brain regions or the cortex, we assessed the correlation between PSA of the early (5–9-msec) portion, the phase space subarea, of the SSEP and NDS “subscores” of interest. The N7 signal at the end of 60 mins has a fair but not significant correlation with the functional subscores corresponding to arousal and brainstem function (Spearman’s ρ = 0.49, p = .066), whereas the sensory-motor subscore showed no significant correlation (Spearman’s ρ = 0.49, p = .30). Groupwise analysis showed that in rats with good outcomes this early signal was more prominent during 25–60 mins postasphyxia (Fig. 5).
Figure 5.
The N7 potential and its recovery. The inset at the top left of the figure shows a typical baseline somatosensory evoked potential (SSEP) waveform showing the characteristic peaks. A very early peak appears about 7 msec poststimulus followed by a biphasic signal characterized by the N10 and P15 potentials. The N7 is the first to recover after ischemic injury and eventually gets submerged as the N10-P15 signal recovers after cardiac arrest. The graph summarizes the evolution of the SSEP N7 phase space subarea during the first hour from the onset of asphyxia. The first 15 min postasphyxia are characterized by suppressed SSEPs, after which the N7 signal begins to recover. As can be seen from the plot, the recovery of N7 stabilizes in all rats by the 60-min mark. Phase space analysis of the N7 peak shows that the recovery of N7 is different between the two outcomes during 35–60 mins postasphyxia (35–60 min, p = .037, one-tailed t test under the assumption of unequal variances under the hypothesis that G2 shows greater recovery than G1). NDS, Neurologic Deficit Score.
DISCUSSION
Bedside neurophysiological monitoring using SSEPs is not used extensively in intensive care units (ICUs) for post-CA evaluation except for prognostication of negative outcomes and withdrawal of life support from comatose survivors. In a 407-patient study conducted in 2006 across 32 ICUs, it was observed that SSEP monitoring is superior to clinical tests in predicting neurologic outcomes, but this evaluation was conducted only at 24, 48, and 72 hrs after injury (33). Furthermore, the monitoring relied only on studying the characteristic N20 peak in this study, where 87% of the patients had a poor neurologic outcome, defined as death or persistent unconsciousness 1 month after CA (33). Our study in the rodent model is the first of its kind to evaluate neural injury and recovery using SSEPs during the early stages (<4 hrs) after CA using a novel quantitative tool. Early evaluation is critical for titration of treatments because excitotoxic-ischemic cascades begin immediately after injury, and it is during this time that interventions like hypothermia and drugs might have the most benefit.
We have shown that early SSEP assessments using an objective measure of signal morphology, PSA, does not require sophisticated peak detection and can track brain injury and recovery in addition to differentiating between good and poor neurologic outcomes. There was no discernable difference in PSAs of the two outcome groups during the first 60 mins postasphyxia as signals begin to reappear in a similar fashion after ROSC, but we observed significantly higher PSAs in the good outcome group during the next 3 hrs. We also noted that the early assessment of SSEPs using PSA was able to predict neurologic outcomes at 72 hrs postasphyxia. This is an important result that highlights the need for SSEP monitoring during early stages of recovery after CA. The most common argument against early monitoring is due to the fact that impaired cerebral perfusion and reperfusion damage might affect SSEP recordings. However, we believe that monitoring early electrical changes that reflect underlying metabolic states may allow for better prognostication and may be useful in subsequent titration of treatment protocol to maximize the chances of neurologic recovery. It should be noted that SSEPs on days 1–3 in our study offered lower prognostic value for differentiating outcomes.
Another study on the same set of 407 patients mentioned earlier (33) used the presence or absence of short (N20)- and long (N70)-latency peaks to show that, while these peaks give information about the likelihood of poor outcomes, they are not precise enough to base treatment decisions solely on their absence and cannot predict good outcomes. Thus, while traditional SSEP monitoring is shown to reliably prognosticate poor outcomes, our quantitative SSEP assessment using PSA shows 78% sensitivity in identifying good outcomes between 85–220 mins with 83% specificity during 85–160 mins and 100% specificity during 175–190 mins postasphyxia. These findings indicate that a holistic approach to studying SSEPs, taking into account the entire morphology of the waveform, in contrast with the binary assessment based on the presence/absence of characteristic peaks, can provide information about good outcomes with high sensitivity without compromising specificity.
An earlier study evaluated SSEPs for reliable outcome prediction as early as 4 hrs after CA, with subsequent recordings at 12, 24, and 48 hrs after cardiopulmonary resuscitation, and recommended waiting for at least 24 hrs to make a reliable prognosis based on SSEP (34). However, a recent study in dogs revealed that there is a critical window of time immediately after CA during which hypothermia has maximum therapeutic potential (35). The sudden disappearance of the evoked response in all rats was analogous to the isoelectric phase observed in the electroencephalograph immediately after an ischemic insult (25). This can be attributed to the depletion of the energy stores of the brain tissue due to lack of oxygen, which leads to an immediate cessation of electrical activity. It is within this window of time that secondary neuronal injury results from the damaging molecular cascades that are initiated by the lack of oxygen. Therapeutic interventions seek to alter the pathophysiological sequence of events to reduce neuronal injury. Therefore, it is essential to evaluate neural activity as soon as possible after injury and to continue monitoring it during and after the application of hypothermia or other pharmacologic treatments.
Reported mechanistic explanations of coma suggest the involvement of structures like the cortex and the thalamus (18). Injury to the thalamocortical pathway and loss of normal rhythms are speculated to result in coma and the characteristic electroencephalographic bursting patterns through the disinhibition of intrinsic “pacemaker” rhythms (36, 37). Under anesthesia-induced burst suppression, spontaneous cortical activity is absent during periods of electrical silence, whereas thalamic potentials persist (38). During bursts, coherent potentials are observed in the thalamus and cortex with the thalamus driving the cortex. These observations indicate that burst suppression coma after CA involves abnormal coupling of the thalamic and cortical potentials and that arousal is dependent upon the restoration of the bidirectional thalamocortical coupling. A possible thalamic component of the auditory evoked potential that preceded the cortical response was reported in rats (17). A negative far-field potential that begins around 16 msec preceding N20 in humans is known to be preserved after thalamic lesions and is suggested to originate in the brainstem. The roles of the thalamus and brainstem in arousal and consciousness make them interesting targets to study in order to gain insight into the mechanism of recovery from ischemic coma. We have shown that there is an association of the very early peak (N7) with the brainstem and arousal components of the NDS in contrast to sensory motor assessment. This finding hints at a noninvasive means to evaluate deep-brain regions critical to arousal and consciousness from comatose states with robust implications for clinical translation. Further, these observations set the stage for future studies on the neuroelectrophysiology of the brainstem, thalamus, and cortex using microelectrode arrays in order to ascertain the origins of the SSEP peaks using spike signals.
The absence of histopathological data is a potential limitation of this study. Whereas we have previously demonstrated that histopathological markers for ischemic cell death correlate with NDS (26, 28, 39, 40), we also acknowledge that postmortem histologic markers in rats have been a poor indicator of clinical significance in human trials and have been less predictive than early behavioral assessments (41–43). As such, we put more emphasis on functional outcome and real-time electrophysiologic measures of recovery (PSA) as a means to clinical translation.
The main barrier to clinical translation of these findings is that, in clinical practice, electrophysiological testing is generally delayed for hours to days after resuscitation. As a basic question, the early evolution of SSEP after CA and its prognostic utility remain largely unknown. Our study assesses the early recovery of SSEP recovery using a novel marker that can be automated to provide a real-time monitor that does not require expert interpretation. This study lays the foundation for a more involved study design that may include therapeutic strategies like hypothermia.
A challenge to the implementation of quantitative SSEP monitoring in the ICU is the lack of a baseline signal in CA patients. A possible solution to this problem is to create standardized cortical SSEP waveforms with typical morphologies that can be used for comparison. Another major challenge is the lack of expertise in interpreting SSEPs in the ICUs. Advanced neural monitoring systems with novel quantitative tools such as PSA can offer health personnel the option to select windows of interest to study different components of SSEPs, simultaneously providing a better means to track injury, recovery, and the effect of neuroprotective interventions in real time and a clinically translational means of evaluating the integrity of deep-brain regions.
CONCLUSION
The area enclosed by the SSEP in the phase space—a space of all possible configurations of magnitudes and slopes in a signal—was introduced as a quantitative descriptor for SSEPs. We demonstrated that this measure was able to objectively track recovery of evoked responses and differentiate between good and poor neurologic outcomes with a good predictive ability. Furthermore, we identified a critical window of interest (1–4 hrs postasphyxia) during which the recovery of SSEPs correlates with neurologic outcome, emphasizing the potential benefits of SSEP monitoring in the ICU soon after CA. Finally, we identified a subcomponent of SSEP that many be associated with deep-brain structures.
Acknowledgments
The authors would like to thank Young-Seok Choi, Dan Wu, Abhishek Rege and Mohsen Mollazadeh of the Johns Hopkins University School of Medicine for discussions and reading of the manuscript.
This work was supported, in part, by grants RO1 HL071568 from the National Institutes of Health (JM, RGG, XJ, NVT) and 09SDG1110140 from the American Heart Association (XJ). The remaining authors have not disclosed any potential conflicts of interest.
Footnotes
Presented, in part, at the Society of Critical Care Medicine’s 39th Critical Care Congress, Orlando, FL, January 9 –13, 2010.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics–2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Edgren E, Kelsey S, Sutton K, et al. The presenting ECG pattern in survivors of cardiac arrest and its relation to the subsequent long-term survival. Brain Resuscitation Clinical Trial I Study Group. Acta Anaesthesiol Scand. 1989;33:265–271. doi: 10.1111/j.1399-6576.1989.tb02905.x. [DOI] [PubMed] [Google Scholar]
- 3.Bedell SE, Delbanco TL, Cook EF, et al. Survival after cardiopulmonary resuscitation in the hospital. N Engl J Med. 1983;309:569–576. doi: 10.1056/NEJM198309083091001. [DOI] [PubMed] [Google Scholar]
- 4.Levy DE, Caronna JJ, Singer BH, et al. Predicting outcome from hypoxic-ischemic coma. JAMA. 1985;253:1420–1426. [PubMed] [Google Scholar]
- 5.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 6.Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 8: Advanced challenges in resuscitation: Section 1: Life-threatening electrolyte abnormalities. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation. 2000;102(Suppl 8):I217–I222. [PubMed] [Google Scholar]
- 7.Taoufik E, Probert L. Ischemic neuronal damage. Curr Pharm Des. 2008;14:3565–3573. doi: 10.2174/138161208786848748. [DOI] [PubMed] [Google Scholar]
- 8.Muthuswamy J, Kimura T, Ding MC, et al. Vulnerability of the thalamic somatosensory pathway after prolonged global hypoxic-ischemic injury. Neuroscience. 2002;115:917–929. doi: 10.1016/s0306-4522(02)00369-x. [DOI] [PubMed] [Google Scholar]
- 9.Dihne M, Grommes C, Lutzenburg M, et al. Different mechanisms of secondary neuronal damage in thalamic nuclei after focal cerebral ischemia in rats. Stroke. 2002;33:3006–3011. doi: 10.1161/01.str.0000039406.64644.cb. [DOI] [PubMed] [Google Scholar]
- 10.Wei L, Ying DJ, Cui L, et al. Necrosis, apoptosis and hybrid death in the cortex and thalamus after barrel cortex ischemia in rats. Brain Res. 2004;1022(1–2):54–61. doi: 10.1016/j.brainres.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 11.Geocadin RG, Muthuswamy J, Sherman DL, et al. Early electrophysiological and histologic changes after global cerebral ischemia in rats. Mov Disord. 2000;15(Suppl 1):14–21. doi: 10.1002/mds.870150704. [DOI] [PubMed] [Google Scholar]
- 12.Smith ML, Auer RN, Siesjo BK. The density and distribution of ischemic brain injury in the rat following 2–10 min of forebrain ischemia. Acta Neuropathol. 1984;64:319–332. doi: 10.1007/BF00690397. [DOI] [PubMed] [Google Scholar]
- 13.Brunko E, Zegers de Beyl D. Prognostic value of early cortical somatosensory evoked potentials after resuscitation from cardiac arrest. Electroencephalogr Clin Neurophysiol. 1987;66:15–24. doi: 10.1016/0013-4694(87)90133-7. [DOI] [PubMed] [Google Scholar]
- 14.Fujioka M, Okuchi K, Sakaki T, et al. Specific changes in human brain following reperfusion after cardiac arrest. Stroke. 1994;25:2091–2095. doi: 10.1161/01.str.25.10.2091. [DOI] [PubMed] [Google Scholar]
- 15.Kinney HC, Korein J, Panigrahy A, et al. Neuropathological findings in the brain of Karen Ann Quinlan. The role of the thalamus in the persistent vegetative state. N Engl J Med. 1994;330:1469–1475. doi: 10.1056/NEJM199405263302101. [DOI] [PubMed] [Google Scholar]
- 16.Ross DT, Graham DI. Selective loss and selective sparing of neurons in the thalamic reticular nucleus following human cardiac arrest. J Cereb Blood Flow Metab. 1993;13:558–567. doi: 10.1038/jcbfm.1993.73. [DOI] [PubMed] [Google Scholar]
- 17.Fisher MA, Perlik SJ. N20 and P40 somatosensory evoked potentials: thalamic lesions and subcortical origin. Acta Neurol Scand. 1985;71:25–30. doi: 10.1111/j.1600-0404.1985.tb03162.x. [DOI] [PubMed] [Google Scholar]
- 18.Young GB. Coma. Ann N Y Acad Sci. 2009;1157:32–47. doi: 10.1111/j.1749-6632.2009.04471.x. [DOI] [PubMed] [Google Scholar]
- 19.Steriade M, Glenn LL. Neocortical and caudate projections of intralaminar thalamic neurons and their synaptic excitation from midbrain reticular core. J Neurophysiol. 1982;48:352–371. doi: 10.1152/jn.1982.48.2.352. [DOI] [PubMed] [Google Scholar]
- 20.Schiff ND, Plum F. The role of arousal and “gating” systems in the neurology of impaired consciousness. J Clin Neurophysiol. 2000;17:438–452. doi: 10.1097/00004691-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Misulis KE, Fakhoury T. Spehlmann’s Evoked Potential Primer. Woburn, MA: Butterworth-Heinemann; 2001. [Google Scholar]
- 22.Hu Y, Luk KD, Lu WW, et al. Comparison of time-frequency analysis techniques in intraoperative somatosensory evoked potential (SEP) monitoring. Comput Biol Med. 2002;32:13–23. doi: 10.1016/s0010-4825(01)00026-9. [DOI] [PubMed] [Google Scholar]
- 23.Hu Y, Luk KD, Lu WW, et al. Application of time-frequency analysis to somatosensory evoked potential for intraoperative spinal cord monitoring. J Neurol Neurosurg Psychiatry. 2003;74:82–87. doi: 10.1136/jnnp.74.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed I. Use of somatosensory evoked responses in the prediction of outcome from coma. Clin Electroencephalogr. 1988;19:78–86. doi: 10.1177/155005948801900209. [DOI] [PubMed] [Google Scholar]
- 25.Geocadin RG, Ghodadra R, Kimura T, et al. A novel quantitative EEG injury measure of global cerebral ischemia. Clin Neurophysiol. 2000;111:1779–1787. doi: 10.1016/s1388-2457(00)00379-5. [DOI] [PubMed] [Google Scholar]
- 26.Jia X, Koenig MA, Shin HC, et al. Improving neurological outcomes post-cardiac arrest in a rat model: Immediate hypothermia and quantitative EEG monitoring. Resuscitation. 2008;76:431–442. doi: 10.1016/j.resuscitation.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia X, Koenig MA, Shin HC, et al. Quantitative EEG and neurological recovery with therapeutic hypothermia after asphyxial cardiac arrest in rats. Brain Res. 2006;1111:166–175. doi: 10.1016/j.brainres.2006.04.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz L, Ebmeyer U, Safar P, et al. Outcome model of asphyxial cardiac arrest in rats. J Cereb Blood Flow Metab. 1995;15:1032–1039. doi: 10.1038/jcbfm.1995.129. [DOI] [PubMed] [Google Scholar]
- 29.Sherman DL, Brambrink AM, Ichord RN, et al. Quantitative EEG during early recovery from hypoxic-ischemic injury in immature piglets: burst occurrence and duration. Clin Electroencephalogr. 1999;30:175–183. doi: 10.1177/155005949903000410. [DOI] [PubMed] [Google Scholar]
- 30.Xiao F, Safar P, Radovsky A. Mild protective and resuscitative hypothermia for asphyxial cardiac arrest in rats. Am J Emerg Med. 1998;16:17–25. doi: 10.1016/s0735-6757(98)90059-6. [DOI] [PubMed] [Google Scholar]
- 31.Zeiner A, Holzer M, Sterz F, et al. Mild resuscitative hypothermia to improve neurological outcome after cardiac arrest. A clinical feasibility trial. Hypothermia After Cardiac Arrest (HACA) Study Group. Stroke. 2000;31:86–94. doi: 10.1161/01.str.31.1.86. [DOI] [PubMed] [Google Scholar]
- 32.Barber CB, Dobkiny DP, Huhdanpaa H. The Quickhull algorithm for convex hulls. ACM Trans Math Softw. 1995;22:469–483. [Google Scholar]
- 33.Zandbergen EG, Hijdra A, Koelman JH, et al. Prediction of poor outcome within the first 3 days of postanoxic coma. Neurology. 2006;66:62–68. doi: 10.1212/01.wnl.0000191308.22233.88. [DOI] [PubMed] [Google Scholar]
- 34.Gendo A, Kramer L, Hafner M, et al. Time-dependency of sensory evoked potentials in comatose cardiac arrest survivors. Intensive Care Med. 2001;27:1305–1311. doi: 10.1007/s001340101008. [DOI] [PubMed] [Google Scholar]
- 35.Nozari A, Safar P, Stezoski SW, et al. Critical time window for intra-arrest cooling with cold saline flush in a dog model of cardiopulmonary resuscitation. Circulation. 2006;113:2690–2696. doi: 10.1161/CIRCULATIONAHA.106.613349. [DOI] [PubMed] [Google Scholar]
- 36.Niedermeyer E, Sherman DL, Geocadin RJ, et al. The burst-suppression electroencephalogram. Clin Electroencephalogr. 1999;30:99–105. doi: 10.1177/155005949903000305. [DOI] [PubMed] [Google Scholar]
- 37.Steriade M, Amzica F, Contreras D. Cortical and thalamic cellular correlates of electroencephalographic burst-suppression. Electroencephalogr Clin Neurophysiol. 1994;90:1–16. doi: 10.1016/0013-4694(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 38.White NS, Alkire MT. Impaired thalamocortical connectivity in humans during general-anesthetic-induced unconsciousness. Neuro-Image. 2003;19:402–411. doi: 10.1016/s1053-8119(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 39.Luft AR, Buitrago MM, Paul JS, et al. Early restitution of electrocorticogram predicts subsequent behavioral recovery from cardiac arrest. J Clin Neurophysiol. 2002;19:540–546. doi: 10.1097/00004691-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Geocadin RG, Malhotra AD, Tong S, et al. Effect of acute hypoxic preconditioning on qEEG and functional recovery after cardiac arrest in rats. Brain Res. 2005;1064:146–154. doi: 10.1016/j.brainres.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 41.Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: Lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- 42.Corbett D, Nurse S. The problem of assessing effective neuroprotection in experimental cerebral ischemia. Prog Neurobiol. 1998;54:531–548. doi: 10.1016/s0301-0082(97)00078-6. [DOI] [PubMed] [Google Scholar]
- 43.Hunter AJ, Mackay KB, Rogers DC. To what extent have functional studies of ischaemia in animals been useful in the assessment of potential neuroprotective agents? Trends Pharmacol Sci. 1998;19:59–66. doi: 10.1016/s0165-6147(97)01157-7. [DOI] [PubMed] [Google Scholar]