Abstract
A functional neuroimaging study examined the long-term neural correlates of early adverse rearing conditions in humans as they relate to socio-emotional development. Previously institutionalized (PI) children and a same-aged comparison group were scanned using functional magnetic resonance imaging (fMRI) while performing an Emotional Face Go/Nogo task. PI children showed heightened activity of the amygdala, a region that supports emotional learning and reactivity to emotional stimuli, and corresponding decreases in cortical regions that support perceptual and cognitive functions. Amygdala activity was associated with decreased eye-contact as measured by eye-tracking methods and during a live dyadic interaction. The association between early rearing environment and subsequent eye-contact was mediated by amygdala activity. These data support the hypothesis that early adversity alters human brain development in a way that can persist into childhood, and they offer insight into the socio-emotional disturbances in human behavior following early adversity.
Early postnatal life is a time of both developmental opportunity and vulnerability, and the timing of experiences is critical for developmental outcome. Non-human animal studies have shown that early rearing conditions can have long-term consequences on emotional behavior (including reactivity and social behavior) and the effects of early experience can be more significant than later experiences (Sabatini et al., 2007). Many of these behavioral outcomes are associated with changes in the amygdala, a biological substrate that supports emotional learning and reactivity to emotional stimuli (Davis, et al., 2001). Amygdala growth and hyperactivity mediate the expression of hyperemotionality as measured by increased anxiety-like behaviors (e.g., decreased exploration of rodents in an open-arm maze) (Vyas, et al., 2004). Numerous non-human animal studies have shown that the amygdala is particularly sensitive to early life rearing conditions (i.e., poor or absent caregiving)(Kikusui, et al., 2009; Plotsky, et al., 2005; Sabatini, et al., 2007). Experimental control of timing is much more difficult in humans since it is necessary to study populations with already existing adverse rearing conditions and early adverse caregiving is often followed by continued adversity throughout life. Institutional care (e.g., orphanage rearing), which is sparse, unstable, and regimented (Gunnar, et al., 2000b), is unfortunately a naturally occurring example of early caregiving adversity in humans affecting millions of children worldwide (http://www.hrw.org). What makes this population unique is that if a child is removed from the orphanage via adoption, the enddate of the adversity is documented, increasing our ability to draw conclusions about the timing of adverse caregiving in a human population. Although institutionalized infants experience an unknown combination of adverse events and we have no means of isolating these unique experiences, poor caregiving is common to all previously institutionalized (PI) children (Gunnar, et al., 2000a), and the odds of developmental delay following this type of caregiving are devastatingly high (Nelson, et al., 2009).
Socio-emotional behavior is especially vulnerable to early-life adversity, and often PI children exhibit elevated emotional reactivity (Colvert, et al., 2008) and low social competence (Hodges, et al., 1989). PI children exhibit more anxiety (Casey, et al., 2009; Zeanah, et al., 2009), internalizing problems (Juffer, et al., 2005) and difficulty regulating behavior in emotionally arousing contexts (Tottenham et al., 2009). PI children also show social impairments (e.g., difficulty with peers and fewer close relationships; Hodges & Tizard, 1989), and impairments in perception of social stimuli have been observed, including reduced cortical activity, as measured by electroencephalogram, in response to human faces (Moulson, et al., 2009), difficulty interpreting the facial expressions of others (Fries, et al., 2004), and inappropriately familiar interactions with strangers (Tizard, et al., 1978). This socio-emotional profile persists for many years. Animal models of early-life stress have shown that amygdala development is altered by poor caregiving, and these alterations are associated with subsequent socio-emotional difficulties (Caldji, et al., 2000; Plotsky, et al., 2005; Sabatini, et al., 2007); however, the means through which the early caregiving environment influences neural development and associated behaviors in humans is unknown. This manuscript describes a functional magnetic resonance imaging (fMRI) study that examines amygdala function in a PI population and its role in atypical socio-emotional behavior.
Most studies of PI children, the present study included, are necessarily quasi-experimental in nature, making it difficult to draw conclusions about the causal effects of institutional care. However, dose-response associations between time in institution and phenotype, which show that longer durations result in worse outcome (Rutter, et al., 2004; Tottenham, Hare, et al., 2009), suggest that institutional care is causing these effects. What is even more compelling is the random assignment design used in the Bucharest Early Intervention Project, showing that those children who remained in institutional care exhibited significantly more emotional difficulties compared to those who were removed via random assignment (Zeanah et al., 2009). These important data lend strong support for a causal link between institutional care and the emotional difficulties observed in this population.
Consistent with the non-human animal findings, hyperemotionality in humans is associated with hyperactivity in amygdala to emotionally-relevant stimuli (e.g., human faces) and corresponding hypoactivity in regions that regulate the amygdala, such as ventromedial prefrontal cortex (vmPFC) (Hare, et al., 2008; Shin, et al., 2005). Oculomotor behavior may be affected by amygdala activity, in that directing gaze away from the most arousing aspects of emotional stimuli is associated with attenuated amygdala activity (van Reekum, et al., 2007). Individual differences in amygdala activity are associated with directing gaze away from arousing (and informative) aspects of human faces (Dalton, et al., 2005), even though doing so can interfere with successful interpersonal communication (Adolphs et al., 2005). Shy individuals, who make less eye-contact than their non-shy peers (Pilkonis, 1977), show impairments in face recognition (Brunet, Mondloch, and Schmidt, 2010) and expression classifications (Battaglia, Ogliari, Zanoni et al., 2004). In typical populations, higher amounts of eye-contact have been associated with social competence and skill, as well as serving a number of social functions like expressing intimacy, exchanging social information, and regulating social exchanges (reviewed in Kleinke et al., 1986). Therefore, exhibiting high levels of eye-contact seems important for successful social behavior. If an association between increased amygdala activity and low levels of eye-contact held for PI children, heightened amygdala activity could be a mechanism by which the association between early experience and socio-emotional behavior is maintained over the course of development.
The primate amygdala develops early in life (Humphrey, 1968; Ulfig, et al., 2003) with the most rapid rate of development occurring during the early postnatal period (Payne et al., 2009), a rate which may heighten the vulnerability of the amygdala to environmental exposures (Lupien, et al., 2009). Gene expression studies show that the sensitivity of the primate amygdala and associated behaviors (self-regulation and social behavior) to adverse rearing conditions may be at a peak during the early postnatal period (Sabatini, et al., 2007), and effects of adverse rearing environment on amygdala development can persist through adulthood (Caldji, et al., 2000). Consistent with these animal models, human neuroimaging has revealed amygdala structural atypicalities and associated emotion difficulties following adverse early caregiving environments that are observable years after the removal from these conditions (Tottenham, Hare, et al., 2009; Mehta et al., 2009). To better characterize the neuroaffective phenotype following adverse rearing conditions, we used a combination of fMRI, eye-tracking and dyadic interaction to test the hypothesis that early life adversity would be followed by amygdala hyperactivity and associated atypical social behavior (decreased eye-contact).
Method
Participants
Imaging data were collected from 55 children (27 comparison (17 female), mean age (standard deviation) =10 years old (0.49); 28 PI (22 female), mean age (standard deviation) = 9 years old (0.43)). Six children (4 comparison, 2 PI) were excluded due to excessive head motion (> 2.5 mm or 2.5° of rotation), four children (4 PI) did not complete the fMRI task, and one child's neuroimaging data (1 comparison) were not usable due to technical problems. The final sample included forty-four children (22 PI; mean age=9.3-years-old, 16 female, mean time in orphanage care=15 months; SD=10 and 22 never-institutionalized comparison; 10.8-years-old, 13 female), whose characteristics are provided in Table 1. PI children were recruited from a group of families who had received local international adoption consultation services or had responded to advertisements placed in the listserves of international adoption family networks. The comparison group was recruited via flyer advertisements within the surrounding community. Children in the comparison group were only included if they were psychiatrically healthy, which was confirmed with parent interview (described below). The families of the PI and the comparison children had household incomes well above the median annual household income in the United States ($48,201; US Census Bureau, 2006), similar to the high socio-economic status that has been observed in another sample of Midwest families who have adopted internationally (e.g., Hellerstedt, et al., 2008).
Table 1.
Subject characteristics
| PI (N=22) | Comparison (N=22) | |
|---|---|---|
| Sex | 16 female; 6 male |
13 female; 9 male |
| Mean age in years (SD) | 9.3 (2.2) | 10.9 (2.4) |
| Mean (SD) IQ | 103.8(15.4) | 110.2 (13.8) |
| Country of origin | 18 East Asia; 4 Eastern Europe |
22 U.S. |
| Ethnic Background | 18 Asian-American 4 European-American |
3 Asian-American 4 African-American 9 European-American 4 Latina/o-American 2 Unknown |
| Mean age when placed in orphanage in months (SD) |
2.8 (6.8) | |
| Mean age when adopted in months (SD) |
17.8 (12.4) | |
| Mean time with family in months (SD) | 96.5 (28.6) | |
| Modal family income range ($) | 150,001 - 200,000 | 150,001 - 200,000 |
Measures
Clinical measures
Parents were interviewed by a trained member of the research team with the Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS-PL; Kaufman et al., 1997) to obtain reports of DSM-IV psychiatric diagnoses including affective, psychotic, anxiety, and behavioral disorders, as well as substance abuse and other disorders. Additionally, parents completed the Self-Report for Childhood Anxiety and Related Disorders (SCARED; Birmaher et al., 1995) and the Conners Parent Rating Scale–Revised: Short Form (Conners, 1997) to provide continuous measures of anxiety and ADHD symptoms, respectively. Parents also completed the Child Behavior Checklist (CBCL; Achenbach, 1991), which provided a standardized score of social competence (i.e., T scores). In order to obtain a measure of child's current mood during the scan, children were asked to rate on a scale from 1-9 how happy they felt (1=very unhappy; 9 = very happy; anchored with unhappy and happy faces) prior to entering the scanner and immediately upon exiting the scanner (when they were asked how they felt while in the scanner).
fMRI Task
Children completed an Emotional Face Go/Nogo task (Hare, et al., 2008) while in the MRI scanner. The event-related task requires pressing a button (“Go” condition) when target facial expressions appeared and inhibiting this behavioral response when distracter (“Nogo” condition) facial expressions appeared. Face stimuli (Tottenham, Tanaka, et al., 2009) were presented singly with a fixed random order and an average interstimulus jitter of 5 seconds (range: 2.5-10 seconds). The face images were grayscale with a visual angle of 15°. Four models (2 female, 2 male) were chosen from each of the following ethnic backgrounds: African-, Asian-, & European-American. A multi-ethnic set was chosen to avoid amygdala differences that might result from in-group/out-group effects (Lieberman, et al., 2005; Phelps, et al., 2000). Images were normalized for size and luminance. Children were instructed to execute the ‘Go’ response quickly for the named target expression (e.g., ‘neutral'), which was presented frequently (70% of the trials), while inhibiting the response when the distracter expression (e.g., ‘fear’) appeared, which was presented infrequently (30% of the trials). Which facial expression participants responded to changed with each run. There were two conditions separated by run, each presented twice – fear faces as the target with neutral faces as the distracter and neutral faces as the target with fearful faces as the distracter – resulting in 120 target trials (60 fearful, 60 neutral) and 52 distracter trials (26 fearful, 26 neutral). The order of runs was counterbalanced across participants. Each face stimulus was presented for 500 milliseconds, and participants were allowed 1500 milliseconds to respond by pressing a button with their index finger.
Subjects viewed images projected onto an overhead liquid crystal display panel with the Integrated Functional Imaging System–Stand Alone (IFIS-SA; MRI Devices, Waukesha, Wisconsin). A fiber optics response box was used for recording behavioral responses. The entire task lasted approximately 20 minutes, and participants were allowed to take breaks if needed between runs. Prior to scanning, children were given opportunity to practice to ensure that they understood and could perform the task.
Eye-contact measures
1) Eye-tracking task
Eye-tracking was employed to obtain high precision measurements of eye-contact during an out-of-scanner emotion-processing task. Eye-tracking capability was not present in the MRI scanner. However, we collected eye-tracking data for a separate behavioral task (data not presented in this manuscript), to provide an index of where children look at faces. After calibration with the eye-tracking camera (ISCAN, Inc), children were shown single presentations of images of faces for 1500 milliseconds that extended a visual angle of 26 degrees. The face image (Tottenham, Tanaka, et al., 2009) was a grayscale face with either happy or fearful open-mouthed expressions. The models were the same as those used in the fMRI-task (Emotional Face Go/Nogo). In total, there were 96 faces during which eye-tracking data were collected. Children were instructed to pay attention to the face for a memory task (data not presented here). There was a total of 36 children (18 comparison, 18 PI) who provided usable eye-tracking data, but there were only 28 children (14 comparison, 14 PI) who provided usable eye-tracking data and usable fMRI data. Eye-tracking data was not obtained from all children either because of an inability to obtain a usable track (N=7), because time did not permit for this procedure (N=7), or because the child participated prior to when this task was added to the protocol (N=2). The eye-tracking measure was obtained by designating two areas of interest (AOI), one around the eye and one around the mouth region of the face stimulus, and calculating the number of frames during which eye gaze was positioned in each AOI relative to the number of frames during stimulus presentation. There was no difference in eye-contact between happy and fearful faces (t=1.40, ns) and therefore, eye-tracking values were collapsed across the two expressions as an index of eye-contact.
2) Live dyadic interaction
Eye-contact during live dyadic interaction was recorded to provide an ecologically valid measure of eye-contact. Children, who had been testing with a member of the research team, were told to reunite with their parent, who had been participating in a videotaped interview with a different member of the research team. This interaction was included as a part of each family's visit for the purpose of allowing the child to rest and “check in” with his or her parent. No instructions were given to the parent or the child, and the members of the research team remained unobtrusive during the interaction by tending to paperwork. Children and their parents were videotaped during the unstructured dyadic interaction lasting approximately 1 minute. A coder, made as blind as possible to the rearing conditions of participants, noted the digitally recorded interactions by recording the time stamps on the video any time when children made eye-contact and when eye-contact ceased. It was impossible to ensure complete blindness since the ethnic backgrounds of some of the PI children would suggest to the coder their condition. There were 32 children (21 PI, 11 comparison) who provided usable eye-contact data during live dyadic interaction, but there were 26 children (18 PI, 8 comparison) who provided both usable eye-contact data and usable fMRI data. The reasons for not obtaining eye-contact data from all children was refusal to be videotaped (N=7) and technical difficulties, including an inability to see the child's face and equipment malfunction (N=11). The eye-contact measure during live dyadic interaction was obtained by calculating the number of seconds the child made eye-contact relative to the entire time that the child was in the room with the parent. Thus, this eye-contact measure was calculated as proportion of eye-contact made relative to total time spent in interaction.
Procedure
Children came to the laboratory for two sessions. In the first session, clinical measures, eye-tracking and dyadic interaction data were collected, and children were acclimated to the scanner environment with an MRI replica. The Emotional Face Go/Nogo task was administered in the MRI scanner on the second visit, which occurred on a separate day. During this second visit, children indicated current mood.
Scanning parameters
Subjects were scanned with a General Electric Signa 3.0-Tesla fMRI scanner (General Electric Medical Systems, Milwaukee, Wisconsin) with a quadrature head coil. Foam padding around the head was used to reduce motion. A whole brain, high resolution, T1-weighted anatomic scan (three-dimensional spoiled gradient; 256 × 256 inplane resolution, 240 mm field of view [FOV]; 124 mm × 1.4 mm axial slices) was acquired for each subject for transformation and localization of functional data into Talairach space (Talairach, et al., 1988). A spiral in-and-out sequence (Glover, et al., 2004) was used to collect functional data (repetition time=2500 msec, echo time=30 msec, FOV=200 mm, flip angle=90°, 64 × 64 matrix). We obtained 34 4-mm-thick coronal slices (skip 0) with an inplane resolution of 3.125 × 3.125 mm covering the entire brain except for the posterior portion of occipital lobe.
fMRI Preprocessing
Functional imaging data were preprocessed and analyzed with the Analysis of Functional NeuroImages (AFNI) software package (Cox, 1996). All included data were free of movement greater than 2.5 mm in any direction. After slice time correction images were registered to the first image volume after the high-resolution anatomical dataset with rigid body transformations and smoothed with anisotropic 6-mm Gaussian kernel. Time series were normalized to percent signal change to allow comparisons across runs and individuals by dividing signal intensity at each time point by the mean intensity for that voxel and multiplying the result by 100. The model included regressors for each of the 2 variable types (2 stimulus types, 2 expressions) by convolving the stimulus timing files with a γ-variate hemodynamic response function. Incorrect trials and 6 motion parameters were included as separate regressors for a total of 11 regressors. General linear modeling was performed to fit the percent signal change time courses to each regressor. Linear and quadratic trends were modeled in each voxel time course to control for correlated drift. Group level analyses were conducted on the regression coefficients from the individual analysis after transformation into the standard coordinate space of Talairach and Tournoux with parameters obtained from the transformation of each subject's high-resolution anatomical scan. Talairached transformed images had a resampled resolution of 3 mm3. A group level linear mixed effects (LME) model conducted with the 3dLME program within AFNI. The 3dLME program uses functions from the R software package (http://www.R-project.org). The LME model included the factors Group (Comparison, PI), Emotion (fear, neutral), and TrialType (target, distracter). Correction for multiple comparisons was applied at the cluster level following Monte Carlo simulations conducted in the AlphaSim program within AFNI. Clusterwise false-positive rates of p<0.01 corrected for multiple comparisons were determined for whole brain analyses and p<0.01 small volume corrected for amygdala (H. Kim, et al., 2004).
Imaging Analysis Plan
An omnibus ANOVA (Group [Comparison, PI] × TrialType [Target, Distracter] × Emotion [Fear, Neutral]) was performed on the neuroimaging data. Our primary region of interest was the amygdala, and amygdala signal change was indexed by blood-oxygen-level dependent (BOLD). However, we present data from all activated regions of interest (ROI) in table format. Our focus was on the statistical interactions with Group and therefore, we do not present the full set of results for interactions that do not include this factor. Post hoc t-tests were performed for each group separately within each of the ROIs resulting from the omnibus ANOVA. These t-tests examined MR signal for the condition of interest relative to baseline. Significant increases or decreases from baseline are presented in table format (Tables 3 & 4). In addition, we extracted the beta values from each ROI per subject and conducted between group t-tests on these values to interpret the direction of any interactions. These between group tests are also presented in table format (Tables 3 & 4). These beta weights were also used in the correlations with behavioral measures.
Table 3.
Talairach coordinates and patterns of activation for regions of interest: Group×Emotion (F = 3.915, p<.01 corrected for whole brain)
| Examination of Groups and Emotions Separately | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fear relative to baseline | Neutral relative to baseline | |||||||||||
| ROI | BA | Side | Cluster Size |
x | y | z | Comp | PI | Between Group Post Hoc tests |
Comp | PI | Between Group Post Hoc tests |
| Ventromedial Prefrontal Cortex (Rostral ACC) |
32 | R/L | 207 | −4 | 41 | 11 | ↓ | -- | PI>Comp, t=1.96, p=.057 | ↓ | ↓ | Ns |
| Ventromedial Prefrontal Cortex (Subgenual ACC) |
32 | R | 20 | 5 | 35 | −7 | -- | -- | Ns | -- | ↓ | Comp>PI, t=3.358, p<0.002 |
| Medial Frontal Gyrus | 6 | R | 20 | 11 | −19 | 50 | -- | -- | Ns | ↓ | -- | Ns |
| ACC | 32 | L | 15 | −10 | 17 | 32 | ↑ | ↑ | Ns | -- | ↑ | PI>Comp, t=3.039, p<0.004 |
| Inferior/Middle Frontal Gyrus |
9 | R | 14 | 44 | 14 | 29 | ↑ | ↑ | Ns | ↑ | ↑ | PI>Comp, t=2.139, p<0.038 |
| Amygdala | R | 35 | 19 | −6 | −10 | -- | ↑ | PI>Comp, t=3.344, p<.002 | ↑ | -- | Ns | |
| Amygdala | L | 70 | −20 | −6 | −11 | -- | ↑ | Ns | ↑ | ↑ | Ns | |
| Medial Temporal Gyrus | 22 | L | 22 | −58 | −52 | 14 | -- | ↑ | PI>Comp, t=2.361, p<0.03 | -- | -- | Ns |
| Inferior Parietal Lobule | 40 | R | 46 | 38 | −49 | 56 | ↑ | ↑ | Ns | ↑ | -- | Ns |
| Inferior Parietal Lobule | 39 | L | 113 | −43 | −58 | 35 | -- | ↑ | Ns | -- | -- | Ns |
| Cerebellar Tonsil | R | 21 | 38 | −43 | −34 | -- | -- | Ns | -- | -- | Ns | |
| Cerebellum | L | 13 | −31 | −46 | −52 | -- | -- | Ns | ↑ | -- | Comp>PI, t=2.199, p<0.003 | |
p<0.01 (corrected for total brain); Peak activity reported; Voxel size 3×3×3; BA = Brodmann Area; Comp = Comparison Group; PI = Postinstitutionalized Group; ACC=Anterior Cingulate Cortex; ↑ = Significant Increases relative to baseline; ↓ = significant decreases relative to baseline
Table 4.
Talairach coordinates and patterns of activation for regions of interest: Group×Trial Type (F = 3.915, p<.01 corrected for whole brain)
| Examination of Groups and Emotions Separately | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Target relative to baseline | Distracter relative to baseline | |||||||||||
| ROI | BA | Side | Cluster Size |
x | y | z | Comp | PI | Between Group Post Hoc tests |
Comp | PI | Between Group Post Hoc tests |
| Superior Frontal Gyrus | 10 | L | 49 | −13 | 65 | 20 | -- | -- | Ns | ↑ | -- | Comp>PI, t=2.156, p<0.05 |
| Middle Frontal Gyrus | 9 | R | 57 | 50 | 17 | 32 | ↑ | ↑ | Comp>PI, t=2.098, p<0.05 | ↑ | ↑ | PI>Comp, t=2.995, p<0.005 |
| Ventrolateral Prefrontal Cortex (Middle Frontal Gyrus) |
47 | R | 36 | 35 | 32 | −4 | -- | -- | Comp>PI, t=2.036, p<0.05 | -- | ↑ | Ns |
| Ventromedial Prefrontal Cortex (Subgenual ACC) |
25 | L | 45 | −4 | 20 | −13 | -- | -- | Ns | ↓ | -- | PI>Comp, t=3.016,p<0.005 |
| Ventromedial Prefrontal Cortex (Rostral ACC) |
24 | R | 37 | 11 | 29 | 11 | -- | -- | Ns | ↓ | ↓ | PI>Comp, t=2.429, p<0.02 |
| Inferior/Middle Frontal Gyrus | 9 | L | 32 | −37 | 8 | 32 | -- | ↑ | Ns | ↑ | ↓ | Ns |
| Inferior Frontal/Precentral Gyrus |
6/9 | R | 30 | 38 | 2 | 23 | ↑ | ↑ | Ns | ↑ | -- | Comp>PI, t=2.504, p<0.02 |
| Precentral Gyrus | 4 | R | 30 | 59 | −16 | 35 | -- | -- | Ns | ↓ | ↑ | PI>Comp, t=2.882, p<0.006 |
| Inferior Frontal Gyrus | 44 | R | 19 | 53 | 5 | 20 | -- | -- | Ns | ↑ | ↑ | Ns |
| Amygdala | R | 37 | 17 | −8 | −13 | ↑ | -- | Ns | ↑ | ↑ | PI>Comp, t=3.971, p <10−4 | |
| Amygdala | L | 16 | −13 | −1 | −13 | ↑ | ↑ | Ns | ↓ | ↑ | PI>Comp, t=3.339, p<0.002 | |
| Superior Temporal Sulcus | R | 48 | 47 | −55 | 14 | ↑ | -- | Ns | ↑ | ↓ | Comp>PI, t=2.371, p<0.03 | |
| Paracentral Lobule | 6 | R | 13 | 11 | −31 | 53 | -- | -- | Ns | -- | -- | Ns |
| Postcentral Gyrus | 7 | R | 170 | 20 | −49 | 65 | -- | -- | Ns | ↑ | ↓ | Ns |
| Postcentral Gyrus | 7 | L | 30 | −19 | −46 | 65 | -- | -- | Ns | ↑ | -- | Ns |
| Inferior Parietal Lobule | 2 | L | 47 | −31 | −28 | 32 | ↑ | ↑ | Ns | ↑ | -- | Ns |
| Inferior Parietal Lobule | 40 | L | 36 | −55 | −25 | 29 | -- | ↑ | Ns | ↑ | -- | Ns |
| Precuneus | 7 | R | 168 | 8 | −49 | 47 | -- | -- | Ns | -- | ↓ | Comp>PI, t=2.417, p<0.02 |
| Fusiform/ Parahippocampal Gyrus |
19/ 37 |
L | 360 | −34 | −49 | −1 | ↑ | ↑ | Ns | ↑ | ↑ | Comp>PI, t=2.644, p<0.02 |
| Cerebellar Tonsil | L | 49 | −31 | −43 | −43 | -- | ↑ | Ns | -- | ↓ | Ns | |
p<0.01 (corrected for total brain); Peak activity reported; Voxel size 3×3×3; BA = Brodmann Area; Comp = Comparison Group; PI = Postinstitutionalized Group; ACC=Anterior Cingulate Cortex; ↑ = Significant Increases relative to baseline; ↓ = significant decreases relative to baseline
Results
Clinical Measures
Based on parent interview (K-SADS-PL), one of the PI children reached criterion for an anxiety disorder, four for attention deficit hyperactivity disorder (ADHD), one for both ADHD and anxiety, one for anxiety and a learning disorder, two for learning disorders, and one for oppositional defiant disorder. Based on parent report using the continuous measures (SCARED and Conners), the PI children exhibited more anxiety symptoms [Panic: mean (SD)=0.95 (1.28); t=2.20, p<0.05; a score of 7 or greater indicates Panic Disorder) & Separation Anxiety: mean (SD) = 3.65 (3.47); t=2.76, p<0.009; a score of 5 or greater indicates Separation Anxiety] than the comparison group [Panic: mean (SD) = 0.24 (0.44) & Separation Anxiety: mean (SD) = 1.12 (1.62)]. PI children also exhibited more ADHD symptoms than the comparison group (PI: mean (SD) = 8.67 (10.23) & Comparison: mean (SD) = 2.06 (2.22); t=2.61, p<0.02) (a score of 34 and greater indicates ADHD; Kumar & Steer, 2003). We describe below the correlations between these scores and the neuroimaging data. The difference in social competence as measured by the CBCL did not reach statistical significance (t=1.75, p=0.09), although the comparison group (mean (SD) standardized T score = 48.1 (7.0)) trended towards a higher social competence score relative to the PI group (mean (SD) standardized T score = 43.1 (9.7)). There were no differences between the groups on current mood (before scan t=1.815, ns: PI mean (SD) = 3.3 (2.9), comparison mean (SD) = 1.8 (1.4); during scan t = 0.03, ns: PI mean (SD) = 3.2 (2.2), comparison mean (SD) = 3.2 (2.8)).
Emotional Face Go/Nogo
Behavior
Outlier response times (more than 3 standard deviations from the mean) were removed from the data. Accuracy was calculated as hits minus false alarms divided by the total number of trials. Two separate repeated measures ANOVAs, with Emotion (fear, neutral) as a within-subjects variable and Group (PI, comparison) as the between subjects variable, were run on the accuracy and reaction time data. There were no main effects or interactions for accuracy (F values <2.06, ns) or for response times (all F values <2.60, ns). The descriptive statistics are listed in Table 2.
Table 2.
Behavioral results (accuracy and response time) from the Emotional Face Go/NoGo
| Condition | PI | Comparison | Sig. Value |
|
|---|---|---|---|---|
| Accuracy (proportion correct) | Fear targets (“Go”) in the context of Neutral distracters (“Nogo”) | 0.71 (0.03) | 0.76 (0.03) | ns |
| Neutral targets (“Go”) in the context of Fear distracters (“Nogo”) | 0.70 (0.03) | 0.76 (0.03) | ns | |
| Response Time (milliseconds) | Fear targets (“Go”) in the context of Neutral distracters (“Nogo”) | 640.90 (177.52) | 600.47 (146.34) | ns |
| Neutral target (“Go”) in the context of Fear distracters (“Nogo”) | 661.32 (190.05) | 612.88 (140.01) | ns |
Neuroimaging
Since the amygdala was of primary interest, we describe significant activations in this region first. The omnibus ANOVA (Group [Comparison, PI] × TrialType [Target, Distracter] × Emotion [Fear, Neutral]) showed a main effect of Group (F (1,42)=4.071, p<.01, Rxyz*=17,−9,−12; Lxyz*=−16,−2,−15) on BOLD signal within the amygdala, where higher amygdala signal was observed in the PI group relative to the comparison group. There was a TrialTypeXEmotion interaction in the amygdala (F=3.915, p< .01; Rxyz*=23,−6,−9; Lxyz*=−21,−7,−10). T-tests (t=1.974, p< .01) examined the source of these interactions. Amygdala signal was higher for a) fear target than fear distracter trials (Lxyz†=−20,−3,−9), b) for fear target than neutral target trials (Rxyz†=22,−7,−5; Lxyz†=−22,−1,−10), c) for neutral distracter than neutral target trials (Rxyz†=23,−6,−7; Lxyz†=−26,−4,−9), d) for neutral distracter than fear distracter trials (Rxyz†=23,−7,−10; Lxyz†=−19,−7,−10), and e) for fear distracter than neutral target trials (Rxyz†=21,−4,−5). There was no difference between fear target and neutral distracter trials.
The factor Group interacted with Emotion and also separately with TrialType on BOLD signal in the amygdala. We will first discuss the GroupXEmotion interaction, which was observed bilaterally in the amygdala (F=3.915, p<.01, Rxyz†=20,−4,−8; Lxyz†=−19,−6,−9). Figure 1 shows the results of the omnibus ANOVA for the amygdala, as well as the data separated by group and emotion. As shown in Table 3, post hoc t-tests between groups (t=1.974, p<0.01) showed that fear faces resulted in greater bilateral amygdala activation in the PI group relative to the comparison group (Rxyz†=17,−9,−12; Lxyz*=−18,−10,−13), but there was no difference between the two groups for neutral faces. Within each group, two separate t-tests performed on the beta weights obtained from the GroupXEmotion ROI showed that there was increased activity for fear faces relative to neutral faces in the PI group in the right hemisphere (t=2.556, p<0.02), but no difference for the comparison group. There was also greater amygdala activity to fear faces for the PI group relative to neutral faces for the comparison group (Rxyz†=14,−6,−14), but no difference between neutral faces for the PI group and fear faces for the comparison group1. Other areas activated for the interaction of Group and Emotion are listed in Table 3. In addition to the results of the omnibus ANOVA, this table also provides the results for each condition relative to baseline. There was an interaction between group and emotion in regions of the prefrontal cortex, including vmPFC, medial frontal cortex, anterior cingulate cortex (ACC), and inferior/middle frontal gyrus, medial temporal cortex, parietal cortex, and cerebellum. As seen in Table 3, between group post hoc t-tests showed that both groups increased activity in anterior cingulate and inferior/middle gyrus for fear relative to baseline, but the PI group increased activity in these regions more for neutral face face stimuli. The PI group also showed relatively greater activity to fearful faces than the comparison group in the vmPFC (rostral ACC), but this difference was due to the comparison group showing a relative decrease in vmPFC activity to fearful faces, whereas the PI group showed no change in vmPFC activity to fearful faces. In contrast, the PI group showed a relative decrease in subgenual ACC activity to neutral faces, whereas the comparison group did not. The comparison group also showed relative increase in cerebellum for neutral faces, whereas the PI group showed no change relative to baseline for neutral faces.
Figure 1.
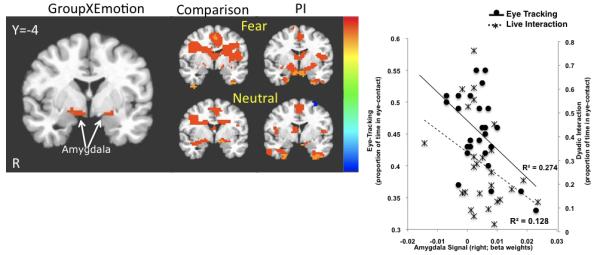
Amygdala signal change to Fearful Faces and Eye-contact. Left: A GroupXEmotion interaction showed that PI children exhibited greater signal change than the comparison group for fearful faces. Middle: Post hoc t-tests showed the results for each emotion relative to baseline separately for PI and comparison children. Right: BOLD signal (beta weights) from the GroupXEmotion amygdala ROI was inversely correlated with amount of eye-contact children made during eye-tracking (proportion of frames spent looking at the eye region). Note: The negative correlation between amygdala signal change and eye-contact during a live dyadic interaction (proportion of time making eye-contact) was not statistically significant.
There was also a GroupXTrialType interaction observed bilaterally in the amygdala (F= 3.915, p< .01; Rxyz†=17,−10,−13; Lxyz†=−19,−1,−22 & −13,−1,−16) (see Fig 2). Post hoc t-tests between groups (t=1.974, p<0.01) showed that a) distracter trials were associated with greater bilateral amygdala response for the PI group relative to the comparison group (Rxyz†=17 −9 −13; Lxyz†=−16 −4 −19), and that b) there was no signal difference in the amygdala between the groups for target trials, c) no signal difference for the contrast of target trials for the PI group and distracter trials for the comparison group and d) no signal difference for the contrast of distracter trials for the PI group and target trials for the comparison group2. Within each group, two separate t-tests performed on the amygdala beta weights obtained from the GroupXTrialType interaction showed that there was greater signal change to target relative to distracter stimuli for the comparison group in both hemispheres (Right: t=2.584, p<0.02; Left: t=2.585, p<0.02), and in contrast, there was greater signal change for distracter relative to target stimuli for the PI group in the right hemisphere (t=4.355, p<10−4). As seen in Table 4, in addition to the amygdala, there was an interaction between Group and TrialType in prefrontal, medial temporal, and parietal regions, as well as cerebellum. Between group post hoc t-tests showed that the PI group exhibited greater signal change in the amygdala, precentral gyrus, and middle frontal gyrus during the presentation of distracter stimuli relative to the comparison group. Distracter stimuli also resulted in greater signal in the PI group relative to the comparison group, but this difference was due to the PI group exhibiting less of a decrease relative to baseline than the comparison group. In contrast, the distracter stimuli resulted in greater relative signal increases in the superior frontal gyrus, inferior/middle frontal gyrus, superior temporal sulcus, and fusiform/parahippocampal gyrus in the comparison group than the PI group. The comparison group also showed more signal change to target stimuli than the PI group did in the middle frontal gyrus and ventrolateral prefrontal cortex. Distracter stimuli also resulted in greater activity in the precuneus for the comparison group relative to the PI group, but this difference was due to the PI group showing a decrease in activity relative to baseline.
Figure 2.
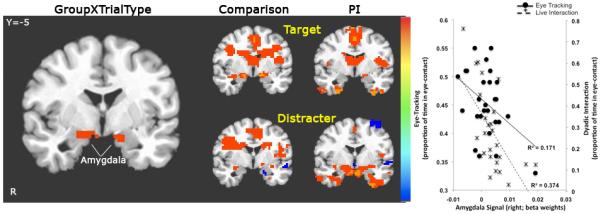
Amygdala signal change to Distracter Faces and Eye-contact. Left: A GroupXTrialType interaction showed that PI children exhibited greater signal change than the comparison group for distracter stimuli. Middle: Post hoc t-tests showed the results for each trial type relative to baseline separately for PI and comparison children. Right: BOLD signal (beta weights) from the GroupXTrialType amygdala ROI was inversely correlated with amount of eye-contact children made during both eye-tracking (proportion of frames spent looking at the eye region) and live dyadic interaction (proportion of time making eye-contact).
Eye-contact measured during eye-tracking and dyadic interaction
We examined behavioral differences in eye-contact that emerged as a function of early rearing condition. Comparison children spent significantly more time looking at the eye region (t=2.998, p<0.005)(mean proportion of time=0.48, SD=0.05) than the PI group (mean proportion of time=0.42, SD=0.06) as measured by eye-tracking, and there was no difference on the amount of time children spent looking at the mouth region (t=0.61, ns; mean (SD) proportion of time, PI=0.45 (0.08), comparison=0.47 (0.06)). The group difference for proportion of time making eye-contact during live dyadic interaction did not reach statistical significance (t=1.490, p=0.149) between the PI (mean=0.25, SD=0.16) and comparison children (mean=0.37, SD=0.25).
Associations between amygdala activity and social behaviors
We examined whether amygdala activity during the Emotional Face Go/Nogo correlated with social competence as measured by the CBCL and the two measures of eye-contact. We were specifically interested in amygdala response to the fear and distracter stimuli since these were the conditions that resulted in-group differences in amygdala response. A correlation analysis showed that social competence (CBCL T score) was negatively correlated with amygdala activity in response to fearful faces (beta weights obtained from GroupXEmotion ROIs; r=−0.35, p <0.05), but not to other conditions in the Emotional Face Go/NoGo. There was also an association between eye-contact and amygdala activity during the Emotional Face Go/Nogo. Specifically, amygdala activity (beta weights obtained from GroupXEmotion interaction) to fearful faces in the right (r=−0.519, p<0.006; Fig 1) and left (r=−0.534, p<0.004) hemispheres was inversely associated with eye-contact when measured during the eye-tracking procedure. The correlation between amygdala activity to fearful faces and eye-contact during live dyadic interaction approached statistical significance on the right (r=−0.36,p=0.07). Amygdala activity to distracter stimuli (beta weights obtained from GroupXTrialType interaction) was also negatively correlated with eye-contact during the eye-tracking procedure in the right hemisphere (r=−0.405, p<0.03; Fig 2) and during the live dyadic interaction both in the right (r=−0.605, p<0.001) and the left (r=−.47, p<0.015) hemispheres.
We also examined the association between the eye-contact measures and amygdala activity within the PI group. Just as was found with the entire sample, within the PI group there was a negative correlation between amygdala response to fearful faces and eye-contact as measured by eye-tracking (right: r=−0.751, p<0.003; left: r=−0.648, p<0.017). There was also a negative correlation between amygdala response to distracter stimuli and eye-contact during live dyadic social interaction within the PI group in the right hemisphere (r=−0.478, p<0.045). There were no other associations between eye-contact and amygdala response to fearful or distracter stimuli.
Hierarchical regression: Amygdala as mediator between early rearing environment and eye-contact
Amygdala activity was tested as a potential mediator of early rearing environment on current eye-contact as measured by eye-tracking, as specified by Baron & Kenny (1986). We examined amygdala activity that differentiated the two groups, namely right amygdala signal (beta weights obtained from the GroupXEmotion interaction) to fearful faces and right and left amygdala response to distracter stimuli (beta weights obtained from the GroupXTrialType interaction) in three separate hierarchical regression analyses. First, we will describe the results for the model that included right amygdala signal in response to fearful faces. This analysis showed that early rearing environment was a significant predictor of the amount of eye-contact made by children (beta=0.375, p=0.014) (see Table 5). In the second step of the model, rearing environment was simultaneously regressed on eye-contact along with amygdala signal as the mediator variable. The association between early rearing environment and amygdala signal was significant as was the association between amygdala signal and eye-contact (Fig 3). Moreover, the association between early rearing condition and eye-contact was mediated by amygdala signal (β=−.422, p<0.05), which when included in the analysis, explained the majority of the variance attributed to rearing environment, and the coefficient between rearing environment and eye-contact became non-significant when amygdala signal was included (β=0.191, ns). Rearing environment alone explained 17% of the variance in eye-contact, whereas rearing environment in conjunction with amygdala signal accounted for 30% of the variance. The Sobel test was employed to determine whether the inclusion of the mediator significantly attenuated the contribution of rearing environment in the prediction of eye-contact (Sobel, 1982). The regression coefficients for rearing environment decreased and were no longer significant after inclusion of amygdala activity, indicating that amygdala signal mediated the association between early rearing environment and eye-contact. The two other analyses that included amygdala response to distracter stimuli showed that these values did not significantly mediate the association between early rearing environment and eye-contact (Right: F=2.702, p=0.087; Group coefficient=0.04, p>0.05, Amygdala coefficient=−3.590, p>0.05 & Left: F=1.430, p=0.258; Group coefficient=0.036, p>0.05, Amygdala coefficient=−0.206, p>0.05).
Table 5.
Hierarchical Regression: Amygdala Activity to Fearful Faces Mediates association between Early Rearing Environment and Current Eye Contact.
| Variables in model | B | SE B | β | ΔR2 | Sobel Test |
|---|---|---|---|---|---|
| Step1 | |||||
| Early Rearing Environment | 0. 046 | 0.021 | 0.407* | 0.165* | |
|
| |||||
| Step 2 | |||||
| Early Rearing Environment | 0.021 | 0.022 | 0.191 | ||
| Amygdala Activity | −3.676 | 1.739 | −0.422* | 0.296* | 1.93 (p=0.05) |
p<0.05
Figure 3.
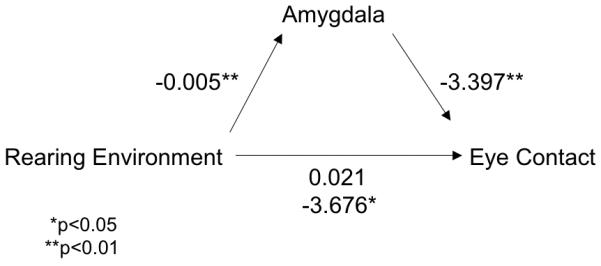
Amygdala response to fearful faces mediates association between early rearing environment and amount of current eye-contact. The unstandardized coefficients are shown for each association. The significant coefficient between rearing environment and eye-contact at the time of data collection was reduced and became non-significant when amygdala activity was included in the model, showing that the association between early rearing environment and eye-contact was mediated by the amygdala.
Discussion
Here we show that adverse rearing conditions in the postnatal period is followed by heightened amygdala activity during childhood. We have previously shown that institutional care during infancy is followed by amygdala hypertrophy in childhood, which is associated with emotional difficulties exhibited by the child (Tottenham, Hare, et al., 2009), and the current functional findings add to these structural findings. Although an earlier report using PET scanning found amygdala hypoactivity in PI children relative to an adult and epileptic population (Chugani, et al., 2001), here we show that when compared to healthy, same aged peers, PI children show atypically high amygdala activity. Behavioral studies that employ random assignment have found that anxious phenotypes, which seem to be associated with elevated amygdala activity (Thomas et al, 2001b), are caused by institutional care (Zeanah et al., 2009), providing support for the notion that the elevated amygdala activity observed in the current PI sample is also caused by institutional care. Non-human animal studies suggest the early, rapid development of the amygdala (Avishai-Eliner, et al., 1996; Payne, et al., 2009; Vazquez, et al., 2006) may increase its vulnerability to environmental pressures, resulting in elevated endogenous stress hormone, decreased gene expression (guanylate cyclase1∝3), precocious structural development, and altered future functioning (Becker, et al., 2007; Kikusui & Mori, 2009; Moriceau, et al., 2009; Plotsky, et al., 2005; Sabatini, et al., 2007), and our neuroimaging findings are consistent with vulnerability of the amygdala early in life.
PI children showed heightened amygdala activity relative to the comparison group in response to both fearful faces and distracter stimuli, while neutral and target stimuli did not produce group differences. In fact, the comparison group did not show differential amygdala response for fear and neutral faces, which is consistent with previous reports of typical children not showing greater amygdala response to fearful faces relative to neutral as adults do (Thomas et al., 2001). Therefore, it is noteworthy that the PI group exhibits the adult-like amygdala activation for fearful faces above neutral faces, which may be an indication of precocious amygdala development that has been shown to follow early life stressors in rodents (Kikusui & Mori, 2009; Moriceau et al., 2009). PI children also showed increased amygdala signal for distracter stimuli relative to the comparison group. The increased signal change in the PI group to distracter faces suggests that the comparison children were better able to ignore the emotional content of the distracter stimuli, while the PI children were not. This increased amygdala engagement to distracting emotional information may in part explain the emotional lability described in stressed populations (Lemieux & Coe, 1995), including PI children (Gunnar, Bruce, & Grtevant, 2000). Heightened amygdala activity has been associated with increased vigilance to emotionally significant stimuli, and this neural phenotype may increase vigilance for environmental threat in a PI population.
The Emotion of the face stimuli also interacted with Group in several other regions, including the vmPFC (including rostral anterior cingulate cortex), a region that has strong bidirectional connections with the amygdala, often playing a modulatory role over the amygdala in healthy adults (Phelps, et al., 2004; Quirk, et al., 2006). This difference was the result of comparison children showing a relative decrease in vmPFC activity during the presentation of fearful faces. In healthy populations, the amygdala and vmPFC show inverse activity (Phelps, et al., 2004), which might be mediated by the integrity of the white matter tracts between them (Kim, et al., 2009). However, pathological populations show less inverse coupling between the two regions (Marsh, et al., 2008; Shin, et al., 2006), and decreased coupling has been associated with increased trait anxiety (Hare et al., 2008). Unlike the comparison children, the PI group showed no change in vmPFC with increased amygdala activity. These data are consistent with poor communication between the two regions in the PI group, and recent diffusion tensor imaging has identified reduced white matter between amygdala and prefrontal cortex in PI children (Govindan, et al., 2009).
Additionally, Emotion interacted with Group in regions, including the dorsal prefrontal cortex, subgenual anterior cingulate, temporal cortex, parietal cortex, and cerebellum. In general, the differences in the dorsal regions of prefrontal cortex were most obvious for the neutral conditions, where the PI group showed greater increases relative to baseline for neutral faces as compared to the comparison group. Although we were surprised to see increased prefrontal activity in the PI group, the fact that these differences appeared for the neutral face stimuli may reflect group differences in response to neutral faces. In typical populations of children, neutral faces are more likely to engage subcortical regions, like the amygdala, than fear faces (Thomas et al., 2001a). (Note that only the comparison group showed increased activity in the right amygdala for neutral faces.) Given the often inverse activity between cortical and subcortical regions, neutral faces may reduce cortical activity for the comparison children. The PI group also showed increased activity in temporal cortex for fearful faces. Group differences in parietal activity are also suggested by the GroupXTrialType interaction and the paired comparisons between emotion and baseline. Activity in temporal and parietal regions may reflect increased emotional arousal that has been observed in other anxious developing populations (Krain, et al., 2008). The between group t-test showed greater cerebellar activity for the neutral faces than the PI group. Cerebellar group differences are consistent with recently reported cerebellar volumetric differences identified in PI children relative to a comparison group (Bauer, et al., 2009), although clearly more research is necessary to interpret these findings. Thus, early adverse caregiving is followed by differences in brain activity to facial emotions, like fear and neutral, that includes amygdala, frontal, temporal, parietal, cerebellar regions.
Previously we reported more errors for negatively valenced faces in the PI group using a more difficult behavioral version of the Emotional Face Go/Nogo task (shorter intertrial intervals) (Tottenham, Hare et al., 2009). In the present fMRI compatible version of the task (longer intertrial intervals), we did not observe performance differences between groups, perhaps reflecting the decreased difficulty of the scanner version of the task. Nonetheless, we observed increased amygdala activity to distracter trials for the PI children relative to the comparison group. Similar to the vmPFC response to fearful faces, the vmPFC response to distracter stimuli showed greater decrease relative to baseline for the comparison group than it did for the PI children, again indicating less effective communication between the two regions for the PI group. In general, the comparison group showed greater signal increases in cortical regions including the superior frontal gyrus, middle frontal gyrus, ventrolateral prefrontal cortex, inferior/precentral gyrus, parietal cortex, superior temporal sulcus, and fusiform gyrus to distracter (i.e., nogo) stimuli. These frontal and parietal regions have been implicated in cognitive control processes (Bunge, et al., 2002; Durston, et al., 2006), and the occipitotemporal regions (fusiform gyrus and superior temporal sulcus) have been implicated in representing the structural aspects of faces (Haxby, et al., 2001). The more robust cortical activity in the comparison group suggests they are more likely to engage cognitive and perceptual processes than those children with adverse caregiving histories. These differences show that despite similar performance on the current Emotional Face Go/Nogo task (perhaps related to the decreased sensitivity of this version of the task to detect group differences at the behavioral level), the two groups exhibit different patterns of neural activity and that face distracters, regardless of valence are effective in increasing amygdala activity in the PI group. Thus, early adverse caregiving is followed by differences in brain activity to distracting social stimuli that includes amygdala, frontal, temporal, parietal, cerebellar regions. These neural differences may lead to difficulty with self control in emotional contexts observed in this population (Tottenham et al., 2009).
It has been shown in previous PI samples (Hodges & Tizard, 1989) that difficulty with social relationships is common. In the current study, we examined indices of social behavior including social competence and eye-contact. Social competence was negatively correlated with amygdala response to fearful faces, such that higher amygdala response to faces was associated with lower social competence. Eye-contact, a critical aspect of social behavior, was also negatively correlated with amygdala response to faces (both fear and distracters), such that higher amygdala responses to faces were associated with less eye-contact. This association was observed during two independent measures of eye-contact, one with high precision (eye-tracking) and one with ecological validity (eye-contact during a live dyadic social interaction). As a group, PI children made less eye-contact as measured with eye-tracking. Importantly, amygdala activity mediated the association between early rearing conditions and the observed decreased eye-contact during childhood. The significant contribution of amygdala activity in explaining the association between early rearing environment and decreased eye-contact suggests that the often observed social difficulties in PI children (Hodges & Tizard, 1989) are, in part, the result of amygdala hyperactivity.
Studies in adults suggest that looking at the eye region is particularly effective in increasing amygdala activity (Whalen, et al., 2004), activity that can increase subjective experiences of negative emotion (Lanteaume, et al., 2007). Therefore one means of decreasing amygdala activity may be to direct gaze away from the most arousing aspects of stimuli (Dalton, et al., 2005; van Reekum, et al., 2007). Since we found a similar association in the current study between amygdala activation and eye-contact, one possible explanation of this finding is that minimizing eye-contact may be an attempt on the child's part to regulate overarousal caused by face-to-face interactions. In light of the critical role that face processing plays in successful social interaction, these findings suggest a neurobehavioral mechanism by which early adversity is followed by poor socio-emotional health. Thus, beyond social deprivation experienced during institutional care, PI children continue to have daily atypical experience with faces, a finding which may explain the decreased fusiform activity in the PI group, a brain region that supports developing expertise with faces (Golarai, et al., 2007; Tarr, et al., 2000). The eye-contact measures and parent report of social competence used in the current study are distal measures of social competence; however, our findings might provide insight into the constellation of socio-emotional atypicalities observed in children with a history of adversity including atypical friendliness towards unfamiliar adults, atypical affective attachments to parents, and atypical social relationships with peers (as reviewed in Gunnar, et al., 2000b)).
There are many limitations of this study. We cannot ever determine the specific events infants experience pre-adoption, nor do we have insight into the prenatal conditions or the genetic profile of this population, making conclusive statements about causality difficult. However, a rapidly growing literature suggests that institutional rearing results in adverse developmental outcomes (Colvert, et al., 2008; Fries & Pollak, 2004; D. E. Johnson, et al., 1999; Kertes, et al., 2008; Tottenham et al., 2009; Zeanah, et al., 2009), and recent advances in the ability to use random assignment enhance our ability to make claims about the directionality of events (Zeanah, et al., 2009).
Secondly, we cannot control the sample's characteristics, some of which may confound our findings. The numerous levels of privation of the PI group present a challenge for identifying all of the appropriate controls, and we utilize a comparison group who differs in many ways from the PI group. Since we cannot conclude that any one factor caused the observed effects, the present results must be interpreted with appropriate caution. In the current study, the comparison group was of a similar age, sex, and IQ as the PI group, although they differed on ethnic background, and future attempts to match on ethnic background will be necessary if amygdala activity differences are identified between ethnically dissimilar children. However, there are no data to suggest that ethnically Asian individuals show elevated amygdala activity except in cases when the elevated activity is an artifact of decreased familiarity with the ethnicities of the models used in the experiment (Chiao et al., 2007; Denrtl et al., 2009). Although, there was a high representation of girls adopted from China in this sample, the large representation of Chinese children may reduce some of the levels of privation in typically found in samples of PI children. It has been reported that Chinese infants are typically healthy upon arrival at the orphanage overwhelmingly being abandoned by non-impoverished two-parent households, where the decision to abandon was most often made by the birthfather – the primary reason for abandonment having to do with the preference for a son (Johnson, et al., 1998). Children adopted from China are more likely to show elevated lead levels, anemia, and hepatitis B, but they often experience better prenatal conditions and spend less time in institutional care than other PI children (Miller, et al., 2000). While this caregiving history is somewhat less bleak than has been reported in populations of children adopted from Eastern European countries (Saiman, et al., 2001), we have nonetheless observed elevated amygdala responses in this sample. This functional difference is consistent with amygdala structural atypicalities reported previously (Tottenham, Hare, et al., 2009), as well as social-emotional difficulties such as impaired emotion regulation (Tottenham, Hare, et al., 2009) and greater trait anxiety (Casey, et al., 2009). Many of the children in the PI sample had a mental illness (particularly anxiety and impulse control problems). Because children with anxiety disorders show elevated amygdala response to fearful faces (Thomas et al., 2001b), we performed analyses removing children with anxiety disorders and found the same results as when they were included. We were less concerned about impulse control artifacts in the observed amygdala activations since children with impulse control problems (e.g., ADHD) show typical amygdala responses to fearful faces (Marsh et al., 2008). Nonetheless, our analyses that examined the association between continuous measures of these phenotypes (both anxiety and ADHD) showed that the heightened amygdala response observed in the PI group was not an artificial result of these children being included in the sample. Instead, our findings suggest that the observed amygdala effects were directly associated with early adverse caregiving.
Another source of concern might be group differences in habituation of the amygdala since amygdala response to fearful faces in the scanner occurred after the eye-tracking procedure (when children also saw fearful faces). However, the two presentations of fearful faces never occurred on the same day, and previous work in healthy adults has shown that the amygdala response to fear does not habituate when test sessions are separated by a period of days or weeks (Johnstone et al., 2005). Instead, the response is highly stable across days. Therefore, the chance that the initial presentation of fearful faces during the eye-tracking resulted in a decrement in the amygdala response during the scanner is unlikely.
These data are consistent with the hypothesis that early life is a time when poor caregiving can have significant long-term effects on neural development, and that these experiences can impact socio-emotional behavior. Alterations in these phenotypes following early-life adversity appear resistant to change when measured in childhood.
Acknowledgements
Supported by the National Institute of Mental Health (R01 MH73175, P50 MH 079513). Many thanks to Drs. Jane Aaronson, Douglas Ballon, and Henning Voss, to Siobhan O'Herron, and to the Weill Cornell Medical College Citigroup Biomedical Imaging Center (Douglas Ballon, director).
Footnotes
small volume corrected
whole brain corrected
Since three of the PI children had clinical anxiety disorders and anxiety has been associated with heightened amygdala activity in children (Thomas et al., 2001b), this analysis was performed excluding children with an anxiety disorder (n=3). We conducted between group t-tests on the amygdala beta weights obtained from the GroupXEmotion interaction without these children. Results show that the effects remained in the amygdala. Fear faces resulted in greater amygdala activation for the PI than the comparison children in the right amygdala (t=1.975, p<.01), and there was no difference between groups for neutral faces. Therefore, the observed effects on amygdala activity were not artificially produced by the three children with an anxiety disorder. We also examined the association between amygdala activity to fear and neutral faces and current mood using child reported mood prior to scanning and during the scanning session. Further, we examined anxiety and ADHD symptoms continuously using the SCARED and the Conners Parent Rating Scale–Revised: Short Form, respectively. There were no associations between these continuous measures and amygdala activity to fear or neutral faces (all p's >0.2).
This analysis was repeated excluding children with an anxiety disorder (n=3) using the amygdala beta weights obtained from the GroupXTrialType Interaction. Results show that the effects remained in the amygdala. Distracter faces resulted in greater amygdala activation for the PI than the comparison children on the right (t=2.352, p<.025) and on the left (t=2.297, p<0.029), and there was no difference between groups for target faces. Therefore, the observed effects on amygdala activity were not artificially produced by the three children with an anxiety disorder. We also examined the association between amygdala activity and current mood using child reported mood prior to scanning and during the scanning session. Moreover, we examined anxiety and ADHD symptoms continuously using the SCARED and the Conners Parent Rating Scale–Revised: Short Form, respectively. There were no associations between these continuous measures and amygdala activity to fear or neutral faces (all p's >0.05).
References
- Achenbach TM. Integrative Guide to the 1991 CBCL/4-18, YSR, and TRF Profiles. University of Vermont, Department of Psychology; Burlington, VT: 1991. [Google Scholar]
- Avishai-Eliner S, Yi SJ, Baram TZ. Developmental profile of messenger RNA for the corticotropin-releasing hormone receptor in the rat limbic system. Developmental Brain Research. 1996;91(2):159–163. doi: 10.1016/0165-3806(95)00158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Ogliari A, Zanoni A, Villa F, Citterio A, Binaghi F, Fossati A, Maffei C. Children's discrimination of expressions of emotions: relationship with indices of social anxiety and shyness. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43(3):358–365. doi: 10.1097/00004583-200403000-00019. [DOI] [PubMed] [Google Scholar]
- Bauer PM, Hanson JL, Pierson RK, Davidson RJ, Pollak SD. Cerebellar volume and cognitive functioning in children who experienced early deprivation. Biol Psychiatry. 2009;66(12):1100–1106. doi: 10.1016/j.biopsych.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K, Abraham A, Kindler J, Helmeke C, Braun K. Exposure to neonatal separation stress alters exploratory behavior and corticotropin releasing factor expression in neurons in the amygdala and hippocampus. Developmental Neurobiology. 2007;67(5):617–629. doi: 10.1002/dneu.20372. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(10):1230–1236. doi: 10.1097/00004583-199910000-00011. [DOI] [PubMed] [Google Scholar]
- Brunet PM, Mondloch CJ, Schmidt LA. Shy children are less sensitive to some cues to facial recognition. Child Psychiatry and Human Development. 2009;41(1):1–14. doi: 10.1007/s10578-009-0150-0. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33(2):301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The Effects of Early Rearing Environment on the Development of GABA-A and Central Benzodiazepine Receptor Levels and Novelty-Induced Fearfulness in the Rat. Neuropsychopharmacology. 2000;22(3):219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Glatt CE, Tottenham N, Soliman F, Bath K, Amso D, Altemus M, Pattwell S, Jones R, Levita L, McEwen B, Magarinos AM, Gunnar M, Thomas KM, Mezey J, Clark AG, Hempstead BL, Lee FS. Brain-derived neurotrophic factor as a model system for examining gene by environment interactions across development. Neuroscience. 2009;164(1):108–120. doi: 10.1016/j.neuroscience.2009.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhasz C, Nagy F, Chugani DC. Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage. 2001;14(6):1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- Colvert E, Rutter M, Beckett C, Castle J, Groothues C, Hawkins A, Kreppner J, O'Connor T,G, Stevens S, Sonuga-Barke EJ. Emotional difficulties in early adolescence following severe early deprivation: findings from the English and Romanian adoptees study. Development and Psychopathology. 2008;20(2):547–567. doi: 10.1017/S0954579408000278. [DOI] [PubMed] [Google Scholar]
- Conners CK. Conners'Rating Scales–Revised: Technical manual. Multi-Health Systems; North Tonawanda, NY: 1997. [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computations in Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Critchley H, Daly E, Phillips M, Brammer M, Bullmore E, Williams S, Van Amelsvoort T, Robertson D, David A, Murphy D. Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Human Brain Mapping. 2000;9(2):93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, Alexander AL, Davidson RJ. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Durston S, Mulder M, Casey BJ, Ziermans T, van Engeland H. Activation in ventral prefrontal cortex is sensitive to genetic vulnerability for attention-deficit hyperactivity disorder. Biological Psychiatry. 2006;60(10):1062–1070. doi: 10.1016/j.biopsych.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Fries AB, Pollak SD. Emotion understanding in postinstitutionalized Eastern European children. Development and Psychopathology. 2004;16(2):355–369. doi: 10.1017/S0954579404044554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Thomason ME. Improved combination of spiral-in/out images for BOLD fMRI. Magnetic Resonance Imaging. 2004;51(4):863–868. doi: 10.1002/mrm.20016. [DOI] [PubMed] [Google Scholar]
- Golarai G, Ghahremani DG, Whitfield-Gabrieli S, Reiss A, Eberhardt JL, Gabrieli JD, et al. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nature Neuroscience. 2007;10(4):512–522. doi: 10.1038/nn1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan RM, Behen ME, Helder E, Makki MI, Chugani HT. Altered Water Diffusivity in Cortical Association Tracts in Children with Early Deprivation Identified with Tract-Based Spatial Statistics (TBSS) Cerebral Cortex. 2009 doi: 10.1093/cercor/bhp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Bruce J, Grotevant HD. International adoption of institutionally reared children: research and policy. Development and Psychopathology. 2000a;12(4):677–693. doi: 10.1017/s0954579400004077. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Bruce J, Grotevant HD. International adoption of institutionally reared children: Research and policy. Development and Psychopathology. 2000b;(12):677–693. doi: 10.1017/s0954579400004077. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;5539;293:2425–2430. doi: 10.1126/science.1063736. [see comments] [DOI] [PubMed] [Google Scholar]
- Hellerstedt WL, Madsen NJ, Gunnar MR, Grotevant HD, Lee RM, Johnson DE. The International Adoption Project: Population-based Surveillance of Minnesota Parents Who Adopted Children Internationally. Maternal and Child Health Journal. 2008;12(2):162–171. doi: 10.1007/s10995-007-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges J, Tizard B. Social and family relationships of ex-institutional adolescents. Journal of Child Psychology & Psychiatry. 1989;30(1):77–97. doi: 10.1111/j.1469-7610.1989.tb00770.x. [DOI] [PubMed] [Google Scholar]
- Humphrey T. The development of the human amygdala during early embryonic life. The Journal of Comparative Neurology. 1968;132(1):135–165. doi: 10.1002/cne.901320108. [DOI] [PubMed] [Google Scholar]
- Johnson DE, Dole K. International Adoptions: Implications for Early Intervention. Infants and Young Children. 1999;11(4):34–45. [Google Scholar]
- Johnson K, Banghan H, Liyao W. Infant abandonment and adoption in China. Population and Development Review. 1998;24(3):469–510. [Google Scholar]
- Johnstone T, Somerville LH, Alexander AL, Oakes TR, Davidson RJ, Kalin NH, Whalen PJ. Stability of amygdala BOLD response to fearful faces over multiple scan sessions. Neuroimage. 2005;25(4):1112–1123. doi: 10.1016/j.neuroimage.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Juffer F, van Ijzendoorn MH. Behavior problems and mental health referrals of international adoptees: a meta-analysis. Journal of the American Medical Association. 2005;293(20):2501–2515. doi: 10.1001/jama.293.20.2501. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kertes DA, Gunnar MR, Madsen NJ, Long JD. Early deprivation and home basal cortisol levels: a study of internationally adopted children. Development and Psychopathology. 2008;20(2):473–491. doi: 10.1017/S0954579408000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusui T, Mori Y. Behavioural and neurochemical consequences of early weaning in rodents. Journal of Neuroendocinrology. 2009;21(4):427–431. doi: 10.1111/j.1365-2826.2009.01837.x. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, et al. Contextual modulation of amygdala responsivity to surprised faces. Journal of Cognitive Neuroscience. 2004;16(10):1730–1745. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. Journal of Neuroscience. 2009;29(37):11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinke CL. Gaze and eye contact: a research review. Psychological Bulletin. 1986;100(1):78–100. [PubMed] [Google Scholar]
- Krain AL, Gotimer K, Hefton S, Ernst M, Castellanos FX, Pine DS, et al. A functional magnetic resonance imaging investigation of uncertainty in adolescents with anxiety disorders. Biological Psychiatry. 2008;63(6):563–568. doi: 10.1016/j.biopsych.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Kumar G, Steer RA. Factorial validity of the Conners' Parent Rating Scale-revised: short form with psychiatric outpatients. Journal of Personality Assessment. 2003;80(3):252–259. doi: 10.1207/S15327752JPA8003_04. [DOI] [PubMed] [Google Scholar]
- Lanteaume L, Khalfa S, Regis J, Marquis P, Chauvel P, Bartolomei F. Emotion induction after direct intracerebral stimulations of human amygdala. Cerebral Cortex. 2007;17(6):1307–1313. doi: 10.1093/cercor/bhl041. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Hariri A, Jarcho JM, Eisenberger NI, Bookheimer SY. An fMRI investigation of race-related amygdala activity in African-American and Caucasian-American individuals. Nature Neuroscience. 2005;8(6):720–722. doi: 10.1038/nn1465. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, Towbin KE, Leibenluft E, Pine DS, Blair RJ. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry. 2008;165(6):712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SC, Rutter M, Sonuga-Barke EJ. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. Journal of Child Psychology & Psychiatry. 2009;50(8):943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- Miller LC, Hendrie NW. Health of children adopted from China. Pediatrics. 2000;105(6):E76. doi: 10.1542/peds.105.6.e76. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Raineki C, Holman JD, Holman JG, Sullivan RM. Enduring neurobehavioral effects of early life trauma mediated through learning and corticosterone suppression. Frontiers in Behavioral Neuroscience. 2009;3:22. doi: 10.3389/neuro.08.022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulson MC, Fox NA, Zeanah CH, Nelson CA. Early adverse experiences and the neurobiology of facial emotion processing. Developmental Psychology. 2009;45(1):17–30. doi: 10.1037/a0014035. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Furtado EA, Fox NA, Zeanah CH. The Deprived Human Brain: Developmental deficits among institutionalized Romanian children—and later improvements—strengthen the case for individualized care. American Scientist. 2009;97:222–229. [Google Scholar]
- O'Connor TG, Marvin RS, Rutter M, Olrick JT, Britner PA. Child-parent attachment following early institutional deprivation. Development and Psychopathology. 2003;15(1):19–38. doi: 10.1017/s0954579403000026. [DOI] [PubMed] [Google Scholar]
- Payne C, Machado CJ, Bliwise NG, Bachevalier J. Maturation of the hippocampal formation and amygdala in Macaca mulatta: A volumetric magnetic resonance imaging study. Hippocampus. 2009 doi: 10.1002/hipo.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Cunningham WA, Funayama ES, Gatenby JC, Gore JC, et al. Performance on indirect measures of race evaluation predicts amygdala activation. Journal of Cognitive Neuroscience. 2000;12(5):729–738. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- Pilkonis P. The behavioral consequences of shyness. Journal of Personality. 1977;45(4):596–611. [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30(12):2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16(6):723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rutter M, O'Connor TG. Are there biological programming effects for psychological development? Findings from a study of Romanian adoptees. Developmental Psychology. 2004;40(1):81–94. doi: 10.1037/0012-1649.40.1.81. [DOI] [PubMed] [Google Scholar]
- Sabatini MJ, Ebert P, Lewis DA, Levitt P, Cameron JL, Mirnics K. Amygdala gene expression correlates of social behavior in monkeys experienceing maternal separation. Journal of Neuroscience. 2007;27(12):3295–3304. doi: 10.1523/JNEUROSCI.4765-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiman L, Aronson J, Zhou J, Gomez-Duarte C, Gabriel PS, Alonso M, et al. Prevalence of infectious diseases among internationally adopted children. Pediatrics. 2001;108(3):608–612. doi: 10.1542/peds.108.3.608. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of the New York Academy of Sciences. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Sobel ME. Asymptotic intervals for indirect effects in structural equations models. In: Leinhart S, editor. Sociological methodology. Jossey-Bass; San Francisco: 1982. pp. 290–312. [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- Tarr MJ, Gauthier I. FFA: a flexible fusiform area for subordinate-level visual processing automatized by expertise. Nature Neuroscience. 2000;3(8):764–769. doi: 10.1038/77666. [DOI] [PubMed] [Google Scholar]
- Tizard B, Hodges J. The effect of early institutional rearing on the development of eight year old children. Journal of Child Psychology & Psychiatry. 1978;19(2):99–118. doi: 10.1111/j.1469-7610.1978.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, Casey BJ. Amygdala response to facial expressions in children and adults. Biological Psychiatry. 2001a;49(4):309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Archives of General Psychiatry. 2001;58(11):1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry T, Nurse M, Gilhooly T, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and emotion regulation difficulties. Developmental Science. 2009:1–15. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfig N, Setzer M, Bohl J. Ontogeny of the human amygdala. Annals of the New York Academy of Sciences. 2003;985:22–33. doi: 10.1111/j.1749-6632.2003.tb07068.x. [DOI] [PubMed] [Google Scholar]
- van Reekum CM, Johnstone T, Urry HL, Thurow ME, Schaefer HS, Alexander AL, et al. Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. Neuroimage. 2007;36(3):1041–1055. doi: 10.1016/j.neuroimage.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Vazquez DM, Bailey C, Dent GW, Okimoto DK, Steffek A, Lopez JF, et al. Brain corticotropin-releasing hormone (CRH) circuits in the developing rat: effect of maternal deprivation. Brain Research. 2006;1121(1):83–94. doi: 10.1016/j.brainres.2006.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128(4):667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Kagan J, Cook RG, Davis FC, Kim H, Polis S, et al. Human amygdala responsivity to masked fearful eye whites. Science. 2004;5704;306:2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Egger HL, Smyke AT, Nelson CA, Fox NA, Marshall PJ, et al. Institutional rearing and psychiatric disorders in romanian preschool children. American Journal of Psychiatry. 2009;166(7):777–785. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]


