Summary
The generation of B cell precursors (BCP) from lymphohematopoietic progenitors (LHP) in bone marrow is dependent on signals provided by the receptor tyrosine kinase Flt3 and its ligand, Flt3-ligand (FL). Mice deficient in FL exhibit striking reductions in LHP and BCP. Currently the mechanism by which Flt3 regulates lymphoid lineage/B cell development is unknown. Here we show that haploinsufficiency of FL (FL+/−) reduced LHP, CLP and Pro-B cells, suggesting that FL levels set a threshold for B lymphopoiesis. Limiting dilution analysis confirmed reduced BCP frequency in FL+/− mice. Real-time PCR of LHP from FL+/− animals showed increased transcripts of the B lineage inhibitor id1. However, targeted deletion of id1 did not restore the lymphoid/B lineage deficiencies in FL−/− mice, supporting Id1-independent mechanisms. BrdU incorporation studies established that FL is not essential for the proliferation of Flt3+ multipotential progenitors. Analysis of FL−/− progenitors expressing low levels of Flt3 revealed decreased levels of the pro-survival factor Mcl1. Consequently, the Flt3+ LHP progeny of Flt3low LSK+ cells exhibited increased Annexin V staining. Together, these data suggest that Flt3 signaling initiates a cascade of events in Flt3low precursors that promote the survival of LHP from which BCP are derived.
Keywords: Flt3-ligand, cell differentiation, B cell development, apoptosis
Introduction
Lymphopoiesis is a stepwise process dependent on signals from the microenvironment. In bone marrow (BM), differentiating multipotential progenitors (MPP) integrate microenvironmental signals to generate lymphoid progenitors from which BCP are derived. Fms-like tyrosine kinase (Flt3) and its ligand, Flt3-ligand (FL), are critical regulators of lymphoid progenitors and their B lineage progeny. However, the mechanism by which Flt3 signaling regulates the generation of BCP from LHP in vivo is not well understood.
Hematopoietic stem cells (HSC) and MPP can be identified in BM by a lack of lineage markers (Lin-), expression of stem cell antigen-1 (Sca-1), and high levels of the receptor tyrosine kinase, c-kit [1]. These cells are collectively termed LSK+. Lymphohematopoietic progenitors (LHP) are Flt3+ MPP that are functionally distinct from Flt3−/low MPP in having lost megakaryocyte/erythroid differentiation potential [2]. LHP can be distinguished within MPP by a variety of criteria including green fluorescent protein (GFP) knocked into the recombinase-activating gene one (RAG1) coding region [3, 4]. LHP defined by these criteria lack surface expression of the IL-7R. Another way to distinguish LHP in vivo is differential expression of Flt3 and VCAM-1 [5]. The ability to distinguish LHP within MPP provides a means to identify and characterize cellular and molecular circuits that regulate lymphoid lineage development.
Abundant experimental evidence suggests that the molecular circuitry initiating lymphoid lineage specification within MPP correlates with expression of Flt3. Targeted-deletion of Flt3 or Flt3-ligand resulted in profound deficiencies in LHP and/or BCP [6–9]. Molecular analysis of the residual Flt3+ MPP in FL−/− mice revealed severe reductions in lymphoid transcripts [9]. These data were interpreted to suggest that Flt3 signaling regulates lymphoid priming in MPP. However, upregulation of Flt3 could be concomitant with lymphoid priming and not directly regulate this process. Currently, the role of Flt3/FL in regulation of lymphopoiesis, or a molecular connection between Flt3 signaling and the induction of any B lineage regulatory factor or differentiation-related event, remains to be established.
RAG1 locus activation is a hallmark of lymphoid specification [10]. Through the establishment and analysis of RAG1-GFP/+ reporter mice expressing wildtype, heterozygous, and knockout FL alleles, we show that threshold levels of FL are required for RAG1 locus activation in LHP. Limiting dilution analysis confirmed reduced BCP frequency under B cell promoting conditions in vitro. E2A activation is critical for the generation of LHP [11]. Real-time PCR analysis of Flt3+ LHP from FL+/− mice revealed reductions in transcripts for the E2A targets ebf1 and rag1, consistent with the B cell deficiency in these animals. Id family members and Scl/Tal1 regulate E2A [12]. Real-time PCR analysis of Flt3+ LHP from FL+/− mice revealed increased id1 transcripts. However, deletion of id1 in FL−/− mice did not rescue LHP or BCP, indicating that Flt3 does not regulate lymphopoiesis solely by modulating Id1. FL synergizes with other cytokines to promote the proliferation of MPPs in vitro [13]. BrdU incorporation studies revealed that FL is not essential for the proliferation of Flt3+ MPPs in vivo. However, in the absence of FL, there is preferential expansion of Flt3- MPPs to fill a niche that would be competitively filled by Flt3+ MPPs. We show that Flt3 is critical for the survival of LHP in vivo, as abrogated FL production leads to reductions in Mcl-1 protein and increased evidence of apoptosis.
Results
Haploinsufficiency of FL reduces LHP and BCP
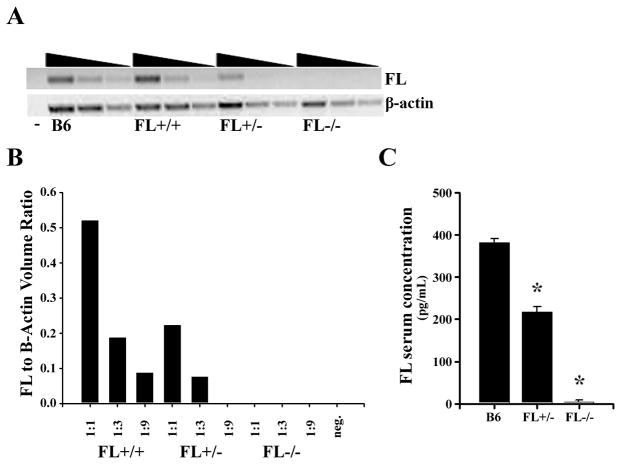
Exogenous administration of FL in vivo increases the frequency of LHP [14, 15]. However, it has not been determined if threshold levels of FL are required for normal lymphocyte production. A RAG1-GFP reporter mouse expressing varying physiological levels of FL provides an in vivo model to determine if haploinsufficiency of FL alters the numbers of LHP and BCP in BM. RAG1-GFP x FL+/+, RAG1-GFP x FL+/−, and RAG1-GFP x FL−/− mice were established. BM from the three genotypes was analyzed for FL transcript abundance. Fig. 1A and 1B illustrates that haploinsufficiency of FL reduced FL transcripts by 50%. ELISA confirmed a similar reduction in FL serum levels in FL+/− mice (Fig. 1C). These data establish that heterozygosity of flt3-l reduces FL production.
Figure 1.
Monoallelic expression of FL reduces FL production. (A) Semi-quantitative RT-PCR of FL transcripts in BM cells from C57BL/6 (B6), FL+/+ x RAG1-GFP/+, FL+/− x RAG1-GFP/+, or FL−/− x RAG1-GFP/+ mice. The cDNA was serially diluted 1:1, 1:3, and 1:9 for semi-quantitative analysis. Beta-actin was used as a loading control. Data are representative of two BM samples for each genotype. (B) Quantification of FL transcripts. Intensity data are the average of two BM samples for each genotype. (C) Concentration of FL (pg/mL) in the serum of C57Bl/6 (B6), FL+/−, and FL−/− mice as determined by ELISA. Data represents the mean ± S.D. (*p ≤ 0.0001) FL concentration in serum from ≥ 5 mice/genotype and 2 independent experiments. P-values were determined using the Students T-test.
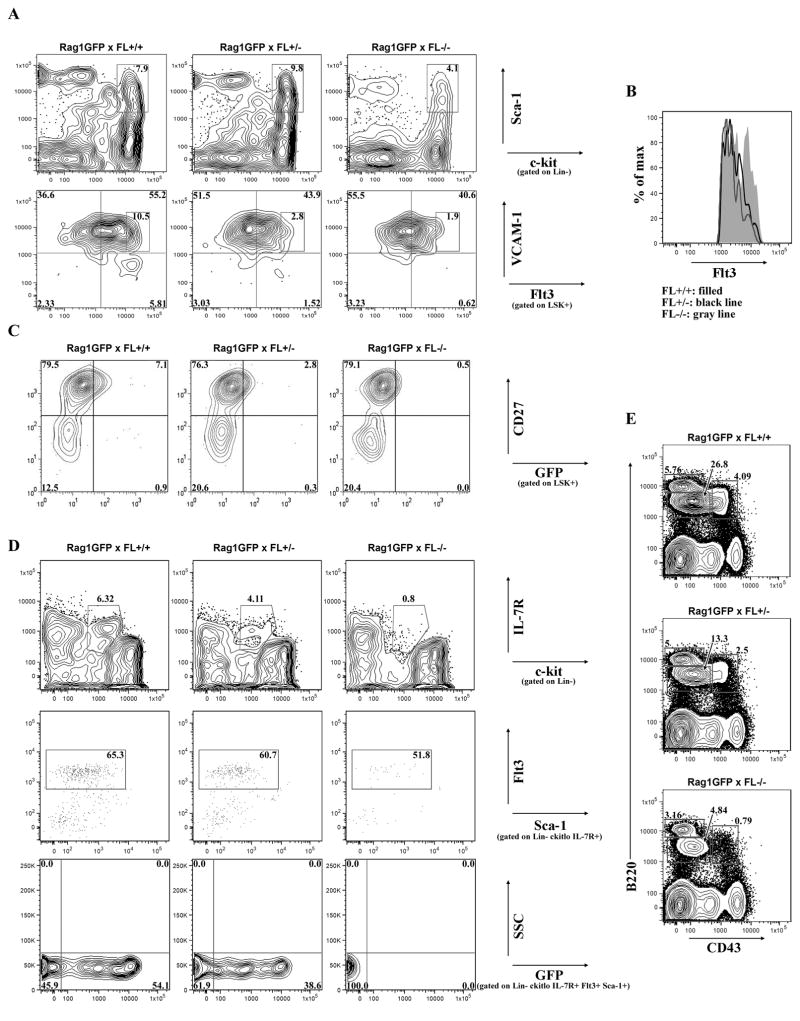
The majority of Flt3+/FL-responsive BM cells are within the LSK+ subset [16, 17]. Percentages of LSK+ cells were not altered by FL-haploinsufficiency in contrast to FL-deficiency (Fig. 2A). Haploinsufficiency of FL had no effect on BM cellularity. In agreement with recent findings [18], percentages and absolute numbers of LSK+ cells were significantly reduced in FL−/− mice (Fig. 2A and absolute numbers (mean±s.d./hind leg bone): FL+/+ (n=10): 1.8×104±8.9×103; FL+/− (n=15): 1.9×104±7.9×103; FL−/− (n=11): 9.3×103±4.8×103, p≤0.01). The reductions in LSK+ cells in FL−/− mice was not a consequence of crossing these animals to RAG1-GFP/+ mice, as we observed statistically significant reductions in LSK+ cells in FL−/− mice not bred to RAG1-GFP/+ (data not shown).
Figure 2.
FL haploinsufficiency reduces LHP and RAG1 locus activation. (A) Flow cytometric analysis of LSK+ BM cells from RAG1-GFP x FL+/+, RAG1-GFP x FL+/−, and RAG1-GFP x FL−/− mice (top panels). Differential expression of Flt3 and VCAM-1 in LSK+ BM cells to discriminate HSC/MPP, GMLP, and LMPP (bottom panels). Boxed regions indicate Flt3hi GMLP. (B) Histogram of boxed region from (A) bottom panels, indicative of Flt3 expression in Flt3hi GMLP in different FL genotypes. (C) Flow cytometric analysis of LSK+ BM cells from RAG1-GFP+ x FL mice to distinguish HSC, MPP, and LHP. A littermate GFP- control was analyzed in each experiment to determine GFP+ gates (data not shown). (D) Flow cytometric analysis of Lin- BM cells to distinguish CLP: Lin- c-kitlo IL-7R+ (Top panels). CLP were further discriminated by Flt3 and Sca-1 expression (middle panels). GFP expression within Lin- c-kitlo IL-7R+ Flt3+ Sca-1+ CLP (bottom panels). GFP+ gates were determined by analysis of Lin- c-kitlo IL-7R- Flt3+ cells which do not express GFP (data not shown). (E) Flow cytometric analysis of BCP. Pre-Pro-B/Pro-B cells are B220+ CD43+, Pre-B cells are B220+ CD43-, and naïve/mature B cells are B220hi CD43-. Data are representative of ≥ 5 mice/genotype and ≥ 3 independent experiments.
Next, we used differential cell surface expression of Flt3 and VCAM1 to determine the sensitivities of LSK+ fractions to threshold levels of FL [5]. HSC/MPP were identified as LSK+ VCAM1+ Flt3−/lo (Fig. 2A, upper left quadrant), GMLP (granulocyte-macrophage-lymphoid progenitor) as LSK+ VCAM1+ Flt3+ (Fig. 2A, upper right quadrant), and LMPP (lymphoid-biased multipotent progenitor) as LSK+ VCAM1- Flt3+hi (Fig. 2A, lower right quadrant). Fig. 2A shows that Flt3−lo HSC/MPP were significantly increased in FL−/− mice (FL+/+ (n=6): 34.6±4.8%; FL+/− (n=5): 50.5±7.9%, p=0.0026; FL−/− (n=6): 66.5±7.1%, p<0.0001). In contrast, percentages (FL+/+ (n=6): 54.5±2.6%; FL+/− (n=5): 41.3±9.4%, p=0.009; FL−/− (n=6): 29.0±8.4%, p<0.0001) and absolute numbers (data not shown) of GMLP were significantly reduced in FL+/− and FL−/− mice with dramatic reductions in GMLP expressing the highest levels of Flt3 (Fig. 2A, bottom panel, boxed region and 2B). Importantly, FL+/− and FL−/− mice exhibited a dose-dependent reduction in LMPP (Fig. 2A), confirming that threshold levels of FL are required for lymphopoiesis.
An alternative immunophenotyping schema used to fractionate HSC, MPP, and LHP is differential expression of CD27 and GFP within LSK+ of RAG1-GFP reporter mice expressing various levels of FL [10]. The LSK+ CD27- subset is enriched for HSC and we observed a consistent increase in percentages of LSK+ CD27- HSC in FL-deficient animals (Fig. 2C)[19]. The LSK+ CD27+ subset includes Flt3lo MPP and Flt3+ GMLP and no significant difference in frequencies of these subsets was found. However, consistent with the dose dependent reduction in LSK+ Flt3+hi cells we showed above, percentages and absolute numbers (mean±s.d./hind leg bone) of LHP defined as LSK+ CD27+ GFP+ were significantly reduced with FL haploinsufficiency (Fig. 2C and FL+/+ (n=9): 1.2×103±5.1×102; FL+/− (n=13): 6.5×102±3.7×102, p≤0.01; FL−/− (n=8): 1.2×102±1.0×102, p<0.0001). Thus, RAG1 locus activation, a hallmark of lymphoid differentiation, is dependent on threshold levels of FL.
BCP are enriched in common lymphoid progenitors (CLP) [20]. Sixty percent of CLP express Flt3, half express the RAG1-GFP reporter, and ebf1 transcripts are upregulated [10, 21, 22]. FL is required for the regulation of CLP [6]. However, CLP also express the IL-7R, which could functionally substitute for FL [20]. Therefore, we asked if haploinsufficiency of FL affected CLP. Indeed, FL+/− mice exhibited a 40% reduction in CLP and percentages of Flt3+ CLP expressing GFP were reduced (Fig. 2D). Thus, the regulation of CLP, as well as RAG1 locus activation within CLP, requires threshold levels of FL.
Downregulation of Flt3 accompanies B lineage commitment [23]. Therefore, BCP subsets, or RAG1 expression within BCP, may not be sensitive to reductions in FL. Shown in Fig. 2E, B220+ CD43+ Pre-Pro-B/Pro-B and B220+ CD43- Pre-B cells were reduced by FL haploinsufficiency. FL+/− mice had significant reductions in percentages of Pre-Pro-B/Pro-B cells and Pre-B cells. In contrast, naïve and mature B cell frequency was not significantly affected by loss of FL. Analysis of GFP within the Pre-Pro B/Pro-B and Pre-B compartments revealed no significant difference in percentages of GFP+ cells (data not shown) indicating that RAG1 activation in BCP is not sensitive to reduction of FL.
Threshold levels of FL are required to establish the B cell developmental potential in MPP
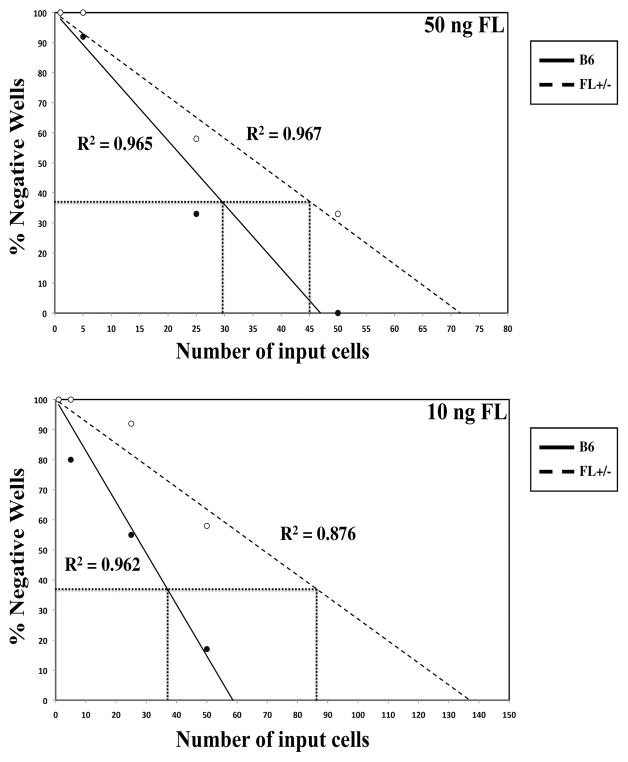
We next asked if reductions in FL altered the B lineage differentiation potential of MPP. GMLP were sorted from WT and FL+/− mice and limiting dilution assays (LDA) were performed under B lineage promoting conditions. Fig. 3 shows that FL+/− GMLP have reduced B cell developmental potential compared to WT, with 30% more input GMLP required to generate one B220+ cell (Fig. 3, top panel). Addition of 100ng/ml of FL did not further increase the B cell precursor frequency beyond that obtained with 50ng/ml, suggesting that 50ng/ml of FL is saturating for B lymphopoiesis in this assay. In contrast, reductions in FL amplified the effect as 60% more input FL+/− GMLP were necessary to generate one B220+ cell (Fig. 3, bottom panel). These experimental data reinforce the importance of threshold levels of FL for the generation of BCP.
Figure 3.
Reduced BCP frequency in FL+/− GMLP. GMLP from FL+/− and C57Bl/6 mice were FACS sorted and cultured in IL-7, SCF, and high (50ng) (top panel) or low (10ng) amount of FL (bottom panel). The solid (C57Bl/6) and dashed (FL+/−) lines represent the % negative wells per number of input cells. The line at 37% negative wells indicates precursor frequency, or the number of input C57Bl/6 or FL+/− cells necessary to generate one BCP. Data are representative of cells pooled from ≥ 5 mice/genotype and 2 independent experiments.
Loss of Id1 does not rescue LHP in FL−/− mice
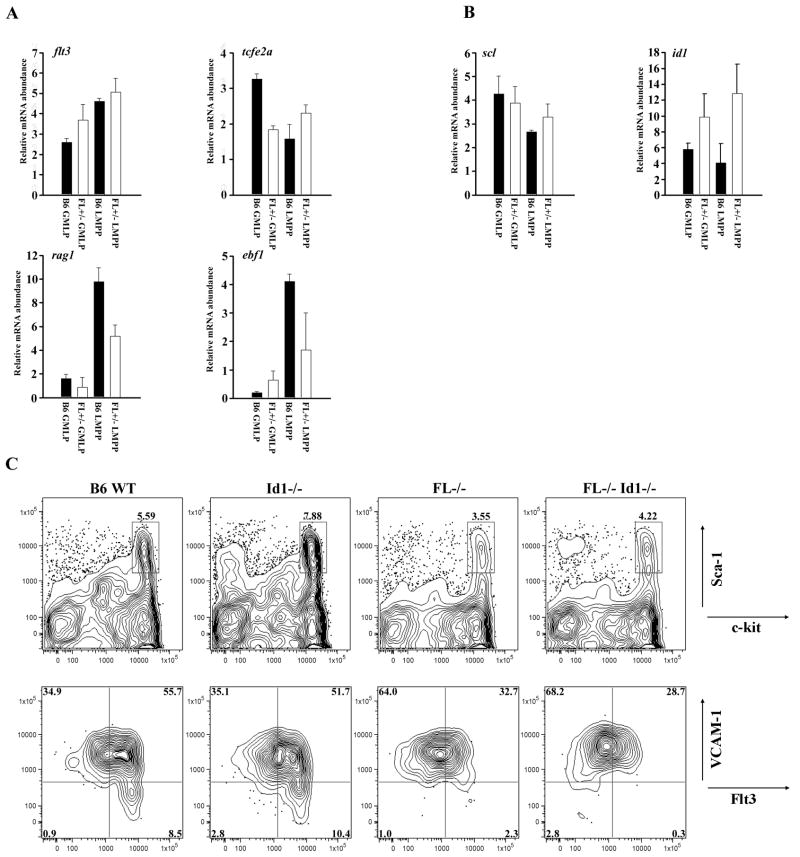
Lymphoid priming is critically dependent on the transcription factor E2A [11]. E2A activity is negatively controlled via dimerization with Id1 and Scl/Tal1 [12]. Quantitative comparison of id1 and scl/tal1 transcripts could be informative with regard to mechanisms by which FL promotes lymphoid specification. GMLP and LMPP were sorted from WT and FL+/− mice. Real-time PCR showed equivalent levels of transcripts corresponding to flt3, variable levels of tcfe2a, and decreased transcripts for rag1 and ebf1 (Fig. 4A) in FL+/− mice. Rag1 transcripts were reduced in GMLP and LMPP, which further validates the RAG1-GFP reporter flow cytometry data (Fig. 4A and 2C). Reductions in ebf1 transcripts in LMPP are consistent with the B lineage developmental defect in FL+/− mice and the LDA results (Fig. 2 and 3). Next, we compared transcript abundance for id1 and scl/tal1. In two independent experiments we found increased transcripts corresponding to id1 in GMLP and LMPP from FL+/− mice (Fig. 4B). In contrast, transcripts for scl/tal1 were unaffected by FL haploinsufficiency (Fig. 4B). In preliminary studies, we observed increases in id2 transcripts in LMPP, but not GMLP, in FL+/− mice (data not shown). Id3 levels were unaffected in either GMLP or LMPP by FL haploinsufficiency (data not shown).
Figure 4.
Id1 does not rescue the lymphoid defect in FL−/− mice. (A) Flt3, tcfe2a, rag1, and ebf1 and (B) Scl and id1 were analyzed from FACS sorted GMLP and LMPP from C57Bl/6 (B6) and FL+/− mice by quantitative PCR. (A+B) Data are representative of 2 independent experiments with BM pooled from ≥ 5 mice/genotype. Error bars represent the mean ± SEM. (C) Flow cytometric analysis of BM from C57Bl/6 (B6), Id1−/−, FL−/−, and FL−/− Id1−/− mice stained with antibodies for lineage markers, c-kit, and Sca-1 to determine LSK+ cells (top panels). LSK+ cells stained with antibodies to Flt3 and VCAM-1 to distinguish HSC/MPP, GMLP, and LMPP (bottom panels). Data are representative of ≥ 3 mice/genotype and ≥ 3 independent experiments.
The increase in id1 transcripts in GMLP and LMPP from FL+/− mice suggested that FL might regulate lymphopoiesis by repressing id1 transcription. To test this hypothesis, FL−/− mice were bred to Id1−/− mice and backcrossed to generate FL−/− x Id1−/− animals. BM cells were harvested and stained with combinations of antibodies to resolve HSC/MPP, GMLP, and LMPP. Loss of id1 did not rescue the deficiencies in GMLP, LMPP, CLP or BCP in FL−/− mice or FL+/− animals (Fig. 4C and data not shown). These data indicate that FL regulates lymphopoiesis through mechanisms independent of suppression of id1 transcription.
FL is not essential for the proliferation of Flt3+ LHP
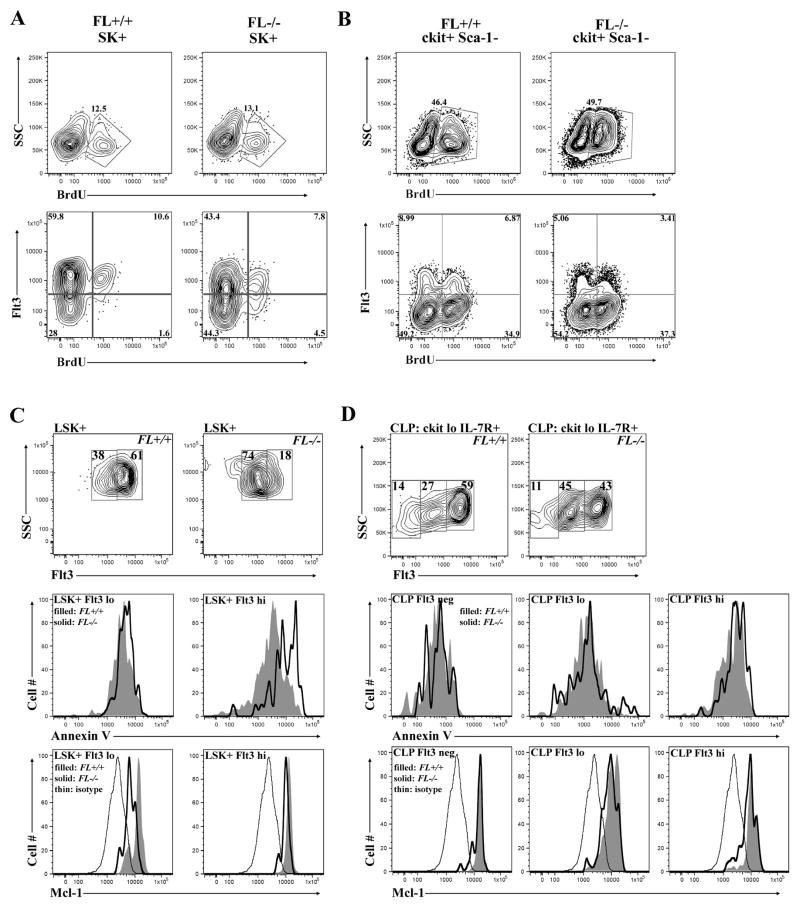
Cytokines regulate hematopoiesis, in part, by controlling the proliferation of hematopoietic progenitors [24]. Numerous studies showed administration of FL increased numbers of hematopoietic progenitors in vivo [25, 26]. Therefore, we determined if FL was required for the proliferation of LHP by evaluating BrdU incorporation. Percentages of BrdU+ cells within the SK+ and SK+ Flt3+ were compared between the FL genotypes. Percentages of BrdU+ cells within the SK+ compartment were not reduced as a consequence of FL deficiency (Fig. 5A). Relatively equivalent ratios of SK+ Flt3+ cells incorporating BrdU+ (an average of 1 in 6 cells) were detected in FL+/+, FL+/−, and FL−/− bone marrow (Fig. 5A and data not shown). We note that percentages of Flt3+ cells were significantly reduced in FL−/− mice, consistent with the data presented above (Fig. 2) and previous findings by others [9, 18]. However, the ratio of BrdU+ to BrdU− cells within the Flt3+fraction was not diminished as a consequence of FL deficiency. Alternatively, we consistently observed an increase in the frequency of SK+ Flt3- cells that incorporated BrdU, suggesting that FL deficiency allows preferential expansion of Flt3- MPP (Fig. 5A, bottom panel, lower right quadrant). Analysis of subsets enriched for myeloid progenitors, c-kit+ Sca-1-and c-kit+ Sca-1- Flt3+, revealed identical results across the three genotypes (Fig. 5B and data not shown). These results indicate that the deficiency in Flt3+ LHP cells in FL−/− mice is not due to defects in proliferation.
Figure 5.
Flt3 regulates the survival, but not the proliferation of LHP. (A-B) Flow cytometric analysis of bone marrow to examine BrdU incorporation 12 hours after 2 mg were injected i.p. into FL+/+ and FL−/− mice in c-kit+ Sca-1+ (SK+) (A) and c-kit+ Sca-1- (B). (A) Total BrdU incorporation in SK+ cells (top panels). BrdU incorporation in SK+ cells using Flt3 as additional criteria (bottom panels). (B) Total BrdU incorporation in c-kit+ Sca-1- cells (top panels). BrdU incorporation in c-kit+ Sca-1- cells using Flt3 as additional criteria (bottom panels). BrdU quadrants are set on the IgG isotype control for each genotype (data not shown). Data are representative of ≥ 3 mice/genotype and ≥ 3 independent experiments. (C-D) Annexin V staining and intracellular Mcl-1 expression in LSK+ cells (C) and CLP (D). FL+/+ represented by filled histogram, FL−/− indicated by solid line, and thin line depicts isotype control. (C) LSK+ cells were fractionated into Flt3lo and Flt3+hi subsets (top panels). Overlaid histograms depicting Annexin V staining in LSK+ Flt3lo and LSK+ Flt3+hi cells (middle panels). Overlaid histograms depicting intracellular Mcl-1 staining in LSK+ Flt3lo and LSK+ Flt3+hi cells (bottom panels). (D) CLP fractionated into three subsets: Flt3neg, Flt3lo, and Flt3hi (top panels). Overlaid histograms depicting Annexin V staining in CLP subsets (middle panels). Overlaid histograms depicting intracellular Mcl-1 staining in CLP subsets (bottom panels). Data are representative of 3 independent experiments (C+D).
Flt3 signaling is required for the survival of LHP
A recent study showed Flt3 signaling prevented spontaneous apoptosis of human stem and progenitor cells in vitro [27]. However, similar studies have not been performed in vivo in the mouse. Therefore, we determined if Flt3 signaling is required for the survival of murine hematopoietic progenitors in vivo. Pre-apoptotic cells can be visualized in BM with Annexin V. Percentages of Annexin V+ cells within HSC/MPP and LHP were compared in WT and FL−/− BM (Fig. 5C). No change in percentages of Annexin V+ cells were observed in HSC/MPP (Fig. 5C). However, FL−/− BM had significantly increased percentages of Annexin V+ LHP suggesting that FL is critical for the survival of LHP (Fig. 5C). We did not observe differences in Annexin V staining in FL+/− LHP (data not shown). Interestingly, analysis of Annexin V+ staining in FL−/− CLP revealed no significant differences compared to WT CLP (Fig. 5D). CLPs express Flt3 and IL-7R. The lack of Annexin V+ cells within the residual FL−/− CLP likely reflects survival signals provided by IL-7R. Consistent with that hypothesis, no differences in Annexin V staining were found in CLP in FL+/− animals (data not shown).
Next we sought to determine the mechanisms by which Flt3 contributes to the survival of LHP. Mcl-1 is a critical survival factor for HSC [28]. Therefore, we determined if Mcl-1 expression was altered in FL-deficient mice. Flow cytometry revealed reduced Mcl-1 expression in FL−/− LSK+ Flt3lo cells (Fig. 5C). FL−/− LSK+ Flt3hi cells also exhibited reduced Mcl-1 expression (Fig. 5C). CLP showed no differences in Mcl-1 expression (Fig. 5D). These data suggest that Flt3 signaling in LSK+ Flt3lo cells provides survival signals through induction of Mcl-1. Intracellular staining showed no change in Bcl-2 expression in LHP or CLP (data not shown), indicating that Flt3 signaling likely does not work through Bcl-2 to promote LHP survival in vivo.
Discussion
The goal of this study was to determine how Flt3 regulates lymphopoiesis and B cell development. Comparison of mice producing varying levels of FL revealed that LHP genesis is exquisitely sensitive to FL availability. Threshold levels of FL are necessary for the initiation and maintenance of RAG1 locus activation in lymphoid progenitors (LHP, CLP), but not in later stage BCP. LDA confirmed FL+/− GMLP had diminished ability to become B220+ BCP. qRT-PCR analysis of sorted GMLP and LMPP from FL+/− mice showed increased transcripts for id1, an established inhibitor of B lymphopoiesis. However, loss of Id1 did not rescue the lymphoid progenitor deficiency in FL−/− mice, suggesting Id1 independent differentiation mechanisms. Previous studies have shown that FL is important for the proliferation of MPPs and CLP in vitro. Here we show that FL is dispensable for the proliferation of Flt3+ MPPs in vivo. We showed increased Flt3+ LHP initiating apoptosis, suggesting that FL is critical for the survival of LHP. Consistent with that finding, Mcl-1 expression was reduced in LSK+ Flt3lo cells, suggesting that signals from Flt3 are important for expression of this critical pro-survival factor. Together, these findings provide mechanistic insight into the role of Flt3 in regulating lymphopoiesis.
Limiting expression of Flt3 has been suggested to control the threshold of Flt3 signaling [25]. Albeit scarce, previous studies have shown that alterations in cytokine levels have consequences, especially those that impact hematopoiesis and/or immune cell function. For example, haploinsufficiency of B-cell-activating-factor (BAFF) decreased serum immunoglobulin levels, consistent with a critical role for BAFF in B cell homeostasis [29]. Similarly, reductions in IL-7 responding cells in BM of pregnant mice was restored by exogenous administration of IL-7 [30]. Exogenous administration of FL has been shown to increase numbers of LSK+ Flt3+ and B220+ ckitlo Flt3+ BCP in BM in vivo [14, 15]. Mice heterozygous for Flk2/Flt3 do not have deficiencies in BCP [7]. However, flk2/flt3−/− mice do not exhibit as severe a deficiency in LHP or BCP as FL−/−. These data lend credence to our findings that FL availability is the limiting factor. To our knowledge, the current study is the first to demonstrate that alteration in systemic levels of FL alters steady-state lymphopoiesis.
We showed that threshold levels of FL are required for expression of the E2A target genes ebf1 and rag1. A regulatory connection between Flt3 signaling and E2A function has not been reported. It is interesting to note that Tcfe2a+/− mice have a similar phenotype to FL+/− mice [11]. These parallel findings support a regulatory connection between Flt3 signaling and E2A function and provide an explanation for the deficiency in LHP in FL+/− mice. Although we found that FL deficiency resulted in elevated id1 transcripts, loss of id1 did not restore GMLP or LMPP in FL−/− mice, suggesting alternate molecular mechanisms. Loss of multiple id family members might be necessary to provide rescue given their functional redundancy. We did document increased id2 transcripts in FL+/− LMPP in preliminary studies. Id2 is required for NK cell development [31]. The increase in id2 transcripts we observed in vivo is more variable and could reflect increased frequencies of NK progenitors and not increased id2 transcription upon FL-deficiency. Future studies will address if combined loss of id1 and id2 alters the lymphoid defect in FL−/− mice.
It is well established that FL functions synergistically with various hematopoietic cytokines to promote the expansion of MPPs and CLPs in vitro [32–34]. Thus, FL deficiency might be predicted to alter the proliferation of Flt3+ MPPs in vivo. However, BrdU analysis of residual Flt3+ MPPs in FL−/− mice did not reveal a proliferation defect. This is not necessarily an unexpected finding, given that Flt3+ MPPs are responsive to a number of hematopoietic growth factors that can provide proliferative signals [32, 34]. Unexpectedly, we saw increased percentages of Flt3- MPPs incorporating BrdU in FL−/− mice. This observation suggests that the absence of FL allows a proliferation advantage to Flt3- MPPs to fill a niche that in FL sufficient BM would be occupied by Flt3+ MPPs. Interestingly, close scrutiny of LSK+ cells from tcfe2a−/− mice revealed increased BrdU incorporation in Flt3- LSK+, compared to Flt3+ LSK+ cells [11]. These similar findings provide additional support that deficiency in lymphoid progenitors results in preferential expansion of Flt3- MPPs.
Mcl-1 is a critical regulator of murine lymphopoiesis [28, 35]. Mcl-1 is highly expressed in LSK+ cells and downregulated in CLP [28]. Conditional-deletion of floxed Mcl-1 established the importance of Mcl-1 in the survival of HSC, CLP, CD19+ BCP [28, 35]. Deficiency in FL resulted in decreased Mcl-1 in Flt3lo MPP accompanied by increased Annexin V+ Flt3hi MPP. Our findings suggest that Flt3 signaling initiates a cascade of events in Flt3lo MPP that selectively promotes the survival of cells primed to enter the lymphoid pathway. Failure to signal via Flt3 leads to diminished Mcl-1 expression in Flt3lo MPPs and increased propensity for apoptosis in Flt3hi MPP, resulting in selective deficiencies in lymphoid progenitors. Diminished numbers of Flt3+ MPPs allows expansion of Flt3- MPPs to fill an unoccupied niche. Taken together, these studies provide new insight in to the role of Flt3/FL signaling in regulating the survival of lymphoid progenitors by controlling the size of the niche they occupy. Based on our findings that FL haploinsufficiency impacts the numbers of LHP and BCP in BM, it will be interesting to extend these studies to examine the consequences of FL haploinsufficiency on immune function.
Materials and Methods
Mice
C57Bl/6 (FL+/+) and FL−/− mice were obtained from Taconic Farms (Germantown, NY). RAG1-GFP/+ and Id1−/− mice have been described [36, 37]. FL−/− and Id1−/− mice were bred to generate FL−/− x Id1−/− mice. RAG1-GFP/+ and FL−/− were bred to create RAG1-GFP/+ x FL+/+, RAG1-GFP/+ x FL+/−, and RAG1-GFP/+ x FL−/− mice. C57Bl/6 mice were bred to FL−/− mice to generate FL+/− mice. Age-matched or littermate controls were used for individual experiments, and mice ranged from 4–12 weeks of age. All animals were bred and maintained at the Mayo Clinic animal facility and experiments carried out according to the Institutional Animal Care and Use Committee guidelines.
Genotyping
RAG1-GFP/+ x FL+/+, RAG1-GFP/+ x FL+/−, and RAG1-GFP/+ x FL−/− mice were identified by separate genotyping reactions with three primer sets: RAG1, GFP, and FL. Genotyping was performed on tail DNA using the following primer combinations: forward FL WT, 5′-AGGAACACTGATAGC ATGAG-3′; reverse FL WT, 5′-GAAAGCCAAAGCTGGATGAC-3′; forward FL mutant, 5′-CAGCTCTATGTGGAGGGGTGAGGT-3′; reverse FL mutant, 5′-CAGAAAGCGAAGGAGCAAAG CTG-3′. The WT primers generate a 910 bp product and the mutant primers a 1073 bp product. The wildtype forward primer binds 5′ of the FL coding region and the 3′ primer within the FL coding region. The mutant forward primer binds within the FL genomic locus and the mutant reverse primer binds within the inserted PGK-neo cassette [38]. Tail DNA was amplified using FL WT primers as follows: 95 °C for 5 min, followed by 35 cycles of 95 °C for 30 sec, 61 °C for 45 sec, and 72 °C for 1 min. Using the FL mutant primers, the PCR parameters are: 95 °C for 5 min, followed by 35 cycles of 95 °C for 30 sec, 58 °C for 45 sec, and 72 °C for 1.5 min. Published RAG1 and GFP primers were used to confirm the RAG1 and GFP genotypes [36]. Id1 primer sequences were provided by R. Benezra.
RT-PCR
RNA was isolated from BM using TRIzol and cDNA synthesized using the SuperScript III Reverse Transcriptase kit and random primers. Serial dilutions of cDNA were amplified by semi-quantitative PCR to measure FL transcript levels compared to β-actin. The following parameters were used for amplification: 95°C for 30 sec, 60°C for 30 sec, and 72°C for 45 sec for 30 cycles. FL RT-PCR primers are published [39]. The Gel Doc XR system (Bio Rad, Hercules, CA) and Quantity One software (Bio Rad) was used to analyze and quantify bands. For quantitative PCR analysis, RNA was isolated from sorted GMLP (LSK+ Flt3+ VCAM-1+) and LMPP (LSK+ Flt3+ VCAM-1-) from B6 and FL+/− mice using Qiagen RNeasy Micro kit. RNA was treated with DNase I kit to remove gDNA and cDNA synthesized. Quantitative real time RT-PCR (qRT-PCR) was performed using GMLP and LMPP cDNA and gene-specific primers (Flt3, tcfe2a, Rag1, ebf, Scl, Id1, and GAPDH). The primers used are: Flt3 (F) 5′-CATCCAAGACAACATCTCCT-3′, (R) 5′-CCCTGAAGTCAACGTAGAAG-3′; tcfe2a (F) 5′-CCAGTCTTTTGCATAACCAT-3′, (R) 5′-AGGTCCTTCTTGTCCTCTTC-3′; rag1 (F) 5′-CTGCAGACATTCTAGCACTC-3′, (R) 5′-AACTGAAGCTCAGGGTAGAC-3′; id1 (F) 5′-CTGAAC GGCGAGATCAGT-3′, (R) 5′-GCCTCAGCGACACAAGAT-3′; scl (F) 5′-ATGTTCACCAACAACAA CC-3′, (R) 5′-GTGTGAGGACCATCAGAAAT-3′. Ebf1 and GAPDH are published [5, 39]. GAPDH was the reference for all qRT-PCR experiments. Comparisons were made using the 2−ΔΔCT or ΔCT method [40].
Flow cytometry
BM was harvested and stained with combinations of the following mAbs: B220 (APC, PE), Flt3 PE, CD27 PE, bio IL-7R, bio VCAM-1, Sca-1 (bio, PeCy5.5, FITC, PerCp-Cy5.5), CD43 PE, c-kit (APC, PeCy7), and Mac-1 PE. Incubation with streptavidin conjugates (SA-APC, SA-PerCP, SA-780) was used to visualize biotinylated antibodies. All antibodies were obtained from eBioSciences or BD Pharmingen. In experiments where lineage-positive cells were excluded, a PeCy7 antibody cocktail was used: B220, CD3ε, Mac-1, Gr-1, and Ter119. For Annexin V staining, BM stained with primary antibodies was incubated with Annexin V FITC per manufacturers’ instructions (eBioSciences). For intracellular Mcl-1 staining, BM cells stained with primary antibodies were fixed and permeabilized with the Cytofix/Cytoperm kit (BD Biosciences), then incubated with rabbit anti-mouse Mcl-1 (Rockland Immunochemicals), followed by incubation with goat anti-rabbit IgG FITC (Southern Biotech). For cell sorting, positive selection was used to enrich c-kit+ cells from BM. BM was incubated with CD117 MACS microbeads and separated using VarioMACS or OctoMACS cell separator. CD117+ cells were stained with mAbs distinguish GMLP (LSK+ Flt3+ VCAM-1+) and LMPP (LSK+ Flt3+ VCAM-1−) and sorted on the FACS Aria (Becton Dickinson) for limiting dilution analysis and qRT-PCR. Surface marker expression was visualized with the FACS Canto, LSRII, or Calibur cytometers (Becton Dickinson). Data was analyzed with FloJo software (Treestar, Inc., San Carlos, CA), and mononuclear and doublet exclusion gates were used to initially enrich for cells of interest.
Limiting dilution analysis
Varying numbers of GMLP from WT and FL+/− mice were sorted directly into 96-well plates in X-VIVO 15 media (Biowhittaker/Lonza, Walkersville, MD) with 10% BSA (Stem Cell Technologies), standard supplements, and the following cytokines: 10 ng/mL IL-7 + 10 ng/mL SCF, +/− 100, 50, or 10 ng/mL of FL (Peprotech). On day 2, 100 uL fresh media was added, and every 3 days onward (days 5, 8, and 11) 100 uL of media was removed and 100 uL fresh media added. On day 14, cells were harvested and stained with antibodies to B220 and MAC1 to determine B220+ progeny. The number of input cells at 37% negative line was used to determine precursor frequency [41].
BrdU analysis
FL+/+, FL+/−, and FL−/− mice were injected intraperitoneally with 2 mg BrdU twelve hours before BM harvest. Following positive selection using CD117 microbeads, CD117+ enriched cells were stained with c-kit APC, bio Sca-1, and Flt3 PE followed by incubation with SA-780 to visualize biotinylated antibodies. Following surface staining, BrdU intracellular staining was performed using FITC BrdU Flow Kit (BD Biosciences). Surface and intracellular marker expression was visualized with the Canto (Becton Dickinson) and analyzed with FloJo software (Treestar, Inc., San Carlos, CA).
ELISA
The serum concentration of FL was calculated by ELISA on serum harvested from FL+/+, FL+/−, and FL−/− mice using mouse Flt3 Ligand Quantikine ELISA Kit (R&D Systems, Minneapolis, MN) per kit instructions. The sensitivity for FL was 5pg/mL.
Statistical analysis
Statistics were performed using the Students T-test. Differences were significant at p ≤ 0.05. All data reflect mean +/− standard deviation (SD) with the exception of Fig. 4 where the error bars represent mean +/− standard error of the mean (SEM).
Acknowledgments
The authors thank the Mayo Clinic Flow Cytometry Core Facility for excellent technical assistance in cell sorting. RAG1-GFP/+ mice were provided by Drs. N. Sakaguchi and P. Kincade. Id1−/− mice were provided by R. Benezra. We thank Virginia Shapiro for her helpful comments in preparing the manuscript. K.L.M. is supported by the Concern Foundation, the Fraternal Order of Eagles Cancer Research Fund, and NIH grant RO1HL096108 from the National Heart, Lung, and Blood Institute.
Abbreviations
- FL
Flt3 ligand
- MPP
multipotential progenitor
- LHP
lymphohematopoietic progenitor
- GMLP
granulocyte-macrophage-lymphoid progenitor
- LMPP
lymphoid-biased multipotent progenitor
- CLP
common lymphoid progenitor
- BCP
B cell precursor
- BrdU
bromodeoxyuridine
Footnotes
Conflict of Interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Okada S, Nakauchi H, Nagayoshi K, Nishikawa S, Miura Y, Suda T. In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells. Blood. 1992;80:3044–3050. [PubMed] [Google Scholar]
- 2.Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M, Jacobsen SE. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Medina KL, Garrett KP, Thompson LF, Rossi MI, Payne KJ, Kincade PW. Identification of very early lymphoid precursors in bone marrow and their regulation by estrogen. Nat Immunol. 2001;2:718–724. doi: 10.1038/90659. [DOI] [PubMed] [Google Scholar]
- 4.Igarashi H, Kuwata N, Kiyota K, Sumita K, Suda T, Ono S, Bauer SR, Sakaguchi N. Localization of recombination activating gene 1/green fluorescent protein (RAG1/GFP) expression in secondary lymphoid organs after immunization with T-dependent antigens in rag1/gfp knockin mice. Blood. 2001;97:2680–2687. doi: 10.1182/blood.v97.9.2680. [DOI] [PubMed] [Google Scholar]
- 5.Lai AY, Kondo M. Asymmetrical lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. J Exp Med. 2006;203:1867–1873. doi: 10.1084/jem.20060697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sitnicka E, Bryder D, Theilgaard-Monch K, Buza-Vidas N, Adolfsson J, Jacobsen SE. Key role of flt3 ligand in regulation of the common lymphoid progenitor but not in maintenance of the hematopoietic stem cell pool. Immunity. 2002;17:463–472. doi: 10.1016/s1074-7613(02)00419-3. [DOI] [PubMed] [Google Scholar]
- 7.Mackarehtschian K, Hardin JD, Moore KA, Boast S, Goff SP, Lemischka IR. Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity. 1995;3:147–161. doi: 10.1016/1074-7613(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 8.McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, Maliszewski CR, Lynch DH, Smith J, Pulendran B, Roux ER, Teepe M, Lyman SD, Peschon JJ. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–3497. [PubMed] [Google Scholar]
- 9.Sitnicka E, Buza-Vidas N, Ahlenius H, Cilio CM, Gekas C, Nygren JM, Mansson R, Cheng M, Jensen CT, Svensson M, Leandersson K, Agace WW, Sigvardsson M, Jacobsen SE. Critical role of FLT3 ligand in IL-7 receptor independent T lymphopoiesis and regulation of lymphoid-primed multipotent progenitors. Blood. 2007;110:2955–2964. doi: 10.1182/blood-2006-10-054726. [DOI] [PubMed] [Google Scholar]
- 10.Igarashi H, Gregory SC, Yokota T, Sakaguchi N, Kincade PW. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity. 2002;17:117–130. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 11.Dias S, Mansson R, Gurbuxani S, Sigvardsson M, Kee BL. E2A proteins promote development of lymphoid-primed multipotent progenitors. Immunity. 2008;29:217–227. doi: 10.1016/j.immuni.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massari ME, Murre C. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veiby OP, Jacobsen FW, Cui L, Lyman SD, Jacobsen SE. The flt3 ligand promotes the survival of primitive hemopoietic progenitor cells with myeloid as well as B lymphoid potential. Suppression of apoptosis and counteraction by TNF-alpha and TGF-beta. J Immunol. 1996;157:2953–2960. [PubMed] [Google Scholar]
- 14.Ceredig R, Rauch M, Balciunaite G, Rolink AG. Increasing Flt3L availability alters composition of a novel bone marrow lymphoid progenitor compartment. Blood. 2006;108:1216–1222. doi: 10.1182/blood-2005-10-006643. [DOI] [PubMed] [Google Scholar]
- 15.Wils EJ, Braakman E, Verjans GM, Rombouts EJ, Broers AE, Niesters HG, Wagemaker G, Staal FJ, Lowenberg B, Spits H, Cornelissen JJ. Flt3 ligand expands lymphoid progenitors prior to recovery of thymopoiesis and accelerates T cell reconstitution after bone marrow transplantation. J Immunol. 2007;178:3551–3557. doi: 10.4049/jimmunol.178.6.3551. [DOI] [PubMed] [Google Scholar]
- 16.Zeigler FC, Bennett BD, Jordan CT, Spencer SD, Baumhueter S, Carroll KJ, Hooley J, Bauer K, Matthews W. Cellular and molecular characterization of the role of the flk-2/flt-3 receptor tyrosine kinase in hematopoietic stem cells. Blood. 1994;84:2422–2430. [PubMed] [Google Scholar]
- 17.Adolfsson J, Borge OJ, Bryder D, Theilgaard-Monch K, Astrand-Grundstrom I, Sitnicka E, Sasaki Y, Jacobsen SE. Upregulation of Flt3 expression within the bone marrow Lin(−)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz BA, Sambandam A, Maillard I, Harman BC, Love PE, Bhandoola A. Selective thymus settling regulated by cytokine and chemokine receptors. J Immunol. 2007;178:2008–2017. doi: 10.4049/jimmunol.178.4.2008. [DOI] [PubMed] [Google Scholar]
- 19.Wiesmann A, Phillips RL, Mojica M, Pierce LJ, Searles AE, Spangrude GJ, Lemischka I. Expression of CD27 on murine hematopoietic stem and progenitor cells. Immunity. 2000;12:193–199. doi: 10.1016/s1074-7613(00)80172-7. [DOI] [PubMed] [Google Scholar]
- 20.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 21.Kikuchi K, Lai AY, Hsu CL, Kondo M. IL-7 receptor signaling is necessary for stage transition in adult B cell development through up-regulation of EBF. J Exp Med. 2005;201:1197–1203. doi: 10.1084/jem.20050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karsunky H, Inlay MA, Serwold T, Bhattacharya D, Weissman IL. Flk2+ common lymphoid progenitors possess equivalent differentiation potential for the B and T lineages. Blood. 2008;111:5562–5570. doi: 10.1182/blood-2007-11-126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmes ML, Carotta S, Corcoran LM, Nutt SL. Repression of Flt3 by Pax5 is crucial for B-cell lineage commitment. Genes Dev. 2006;20:933–938. doi: 10.1101/gad.1396206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker SJ, Rane SG, Reddy EP. Hematopoietic cytokine receptor signaling. Oncogene. 2007;26:6724–6737. doi: 10.1038/sj.onc.1210757. [DOI] [PubMed] [Google Scholar]
- 25.Parcells BW, Ikeda AK, Simms-Waldrip T, Moore TB, Sakamoto KM. FMS-like tyrosine kinase 3 in normal hematopoiesis and acute myeloid leukemia. Stem Cells. 2006;24:1174–1184. doi: 10.1634/stemcells.2005-0519. [DOI] [PubMed] [Google Scholar]
- 26.Wodnar-Filipowicz A. Flt3 ligand: role in control of hematopoietic and immune functions of the bone marrow. News Physiol Sci. 2003;18:247–251. doi: 10.1152/nips.01452.2003. [DOI] [PubMed] [Google Scholar]
- 27.Kikushige Y, Yoshimoto G, Miyamoto T, Iino T, Mori Y, Iwasaki H, Niiro H, Takenaka K, Nagafuji K, Harada M, Ishikawa F, Akashi K. Human Flt3 is expressed at the hematopoietic stem cell and the granulocyte/macrophage progenitor stages to maintain cell survival. J Immunol. 2008;180:7358–7367. doi: 10.4049/jimmunol.180.11.7358. [DOI] [PubMed] [Google Scholar]
- 28.Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K, Korsmeyer SJ. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307:1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- 29.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 30.Bosco N, Ceredig R, Rolink A. Transient decrease in interleukin-7 availability arrests B lymphopoiesis during pregnancy. Eur J Immunol. 2008;38:381–390. doi: 10.1002/eji.200737665. [DOI] [PubMed] [Google Scholar]
- 31.Boos MD, Yokota Y, Eberl G, Kee BL. Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J Exp Med. 2007;204:1119–1130. doi: 10.1084/jem.20061959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banu N, Deng B, Lyman SD, Avraham H. Modulation of haematopoietic progenitor development by FLT-3 ligand. Cytokine. 1999;11:679–688. doi: 10.1006/cyto.1998.0477. [DOI] [PubMed] [Google Scholar]
- 33.Lyman SD, Jacobsen SE. c-kit ligand and Flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood. 1998;91:1101–1134. [PubMed] [Google Scholar]
- 34.Yonemura Y, Ku H, Lyman SD, Ogawa M. In vitro expansion of hematopoietic progenitors and maintenance of stem cells: comparison between FLT3/FLK-2 ligand and KIT ligand. Blood. 1997;89:1915–1921. [PubMed] [Google Scholar]
- 35.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 36.Kuwata N, Igarashi H, Ohmura T, Aizawa S, Sakaguchi N. Cutting edge: absence of expression of RAG1 in peritoneal B-1 cells detected by knocking into RAG1 locus with green fluorescent protein gene. J Immunol. 1999;163:6355–6359. [PubMed] [Google Scholar]
- 37.Jankovic V, Ciarrocchi A, Boccuni P, DeBlasio T, Benezra R, Nimer SD. Id1 restrains myeloid commitment, maintaining the self-renewal capacity of hematopoietic stem cells. Proc Natl Acad Sci U S A. 2007;104:1260–1265. doi: 10.1073/pnas.0607894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colucci F, Samson SI, DeKoter RP, Lantz O, Singh H, Di Santo JP. Differential requirement for the transcription factor PU.1 in the generation of natural killer cells versus B and T cells. Blood. 2001;97:2625–2632. doi: 10.1182/blood.v97.9.2625. [DOI] [PubMed] [Google Scholar]
- 39.Medina KL, Pongubala JM, Reddy KL, Lancki DW, Dekoter R, Kieslinger M, Grosschedl R, Singh H. Assembling a gene regulatory network for specification of the B cell fate. Dev Cell. 2004;7:607–617. doi: 10.1016/j.devcel.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 41.Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981;126:1614–1619. [PubMed] [Google Scholar]