Abstract
The cytokines generated locally in response to infection play an important role in CD8 T cell trafficking, survival, and effector function, rendering these signals prime candidates for immune intervention. Here we show that localized increases in the homeostatic cytokine IL-15 induced by influenza infection is responsible for the migration of CD8 effector T cells (Teff) to the site of infection. Moreover, intranasal delivery of IL-15/ IL-15Rα soluble complexes (IL-15c) specifically restores the frequency of Teff lost in the lung airways of IL-15 deficient animals after influenza infection. Exogenous IL-15c quantitatively augments the respiratory CD8 T cell response, and continued administration of IL-15c throughout the contraction phase of the anti-influenza CD8 T cell response magnifies the resultant CD8 T cell memory generated in situ. This treatment extends the ability of these cells to protect against heterologous infection, immunity that typically depreciates over time. Overall, our studies describe a new function for IL-15 in attracting effector CD8 T cells to the lung airways and suggest that adjuvanting IL-15 could be used to prolong anti-influenza CD8 T cell responses at mucosal surfaces to facilitate pathogen elimination.
Introduction
Current influenza vaccines rely on the induction of a neutralizing antibody response to viral coat proteins that are subject to variation from antigenic drift and shift (1). An alternate vaccination strategy is to immunize against conserved, internal viral proteins recognized by CD8 T cells, a strategy that confers protection against multiple serotypes, or heterosubtypic immunity (2–4). Such a “universal” vaccine could provide cross protection against the emerging and imminent threats of avian H5N1 and swine-origin H1N1 pandemics. However, despite such promise in generating a cross-protective vaccine, the number of memory cells (Tmem) in the lung airways that differentiate from local effector cell precursors declines over time, eventually plateauing ~3 months post infection (5). Curiously, protective heterosubtypic immunity declines concomitantly, despite the large reservoir of Tmem in the spleen that continues to migrate to the lung airways and maintain a local steady-state pool of Tmem (3, 6). Overall, these data suggest that the Teff that gain access to the airways early after infection and differentiate to Tmem in situ are responsible for the limited heterosubtypic immunity. Thus, understanding how to augment the initial seeding pool of Teff in the lung airways may represent a unique methodology to increase the resultant pool of Tmem cells that develops in the respiratory tract and to coordinately prolong protection following a challenge infection.
IL-15 is a common gamma chain cytokine sharing overlapping signaling and biological properties with IL-2 as a result of their mutual usage of the IL-2/15β and common gamma chain (γc) receptor subunits (7–8). Cells co-expressing IL-15 with IL-15Rα on their surfaces present the cytokine in trans to responsive IL-2/15β/γc expressing lymphocytes, a process referred to as transpresentation (9). While production of IL-2 is confined to periods of lymphocyte activation, the constitutive expression of IL-15 maintains the homeostatic proliferation of lymphocytes, most notably memory CD8 T cells, in the steady-state through the sustained expression of bcl-2 (10–13). Pathogenic triggers such as type I interferons (IFNs), however, can induce the expression of IL-15, and Teff upregulate expression of IL-2/15β following activation (12, 14–15). In addition, systemic administration of IL-15 or soluble IL-15/IL-15Rα complexes (IL-15c) during the contraction phase of the immune response against Listeria monocytogenes and Mycobacterium bovis prolongs Teff survival and results in the preferential accumulation of short-lived KLRG-1hi Ag-specific Teff (16). Taken together, these observations suggest that infection-induced alterations in IL-15 expression could modulate regional CD8 Teff responses.
Recent studies indeed demonstrated that a subset of DCs in the lung transpresent IL-15 to Teff that have migrated to the respiratory tract following influenza infection (17). Removal of IL-15 producing DCs from the respiratory tract or IL-15 blockade decreases the frequency of antigen (Ag)-specific CD8 Teff recovered from the lung. While the attrition of Teff in the lung is accelerated in the absence of IL-15, it remains unclear whether this is due solely to the loss of a pertinent survival signal or also the impaired ability of Teff to traffic to the lung airways, as IL-15 is chemotactic in certain inflammatory milieus (18–19). In this article, we demonstrate that influenza infection potentiates IL-15 levels in the respiratory tract that drives the migration of Teff into the lung airways. Moreover, the therapeutic provision of IL-15 during this effector phase of the immune response translates into a greater proportion of locally derived Teff that differentiate into Tmem in situ. Overall, our studies describe a novel function for IL-15 in T cell migration in which IL-15c can be used to augment respiratory CD8 T cell effector responses in order to extend the duration of heterosubtypic immunity and provide long-term immunological protection to subsequent influenza virus infections.
Materials and Methods
Mice, viruses, and infection
C57BL/6 mice were purchased from NCI or Taconic, and age and sex-matched IL-15−/− mice were generously provided by Dr. Leo Lefrançois (University of Connecticut, Farmington, CT) or obtained from Taconic. Influenza viruses A/HK-x31(x31, H3N2) and A/PR/8 (PR8, H1N1) were generously provided by Dr. S. Mark Tompkins (University of Georgia, Athens, GA). The recombinant x31-ova expressing the CD8 H2-Kb restricted SIINFEKL epitope was generously provided by Dr. Peter Dougherty (St. Jude Children’s Research Hospital, Memphis, TN) (20). Animals were infected with either 103 pfu of x31 or 5×103 pfu of PR8 intranasally in 50 µl PBS.
Quantitative RT-PCR
Total RNA was purified from tissues of naïve or 103 pfu x31-infected C57BL/6 mice using the RNeasy Plus Mini Kit (Qiagen). Reverse transcriptions were primed with random primers and performed using the High Capacity cDNA Reverse Transcription Kit from ABI (Foster City, CA). Quantitative PCR assays were done using the ABI TaqMan Gene Expression Master Mix from ABI 7500 Real Time PCR System. Only ratios with a SE<0.2 log (95% confidence limits) were considered for determination of induction levels. Quantitative real-time RT-PCR was performed using IL15-FAM (#mm00434210_m1) and 18s-VIC (#4319413E) assays (ABI). Thermal cycling conditions were 30 min at 48°C, 10 min at 95°C, and 40 cycles of denaturation (95°C for 15sec) and annealing (60°C for 60 sec). Samples were analyzed in triplicate, normalized against 18s and expressed relative to mock infected animals. The results are expressed as fold induction of gene expression (RQ) using the ΔCt method (21).
IL-15c ELISA
The Ready-Set-Go Mouse IL-15/IL-15R Complex ELISA kit was purchased from eBioscience. Whole blood was collected from IL-15−/−, naïve, and influenza-infected animals at various time points post influenza infection, allowed to clot for 1 hour at RT, and clarified via centrifugation at 3,000 g for 10 min. Lungs from the same animals were homogenized in 0.5 ml of PBS and the lysate was used for analysis. Supernatants (100 µl) of samples were used in duplicate. ELISA plates were incubated with samples at RT for 2.5 hours. Plates were read with a Molecular Diagnostics Vmax microplate reader (Molecular Devices Corporation, Sunnydale, CA) at 450 nm and analyzed with Softmax software (Molecular Designs). The standard curve had a minimal regression coefficient of 0.99.
Tissue preparation and flow cytometry
Single-cell suspensions were obtained from spleens and lymph nodes by passing homogenized organs through cell strainers. Spleens were depleted of erythrocytes using Tris-buffered Ammonium Chloride. Peripheral blood was collected from the retro-orbital sinus and also depleted of erythrocytes. Lung airway-resident cells were harvested following intratrachial introduction and recovery of 1ml PBS 3–5×. Following BAL recovery, lymphocytes were isolated from the perfused lung parenchyma. Following perfusion with ~25 mL PBS/heparin, lungs were excised, minced, and incubated at 37°C with 1.25 mM EDTA for 30 min followed by 6 mg/mL collagenase for 60 min at 37°C. After passage through cell strainers, cells were resuspended in 44% Percoll underlayed with 67%, centrifuged, and lymphocytes at the interface were collected.
The influenza NP MHC class I (H-2D(b)/ASNENMETM) tetramer was generated by the NIAID Tetramer Facility (Emory University). Tetramer staining was conducted at RT for 1 hr in conjunction with other mAbs from eBioscience (PerCP/Cy5.5-conjugated αCD4 or αCD8, and Pe/Cy7-conjugated αCD44 or αCD45.1), BD Pharmingen (Pe-Cy7-conjugated αCD11a and APC/Cy7-conjugated αCD8), or Caltag (FITC-conjugated αCD122). Stained cells were analyzed using a BD LSR II digital flow cytometer and BD FACs Diva software.
Enrichment for NP-specific naïve CD8 T cell precursors
Single cell suspensions from spleens and all visible lymph nodes (cervical, axillary, mesenteric, and inguinal) were depleted of erythrocytes, washed, and stained with both PE- and APC-labeled influenza-NP specific MHC class I (H-2D(b)) tetramers, anti-CD8-PerCP, and Fc block for 1 hr RT in 1 mL FACS buffer. Cells were subsequently washed, resuspended in 500 µl MACS buffer (PBS with 0.1% NaN3, 0.5% BSA, and 2mM EDTA, degassed), and labeled with 50 µl anti-PE microbeads (Miltenyi Biotec) for 20 minutes at 4°C. Cells were again washed and passed over a magnetized LS column (Miltenyi Biotech). Bound cells were eluted from the columns and stained with a PeCy7-labeled dump gate (including anti-CD4, 19, and CD11c). The frequency and number of NP-specific cells in each cohort of animals was determined by gating on CD8+, dump− lymphocytes.
Adoptive transfers and migration and proliferation assays
CD8 T cells were enriched to >88% purity from spleens or lung tissue by negative enrichment per the manufacturer’s instructions (Dynal). For in vitro migration assays, cells from the indicated tissues were placed in the top insert of a 0.4 µm chemotaxis Transwell (Fisher Scientific). The bottom chamber contained either warm media alone or supplemented with 100 ng/ml recombinant murine IL-15/IL-15Rα Fc-chimeric complexes (R&D Systems). Percent migration was calculated as # NP-tet+ CD8 T cells in the bottom chamber / # NP-tet+ CD8 T cells in the input sample. For in vivo migration assays, IL-15c consisting of 1.5 µg recombinant murine IL-15 and 7 µg IL-15Rα Fc-chimera (R&D Systems) were generated by incubation at 37°C for 20 min followed by incubation at 4°C for 10 min and delivered i.n. in 72.5 µl PBS. Similarly, IL-2c and IL-7c consisting of 1.5 µg recombinant murine IL-2 (ebioscience) or IL-7 (Peprotech) and 7 µg mAb (R&D Systems) were generated by incubation at 37°C for 20 min followed by incubation at 4°C for 10 min and delivered i.n. in 72.5 µl PBS. IL-15c were also used to treat animals throughout the contraction phase of the anti-influenza response and were delivered i.n. in 36.25 µl PBS every other day for 10 days. To measure cell survival and proliferation, animals were pulsed i.p. with 200 µl of 10 mg/ml of BrdU (Sigma). Cells isolated from these animals 12 hr later were stained with 20 µl 7-AAD (BD Pharmingen) at 4°C for 20 min, surface stained as previously described, and intracellularly stained with a FITC-labeled αBrdU mAb (BD Pharmingen).
Plaque assays
Plaque assays were performed as previously described (22). Briefly, lungs from x31-immune WT and IL-15−/− mice challenged with PR8 were lysed at the indicated times in 1ml 1× MEM+1µg/ml TPCK-treated trypsin. Confluent monolayers of MDCK cells were incubated with 10-fold serially diluted 10% homogenate for 1 hr at 37°C. The inoculums were removed and cells were washed with PBS. Monolayers were then overlaid with MEM containing 1.2% Avicel microcystalline cellulose (FMC BioPolymer, Philadelphia, PA) (22), 0.04M HEPES, 0.02 mM L-glutamine, 0.15% NaHCO3 (w/v), and 1µg/ml TPCK-trypsin. 72 hrs post infection, monolayers were fixed with cold methanol: acetone (60%:40%) and stained with crystal violet.
Statistics
Where appropriate, an unpaired two-tailed student’s T test was applied using Prism Graphpad software. P values are indicated in the figure legend where statistical significance was found. For multiple comparisons, an analysis of variance was applied with a Tukey’s post hoc analysis using Prism Graphpad software. P values less than 0.05 were considered significant.
Results
Infection with influenza virus induces localized IL-15 expression in the respiratory tract
IL-15 is constitutively expressed systemically by DCs and macrophages but can also be expressed in mucosal tissues by epithelial cells (10, 23–24). Moreover, cellular expression of IL-15 is regulated by various pathogenic triggers (15). Namely, the IL-15 promoter contains a type I IFN regulatory element (14–15), and IL-15 expression is increased in response to type I IFNs (12) which are abundant following influenza infection (25). Indeed, influenza infection has been shown to induce the expression and transpresentation of IL-15 in the lungs at day 6 p.i. (16); however, we wished to more carefully characterize the kinetics of IL-15 expression following respiratory infection to better define how pathogen-induced IL-15 impacts local CD8 T cell responses. Since IL-15 protein has been historically difficult to detect in biological solutions, we first monitored IL-15 mRNA expression in the lung airways (via broncheoalveolar lavage [BAL]), lung parenchyma, lung-draining mediastinal lymph nodes (MdLN), and spleens of C57BL/6 mice following intranasal (i.n.) infection with influenza A/Hong Kong x31(x31). By 3 days post infection (p.i.), there was a four-fold induction in the relative expression of IL-15 in the lung airways of infected animals when compared to naïve, i.e. mock-infected, animals, and IL-15 mRNA levels remained elevated until day 7 p.i. (Fig. 1). While IL-15 transcription in the airways began to drop by day 10 p.i., it remained threefold higher than the levels in naïve mice. Expression of IL-15 also increased in the lung parenchyma, MdLN, and spleen following infection, albeit to a much slighter degree. Additionally, a newly developed ELISA which specifically detects soluble IL-15/IL-15Rα complexes (IL-15c) confirmed that expression of IL-15c in the lung lysate was greatly enhanced and quantitatively much greater (than the serum) of influenza-infected animals on day 3 p.i. when compared to naïve mice (Supplemental Fig. 1). Therefore, infection with influenza virus initiates an inflammatory cascade which results in a local increase in the expression of IL-15 suggesting that this cytokine may specifically regulate anti-influenza CD8 Teff responses in the lung airways.
Figure 1. Influenza infection induces the local expression of IL-15 in the lung airways.
Gene expression levels were quantified from BAL, lung tissue, spleen and MdLN by RT-qPCR at the indicated time points post infection with x31, and are expressed as relative expression above a baseline in naïve animals normalized to 1 using the ΔCt method. Values are represented as mean ± SD (n=3; *p < 0.05 by ANOVA with Tukey’s post hoc analysis) compared with mock-infected mice.
IL-15 is responsible for the early accumulation of anti-influenza Teff in the lung airways
IL-15 prolongs the survival of activated CD8 Teff during the contraction phase of the immune response to systemic viral infection, and systemically administered IL-15 can specifically restore the numbers of Teff lost in IL-15−/− mice (16, 26–28). Recent data also suggests that IL-15 modulates local respiratory CD8 Teff responses via sustained survival signals provided by lung-resident DCs (17). However, given that transient augmentation of IL-15 mRNA expression immediately precedes the initial influx of Teff to the lung airways (Fig 1; Teff first detectable ~5.5 days p.i. (data not shown)), the documented chemotactic properties of IL-15, and the fact that lung parenchymal cells (including influenza-infected lung epithelial cells) can also express IL-15 (29), we hypothesized that in addition to supporting the survival anti-influenza CD8Teff, IL-15 could also participate in the recruitment of the Teff to the site of infection. To that end, IL-15−/− and WT animals were infected i.n. with x31, and the kinetics of the CD8 T cell response against influenza nucleoprotein (NP) was assessed using MHC class I tetramers on lymphocytes isolated from the BAL, lung parenchyma, spleen, and MdLN. Since IL-15−/− animals have half the total number of CD8 T cells as WT animals (11, 30), and correspondingly half the number of NP-specific precursors as wild type mice (but the same frequency) (Supplemental Fig. 2A–C), the anti-NP CD8 T cell response is expressed as a percentage of total CD8 T cells to accurately compare rates of expansion.
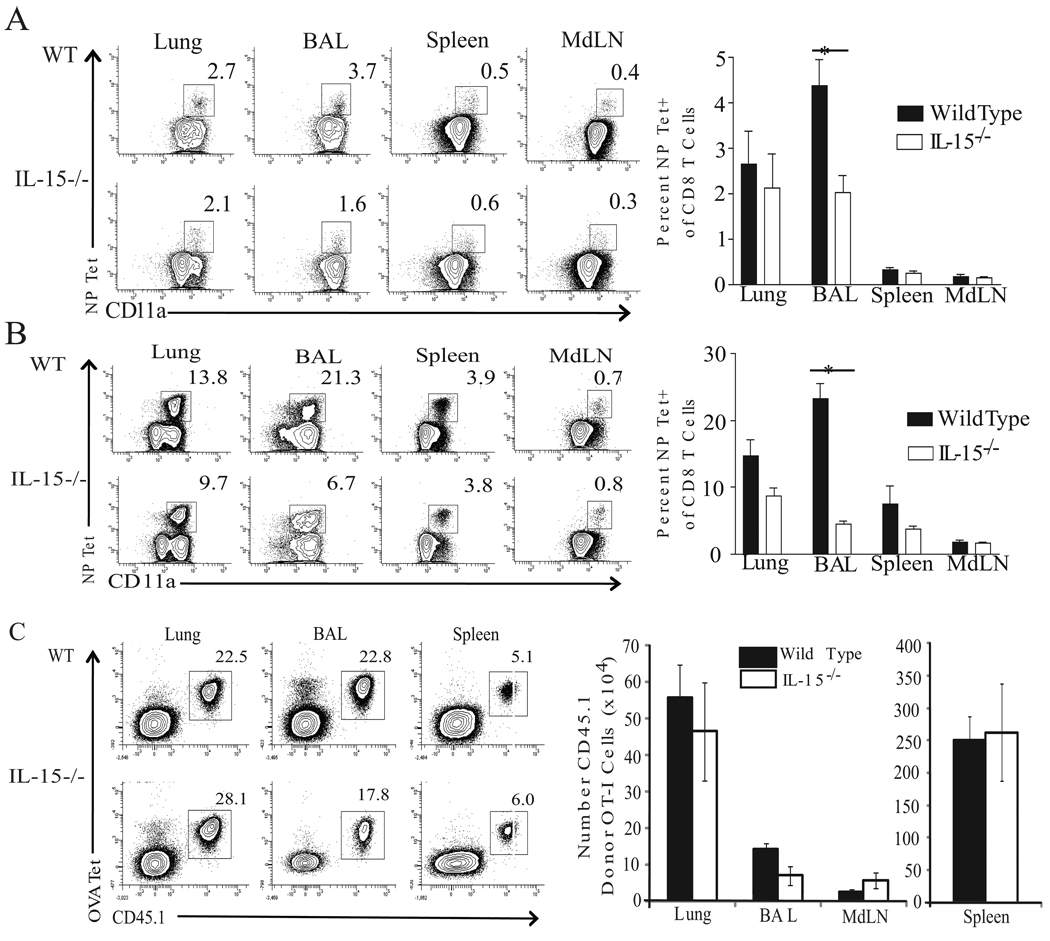
Interestingly, the lung airways of IL-15−/− mice harbored only half the frequency of influenza-specific Teff at day 7 p.i., (Fig. 2A) and only 25% at day 12 p.i. (Fig. 2B). This reduction in the frequency of NP Tet+ CD8 T cells was observed as early as day 6 (~40%) but resolved by day 15 p.i. (data not shown). Moreover, when the total number of NP-specific cells recovered from the airways of IL-15−/− at d6.5 p.i. was assessed, the loss of NP-specific Teff in IL-15−/− mice was greater than the 50% numerical reduction resulting from their reduced NP-specific precursor number (Supplemental Fig. 2D). Only the CD8 and NK cell pools were numerically deficient in the BAL from infected IL-15−/− mice, and the numbers of B220+ B cells and populations of CD11b and CD11c+ cells were unaffected (data not shown). Furthermore, these deficiencies in CD8 Teff accumulation were specific to the lymphocytes recovered from the BAL, as the percentage of influenza-specific Teff in the lymphoid tissues of IL-15−/− mice was not compromised (Fig. 2A–B). The frequency of NP-specific CD8 T cells was also reduced in the lung parenchyma of IL-15−/− animals at day 12 p.i., though not to significant levels. Thus, an IL-15 deficiency resulted in a specific reduction in the frequency of influenza-specific CD8 Teff at the site of infection.
Figure 2. An IL-15 deficiency results in a reduced frequency of influenza-specific effector CD8 T cells in the lung airways.
Lymphocytes from the indicated tissues were isolated and analyzed for tetramer reactivity. Representative flow plots for WT and IL-15−/− animals are shown for day 7 (A) and day 12 (B) p.i. with x31 i.n.. The mean percent tetramer positive among CD8 T cells for WT (shaded bars) and IL-15−/− (open bars) on day 7 p.i. (A) is represented ± SEM (n=3 mice/group; *p=0.0282) and on day 12 p.i. (B) ± SEM (n=3 mice/group; *p=0.0011). Data are representative of two independent experiments. (C) 500 naïve CD45.1+ OT-I CD8 T cells were adoptively transferred into WT or IL-15−/− CD45.2+ recipients subsequently infected with x31-ova. Representative flow plots from the BAL, lung, and spleen are shown (left). The mean number of donor OT-I CD8 Teff isolated from indicated tissues at day 12 p.i. is plotted ± SEM (n=4–5 mice/group) on right. Data are representative of three independent experiments.
In order to confirm our finding that the absence of IL-15 selectively reduces influenza-specific CD8 Teff in the lung airways with equal Ag-specific precursors, we adoptively transferred 500 ovalbumin (ova)-specific CD8 T cells from congenic (CD45.1+) OT-I mice into WT and IL-15−/− CD45.2+ mice. Recipient animals were subsequently infected with x31-ova, and on day 12 p.i., lymphocytes were isolated from the BAL, lung, and lymphoid tissues, and the level of donor OT-I expansion was calculated. The quantity of OT-I cells recovered from the spleen, MdLN, and lung parenchyma was similar between WT and IL-15−/− recipients (Fig. 2C). We did observe, however, a 45% reduction in the number of donor OT-I cells isolated from the BAL of IL-15−/− mice. These data confirm our findings that the absence of IL-15 results in a tissue-specific reduction of Ag-specific CD8 Teff in the lung airways of influenza-infected animals.
Delivery of exogenous IL-15c intranasally enhances the accumulation of influenza-specific CD8 Teff at the site of infection
While intravenous administration of γc cytokines can affect CD8 T cell responses systemically, it is unclear whether such cytokine therapies can be modified to spatially limit their function. Since an IL-15 deficiency resulted in the selective reduction of CD8 Teff in the respiratory tract, we sought to determine whether administration of exogenous IL-15 to the lung airways could rescue this defect. To that end, we intranasally delivered either PBS or IL-15/IL-15Rα complexes (IL-15c) to WT or IL-15−/− animals 12 days p.i. with x31. Delivery of cytokine/receptor complexes increases the cytokine’s potency in vivo presumably by increasing its half-life (31–32). Twelve hours post treatment with IL-15c, cells from the BAL were collected and analyzed for tetramer reactivity. As shown previously, the frequency of NP Tet+ CD8 T cells isolated from the lung airways of IL15−/− mice was reduced by 50% compared to WT animals; however, this percentage was restored to WT levels when exogenous IL-15c was administered to the respiratory tract of these mice (Fig. 3A).
Figure 3. Exogenous IL-15 results in the accumulaton and enhanced survival of influenza-specific CD8 Teff at the site of infection.
(A) Twelve days p.i. with x31, WT or IL-15−/− received PBS or IL-15/IL-5Rα complexes (IL-15c) as indicated below horizontal line. BAL was collected 12 hrs post treatment, and isolated lymphocytes were analyzed for tetramer reactivity. The mean percent NP-tetramer positive among CD8 T cells is represented ± SEM (n=4 mice/group; *p=0.0291). Data are representative of two independent experiments. (B) 6.5 or (C)12 days following x31 infection, animals received either PBS or IL-15c. 12 hours later, BAL were analyzed for CD4, CD8 and CD44 expression and tetramer reactivity. Mean cell number is plotted ± SEM (n=3 mice/group; *p=0.0024) in corresponding right panels. Data are representative of 3 independent experiments. (D) 40 days following x31 infection, animals received either PBS or L-15c. 12 hours later, BAL were analyzed for CD4, CD8 and CD44 expression and tetramer reactivity. Mean cell number is plotted ± SEM. (E) 12 days following x31 infection, animals received PBS (black bar), IL-15c (open bar), IL-7/anti-IL-7 mAb complexes (IL-7c; darkly shaded bar), or IL-2/anti-IL-2 mAb complexes (lightly shaded bar) i.n.. 12 hours later, BAL were analyzed tetramer reactivity. Mean cell number is plotted ± SEM (n-4 mice/group; *p=0.0400). Data are representative of two independent experiments. (F–G) At d 12 p.i., WT mice were pulsed with 2 mg of BrdU i.p. and simultaneously received either PBS, IL-15c, IL-7c, or IL-2c i.n.. Twelve hours later, BAL was collected and stained with 7-AAD and subsequently intracellularly stained for BrdU. Representative flow plots (F) and mean percent of BrdU (top) or 7-AAD (bottom) positive among NP-tet+ CD8 T cells (G) is plotted ± SEM (n=4 mice/group).
IL-15 is known to prolong the survival of CD8 Teff mainly through downstream signaling events that result in the enhanced expression of the anti-apoptotic molecule Bcl-2 (26). Accordingly, it has recently been demonstrated that, following influenza infection, IL-15 transpresented to CD8 Teff in the lung airways is able to protect this population from rapid apoptosis (17). It remains unclear, however, how much of the accumulation of lung-airway-resident CD8 Teff is the result of improved survival and how much is due to IL-15-induced migration into the site. The idea that IL-15 can induce the chemotaxis of lymphocytes is not unprecedented. Natural killer cells, which are dependent on IL-15 for their development, will migrate to IL-15 in an in vitro chemotaxis assay, and overnight treatment of these cells with IL-15 increases their binding to cultured endothelial cells (33). Moreover, T lymphocytes can invade IL-15-containing collagen gels and transmigrate through endothelium in response to IL-15 (19, 34). Despite these in vitro assays demonstrating the chemotactic potential of IL-15, there has been limited inquiry into whether or not IL-15 participates in the recruitment of CD8 T cells to sites of infection in vivo.
Because of the documented chemotactic properties of IL-15, the concentrated regional expression of IL-15 following influenza infection, and the failure of Teff to accumulate in the lung airways of IL-15−/− mice following influenza infection, we hypothesized that IL-15 participates in recruiting influenza-specific CD8 Teff to the site of infection. To test this hypothesis, we first tested whether locally instilled exogenous IL-15 could induce the chemotactic migration of NP-specific CD8 Teff to the lung airways. WT animals were infected with x31 and 6.5, 12, or 40 days later, we intranasally administered either PBS or IL-15c. We monitored alterations in the accumulation of NP-specific CD8 Teff in the lung airways 12 hrs post treatment to distinguish the effects of IL-15c on migration from its potential effects on proliferation or protection from apoptosis. Administration of exogenous IL-15c increased the number of NP-specific CD8 T cells in the lung airways 8 fold (or ~70 × 103 more cells) than PBS treated mice at d12 p.i. (Fig. 3C) while ~50 × 103 NP-specific CD8 Teff were correspondingly lost from the splenic pool of Teff (data not shown) suggesting that this Teff reservoir may be recruited to the BAL in response to IL-15c treatment. Moreover, local delivery of IL-15c induced the migration of activated CD44hi CD8 and CD4 T cells to the lung airways (Fig. 3 C), while naïve (CD44lo) CD8 and CD4 T cells and B220+ cells did not accumulate (data not shown). The specific augmentation in the number of influenza-specific CD8 Teff could only be observed as early as day 6.5 p.i. (Fig. 3B) since few NP-tet+ CD8 T cells could be recovered from the BAL at earlier time points, but this effect was abrogated by the memory phase of the response (day 40 p.i.) (Fig. 3 D). These data indicate that either Tmem are refractory to IL-15-mediated migratory signals or are incapable of accumulating in measurable numbers considering that the vasculature beds may be less permissive for lymphocyte entry in the absence of inflammation, irrespective of a chemotactic signal. IL-15 was specifically responsible for eliciting the migration of influenza-specific CD8 Teff to the lung airways since the intranasal instillation of complexes of the common gamma chain cytokines IL-2 or IL-7 failed to induce the trafficking of CD8 Teff to the lung airways (Fig. 3 E).
Recently, it has been reported that pulmonary DCs promote the survival of influenza-specific CD8 Teff through transpresentation of IL-15 (17). In our studies, it was possible that, in addition to the effect of IL-15 on the migration of CD8 Teff, IL-15-dependent proliferation and/or enhanced survival were also contributing to the observed accumulation of CD8 Teff in the lung airways following IL-15c treatment. To test whether administration of IL-15c affects CD8 Teff proliferation, we infected animals with x31, and, 12 days later, we pulsed animals with 5-bromo-2'-deoxyuridine (BrdU) and simultaneously administered PBS, IL-15, IL-7, or IL-2 complexes i.n.. Compared to PBS treated animals, treatment with any of the γc cytokine complexes did not augment the levels of BrdU incorporation by BAL resident anti-influenza Teff (Fig. 3 F–G). To test whether IL-15c enhanced the survival of Teff otherwise destined to die, CD8 Teff isolated from the lung airways 12 hrs post γc cytokine complex treatment were stained with 7-AAD, a fluorescent dye excluded from viable cells. Analysis of NP-specific CD8 T cells from IL-15c, as well as IL-7 and IL-2 complexes, revealed that all three cytokine treatments enhanced the survival of CD8 Teff since ~50% more of these CD8 T cells excluded 7-AAD (Fig. 3 F–G). In accordance with previous studies, these data confirm that IL-15 provides sustained survival signals to influenza-specific Teff in the absence of proliferation (17). However, IL-2 and IL-7 complexes were also effective at enhancing the survival of influenza-specific Teff without affecting Teff accumulation in the lung airways as observed with IL-15c treatment (Fig. 3 E). Thus, these data show that exogenous IL-15c delivered to the lung airways uniquely induced the migration of CD8 Teff to the site where interaction with IL-15 also augmented survival in situ.
IL-15 induces the migration of influenza-specific CD8 Teff from the systemic Teff pool
Our data demonstrate that localized in vivo administration of exogenous IL-15c recruited influenza-specific CD8 Teff to the lung airways; however, it is unclear whether this IL-15-induced migration was the result of direct chemotaxis to the IL-15c, modification of chemokine receptor expression by IL-15c treatment, or an alteration of a secondary chemotactic signal by an accessory cell affected by the IL-15c treatment. To begin to distinguish between these possibilities we monitored the direct effects of IL-15c on Teff migration in an in vitro chemotaxis assay. Single cell suspensions from the lung parenchyma, BAL, spleen, lung-draining MdLN, and non-draining inguinal lymph nodes of day 12 x31-infected animals were placed in the top chamber of a transwell chemotaxis chamber while media or media supplemented with IL-15c was placed in the bottom. After 90 minutes incubation, cells in the bottom chamber were collected, counted, and the percentage of NP-specific CD8 T cells which had migrated towards the IL-15c was determined. While NP-specific CD8 Teff isolated from both LNs migrated fairly efficiently towards the IL-15c, the Teff isolated from the lung parenchyma and spleen exhibited a 10 fold increase in migration to IL-15c (Fig. 4 A). However, the Teff isolated from the BAL migrated poorly towards IL-15c, presumably because they have down-regulated expression of IL-2/15Rβ (CD122) (Fig. 4 B) following IL-15-induced migration and arrival at their terminal destination.
Figure 4. IL-15 is chemotactic for influenza-specific CD8 Teff in vitro.
(A) 1×106 bulk cells isolated from the indicated tissues of WT animals infected 12 days previously with x31 were placed in the upper chamber of a 0.5µm Transwell with media alone or supplemented with IL-15c in the bottom chamber. Percent migration was calculated as # NP-tet+ CD8 T cells in the bottom chamber / # NP-tet+ CD8 T cells in the input sample. Mean percent migration is plotted ± SEM among three replicates for each tissue sample. (B) The mean percent of CD122 positive among day 12 CD8 Teff was determined and plotted ±SEM (n=3 mice/ group).
Overall, how Teff cells enter the lung airways is not well understood because two routes of entry are available to them. Lymphocytes can enter the lung airways by either traversing the lung parenchyma or by bypassing the lung tissue and entering directly from the circulation (35). Since CD8 Teff isolated from both the spleen and the lung tissue readily migrated to IL-15c in vitro, we asked whether influenza-specific CD8 Teff from one anatomical location preferentially migrated to IL-15c in vivo. To pursue this question, donor CD8 Teff were enriched from the lung or the spleen of day 12 x31-infected CD45.2+ WT donor mice and independently transferred into congenic CD45.1+ recipients that were 12 days p.i. with x31 (Fig. 5 A). At the time of adoptive transfer, recipient animals were given either IL-15c or PBS i.n., and, 12 hours later, the BAL from these animals was collected and analyzed for the presence of donor-derived CD8 Teff. Donor Teff were detected in the BAL of IL-15c treated animals only, confirming that IL-15 is capable of inducing the migration of CD8 Teff in vivo (Fig. 5 B–C). Interestingly, IL-15 elicited the migration of donor Teff isolated from the spleen but not the lungs (Fig. 5 C), suggesting that the majority of cells recruited into the lung airways post influenza infection are likely entering the lung airways from the circulation rather than by trafficking through the lung parenchyma. Together with the data presented in Fig. 2A,B wherein frequencies of NP-specific CD8 T cells are unaltered in the lung parenchyma but reduced in the BAL, these data indicate that circulating, Teff cells are partially dependent on IL-15 for their immigration into the lung airways and those Teff which have mobilized into the lung parenchyma either do not migrate to IL-15 or utilize unique IL-15-independent mechanisms to traverse into the airways.
Figure 5. IL-15 induces the migration of influenza-specific CD8 Teff in vivo.
(A) Methods schematic for panels B and C. (B) 2.5×106 CD8 T cells from the spleens and lungs of CD45.1+ mice 12 days post x31 infection were adoptively transferred i.v. into identically infected CD45.2+ recipients. One hour post transfer, recipients received either PBS or IL-15c i.n. and 12 hours later, BAL was collected and the number of donor (CD45.2+) NP-specific CD8 Teff migrating into the lung airways was determined by flow cytometry. (C) Mean number of donor cells recovered is plotted ± SEM (n=6 mice; p=0.0245).
Mucosal delivery of IL-15c during the effector phase of the anti-influenza CD8 T cell response enhances the resultant Tmem pool
Since IL-15 recruits influenza-specific CD8 Teff to the lung airways and prolongs their survival, adjuvanting IL-15 in the respiratory mucosa could numerically boost the CD8 Tmem generated from Teff maintained at this site. To test this hypothesis, WT mice infected with x31 were given IL-15c or PBS i.n. every other day between day 10–20 p.i.. Animals were rested until memory (day 31 p.i.) when cells from the lung airways and other indicated tissues were collected, counted, and analyzed for the presence of NP-specific CD8 T cells. Animals receiving IL-15c retained a higher frequency (2×) and number (2.5×) of influenza-specific CD Tmem in their lung airways compared to animals receiving PBS alone (Fig. 6A–B). To test the quality of this quantitatively augmented memory CD8 T cell pool, these animals were challenged on day 45 p.i. with a lethal dose of the heterologous H1N1 influenza A/Puerto Rico 8 (PR8). At 3, 6, and 8 days after receiving the challenge infection, lungs from both PBS-treated and IL-15c treated memory mice were collected and viral titers determined. At the earliest time (d3) post infection, the lungs of both WT and IL-15−/− mice contained less virus than those of naive controls; however, the lungs of IL-15c-treated animals had lower viral titers than those of PBS-treated animals (data not shown). More importantly, no detectable virus was isolated from IL-15c treated mice as early as day 6 post challenge while WT mice did not clear virus until 2 days later (Fig. 6C and data not shown). Thus, by altering the dynamics of the CD8 Teff initially seeding the respiratory tract, IL-15c quantitatively and qualitatively augmented the resultant Tmem developing in the lung airways, suggesting IL-15c therapy could be used as a means of prolonging heterosubtypic immunity to influenza infection.
Figure 6. Mucosal delivery of IL-15c during the effector phase of the anti-influenza CD8 T cell response augments the resultant Tmem pool.
Starting 10 days post x31 infection, animals received either PBS or IL-15c every other day for 10 days. Animals were rested for an additional 11 days until memory (day 31 p.i.) when cells from the indicated tissues were collected, counted, and analyzed for CD8 expression and NP-tetramer reactivity. Plotted are the mean frequencies (A) or mean numbers (B) of NP Tet+ CD8 T cells ± SEM (n=6 mice/group; *p=0.0224 (Lung), 0.0019 (BAL), and 0.0002, respectively). Data are representative of two independent experiments. (C) On day 45 post x31 infection, WT and IL-15−/− memory mice treated with PBS (circles) or IL-15c (squares) as in panel (A) and naïve (triangles) animals were challenged with PR8, and 6 days later lung viral titers were determined by plaque assay (n=3 mice/group).
Discussion
The prevention of influenza epidemics is reliant on the generation of a potent and long-lived pool of CD8 Tmem capable of cross-protecting hosts from multiple strains of virus which are continually evolving between and within humans and other mammalian and avian hosts. Unfortunately, the duration of the protection afforded by respiratory CD8 Tmem is limited, as these cells are lost over time (3, 5). Given that the factors responsible for this attrition are ill defined, a reasonable methodology to prolong long-term immunity to influenza infection is to reset the initial frequency of antigen-specific CD8 Teff seeding the lung airways which would coordinately increase the numerical output of Tmem. This methodology requires a deeper understanding of the factors and mechanisms used by antigen-specific CD8 Teff to migrate into and survive within the lung airways following influenza infection for exploitation as a vaccine adjuvant.
In the fifteen years since its discovery, IL-15 has been implicated in the development, activation, or maintenance of many cell types both inside and outside the immune system (10). The ability of IL-15 to mediate so many effects on divergent cell populations requires regulation of both IL-15 and its receptors. Our studies define a new function for IL-15, namely modulating the trafficking of Teff to the lung airways. This function of IL-15 may require regulation distinct from our traditional view of how IL-15 delivers signals for the survival and proliferation of CD8 Teff and Tmem. In these scenarios, IL-15 is transpresented by DCS and macrophages to CD8 Tmem that express CD122 (36–38). While DCs can transpresent IL-15 in the lung airways after influenza infection, and this method supports Teff survival, substantial levels of IL-15 are produced by non-DC populations. Airway epithelial cells (AECs) constitutively express IL-15 and IL-15Rα on their apical surfaces and are directly juxtaposed to the vascular endothelium (39). During inflammation, AECs can be induced to express IL-15 on the basolateral surface, potentially making the cytokine available to cells in the vasculature (39). Viral infection also induces the expression of metalloproteases such as ADAM17, which can cleave IL-15Rα from the surface of cells in order to create soluble IL-15/IL-15Rα complexes, similar to our intranasally delivered IL-15c (40). Indeed, soluble IL-15c can be detected as early as d3 p.i. in the lung as well as in the serum (Supplemental Figure 1). With IL-15 message levels continuing to rise locally in the lung (in the absence of sustained or increasing levels of IL-15c), it is likely that these complexes are shed locally only during a distinct time frame, and subsequent responses to IL-15 in the lung are due to interaction with membrane bound complexes (Fig. 1, Supplemental Figure 1, (16)). These two forms of IL-15 are likely important in creating a chemotactic gradient for migrating CD8 Teff which instructs them leave the circulation and enter the lung airways. The spatial and temporal expression of the soluble vs membrane forms of IL-15 could regulate the migration and pro-survival functions of IL-15, respectively. Ongoing studies will explore these possibilities.
How CD8 T cells “see” these complexes is unclear; IL-15 has dual binding sites for IL-15Rα, and therefore CD8 T cells responding to these IL-15 complexes could potentially receive signals through either IL-15Rα or CD122, which are both expressed on the surface of Ag-experienced CD8 T cells (10, 41). Studies thus far have only focused on IL-15 signaling through CD122, however, IL-15Rα can transmit signals either alone or in conjuction with γc and the newly identified transmembrane tyrosine kinase Axl (42). The differential coupling and signaling through these IL-15 receptors may help integrate IL-15 signals into unique cascades that result in different biological functions, including survival, proliferation, and migration. Future studies in our lab will define how IL-15 signaling regulates Teff migration to the lung airways.
While signaling through CD122 can enhance the survival of KLRG-1hi Teff (16, 43), and pulmonary dendritic cells can provide survival signals to resident influenza-specific CD8 Teff through IL-15 transpresentation (17), our data shows for the first time that IL-15 also evokes the chemotactic migration of pulmonary CD8 Teff to the site of infection. Anti-NP CD8 Teff isolated from secondary lymphoid tissues are only minimally affected by an IL-15 deficiency, despite the fact that influenza infection increased IL-15 mRNA levels in these sites, whereas respiratory bound anti-influenza effectors are numerically compromised. In addition, the timing of the influenza-induced IL-15 expression in the lung airways precedes and coincides with Teff influx and the anti-influenza CD8 Teff in the lung airways are enriched by 8-fold only 12 hours after IL-15c treatment, a timeframe more consistent with enhanced migration than survival (Fig. 3). While our in vitro chemotaxis assays suggest that the enhancement of Teff migration is a direct effect of IL-15 signaling, IL-15 can induce the transcription of chemokine receptors of the CC family in human PBLs (44). Lymphocytes exposed to IL-15 also morphologically resemble motile T cells with polarized expression of chemokine receptors at the leading edge and adhesion molecules on the uropod (45). Moreover, IL-15 specifically increases the expression of LFA-1, an integrin required for lung-specific trafficking of T cells (6, 33); however, none of these IL-15-induced effects on T cell migration have been studied in an infection model.
Perhaps the most significant finding of our work, however, is that IL-15c instilled directly into the respiratory tract can augment regional CD8 Teff responses and coordinately prolongs the persistence of immunological memory to influenza infection. Memory mice treated with IL-15c during the effector phase exhibited more rapid clearance of a heterologous influenza infection (Fig. 6), even when challenged a full 25 days after treatment had discontinued, implying that augmenting the number of Teff gaining access to the lung airways concordantly augments the resultant Tmem that differentiate in situ and mediate protection. Importantly, the use of IL-15 as a therapeutic or vaccine adjuvant may not be limited to influenza virus as infection with dual recombinant vaccinia viral vectors expressing both gp160 and IL-15 resulted in a heightened CD8 Teff and Tmem response against this HIV antigen (46). While γc cytokines, most notably IL-2, have been used as adjuvants with vaccines against specific cancers and infectious diseases (47), toxicity and half-life issues have precluded their success in clinical trials. However, by complexing IL-15 to its receptor and delivering intranasally, direct communication between the cytokine and responding Teff can be established locally and temporally regulated without affecting lymphocytes in other sites.
In summary, our experiments demonstrate for the first time that IL-15c can be used therapeutically to augment the number of Teff that traffic to and survive in the lung airways, suggesting that IL-15c can be used as a vaccine adjuvant to boost site-specific immunity. The implications of continual enhanced recruitment of Teff to the lung airways could be extended to multiple disease models, including cancer and other chronic infections, but is especially relevant to influenza infection where the efficacy of CD8 T cell-mediated protection and viral clearance is linked to the number of virus-specific CTLs present directly in the mucosa prior to challenge (3, 5). Periodic recruitment and augmentation of T cells reactive to conserved influenza epitopes by an adjuvanted IL-15c boost could sustain Teff above numerical thresholds and restore their protective capacity to both seasonal and pandemic strains of influenza.
Supplementary Material
Acknowledgements
We are especially grateful to Dr. R. Tripp and Jamie Barber for access and assistance with the LSRII. We also thank Drs. D. Campbell and L. Lefrançois for their critical reading of the manuscript and Jacob Parnell and Megan Beers for technical assistance.
Supported by the University of Georgia and the National Institutes of Health (AI077038 and AI081800 to K.D.K.)
References
- 1.Ghedin E, Sengamalay NA, Shumway M, Zaborsky J, Feldblyum T, Subbu V, Spiro DJ, Sitz J, Koo H, Bolotov P, Dernovoy D, Tatusova T, Bao Y, St George K, Taylor J, Lipman DJ, Fraser CM, Taubenberger JK, Salzberg SL. Large-scale sequencing of human influenza reveals the dynamic nature of viral genome evolution. Nature. 2005;437:1162–1166. doi: 10.1038/nature04239. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen HH, Moldoveanu Z, Novak MJ, van Ginkel FW, Ban E, Kiyono H, McGhee JR, Mestecky J. Heterosubtypic immunity to lethal influenza A virus infection is associated with virus-specific CD8(+) cytotoxic T lymphocyte responses induced in mucosa-associated tissues. Virology. 1999;254:50–60. doi: 10.1006/viro.1998.9521. [DOI] [PubMed] [Google Scholar]
- 3.Liang S, Mozdzanowska K, Palladino G, Gerhard W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J Immunol. 1994;152:1653–1661. [PubMed] [Google Scholar]
- 4.Yewdell JW, Bennink JR, Smith GL, Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1985;82:1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hogan RJ, Usherwood EJ, Zhong W, Roberts AA, Dutton RW, Harmsen AG, Woodland DL. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J Immunol. 2001;166:1813–1822. doi: 10.4049/jimmunol.166.3.1813. [DOI] [PubMed] [Google Scholar]
- 6.Ely KH, Cookenham T, Roberts AD, Woodland DL. Memory T cell populations in the lung airways are maintained by continual recruitment. J Immunol. 2006;176:537–543. doi: 10.4049/jimmunol.176.1.537. [DOI] [PubMed] [Google Scholar]
- 7.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 8.Bamford RN, Grant AJ, Burton JD, Peters C, Kurys G, Goldman CK, Brennan J, Roessler E, Waldmann TA. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci U S A. 1994;91:4940–4944. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 10.Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17:259–280. doi: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 13.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azimi N, Shiramizu KM, Tagaya Y, Mariner J, Waldmann TA. Viral activation of interleukin-15 (IL-15): characterization of a virus-inducible element in the IL-15 promoter region. J Virol. 2000;74:7338–7348. doi: 10.1128/jvi.74.16.7338-7348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattei F, Schiavoni G, Belardelli F, Tough DF. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J Immunol. 2001;167:1179–1187. doi: 10.4049/jimmunol.167.3.1179. [DOI] [PubMed] [Google Scholar]
- 16.Rubinstein MP, Lind NA, Purton JF, Filippou P, Best JA, McGhee PA, Surh CD, Goldrath AW. IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood. 2008;112:3704–3712. doi: 10.1182/blood-2008-06-160945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGill J, Van Rooijen N, Legge KL. IL-15 trans-presentation by pulmonary dendritic cells promotes effector CD8 T cell survival during influenza virus infection. J Exp Med. 2010;207:521–534. doi: 10.1084/jem.20091711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McInnes IB, al-Mughales J, Field M, Leung BP, Huang FP, Dixon R, Sturrock RD, Wilkinson PC, Liew FY. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat Med. 1996;2:175–182. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson PC, Liew FY. Chemoattraction of human blood T lymphocytes by interleukin-15. J Exp Med. 1995;181:1255–1259. doi: 10.1084/jem.181.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkins MR, Webby R, Doherty PC, Turner SJ. Addition of a prominent epitope affects influenza A virus-specific CD8+ T cell immunodominance hierarchies when antigen is limiting. J Immunol. 2006;177:2917–2925. doi: 10.4049/jimmunol.177.5.2917. [DOI] [PubMed] [Google Scholar]
- 21.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 22.Matrosovich M, Matrosovich T, Garten W, Klenk H-D. New low-viscosity overlay medium for viral plaque assays. Virology Journal. 2006;3:63. doi: 10.1186/1743-422X-3-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma LJ, Acero LF, Zal T, Schluns KS. Trans-presentation of IL-15 by intestinal epithelial cells drives development of CD8alphaalpha IELs. J Immunol. 2009;183:1044–1054. doi: 10.4049/jimmunol.0900420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 25.Feldman SB, Ferraro M, Zheng HM, Patel N, Gould-Fogerite S, Fitzgerald-Bocarsly P. Viral Induction of Low Frequency Interferon-[alpha] Producing Cells. Virology. 1994;204:1–7. doi: 10.1006/viro.1994.1504. [DOI] [PubMed] [Google Scholar]
- 26.Sanjabi S, Mosaheb MM, Flavell RA. Opposing effects of TGF-beta and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity. 2009;31:131–144. doi: 10.1016/j.immuni.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yajima T, Yoshihara K, Nakazato K, Kumabe S, Koyasu S, Sad S, Shen H, Kuwano H, Yoshikai Y. IL-15 regulates CD8+ T cell contraction during primary infection. J Immunol. 2006;176:507–515. doi: 10.4049/jimmunol.176.1.507. [DOI] [PubMed] [Google Scholar]
- 28.Tang C, Yamada H, Shibata K, Yoshida S, Wajjwalku W, Yoshikai Y. IL-15 protects antigen-specific CD8+ T cell contraction after Mycobacterium bovis bacillus Calmette-Guerin infection. J Leukoc Biol. 2009;86:187–194. doi: 10.1189/jlb.0608363. [DOI] [PubMed] [Google Scholar]
- 29.Hocke AC, Hartmann IK, Eitel J, Optiz B, Scharf S, Suttorp N, Hippenstiel S. Subcellular expression pattern and role of IL-15 in pneumococci induced lung epithelial apoptosis. Histochem Cell Biol. 2008;130:165–176. doi: 10.1007/s00418-008-0414-y. [DOI] [PubMed] [Google Scholar]
- 30.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 31.Stoklasek TA, Schluns KS, Lefrancois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177:6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, Sprent J. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha} Proc Natl Acad Sci U S A. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allavena P, Giardina G, Bianchi G, Mantovani A. IL-15 is chemotactic for natural killer cells and stimulates their adhesion to vascular endothelium. J Leukoc Biol. 1997;61:729–735. doi: 10.1002/jlb.61.6.729. [DOI] [PubMed] [Google Scholar]
- 34.Sancho D, Yanez-Mo M, Tejedor R, Sanchez-Madrid F. Activation of peripheral blood T cells by interaction and migration through endothelium: role of lymphocyte function antigen-1/intercellular adhesion molecule-1 and interleukin-15. Blood. 1999;93:886–896. [PubMed] [Google Scholar]
- 35.Galkina E, Thatte J, Dabak V, Williams MB, Ley K, Braciale TJ. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J Clin Invest. 2005;115:3473–3483. doi: 10.1172/JCI24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200:825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato N, Patel HJ, Waldmann TA, Tagaya Y. The IL-15/IL-15Ralpha on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proc Natl Acad Sci U S A. 2007;104:588–593. doi: 10.1073/pnas.0610115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stonier SW, Ma LJ, Castillo EF, Schluns KS. Dendritic cells drive memory CD8 T-cell homeostasis via IL-15 transpresentation. Blood. 2008;112:4546–4554. doi: 10.1182/blood-2008-05-156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hocke AC, Lampe MP, Witzenrath M, Mollenkopf H, Zerrahn J, Schmeck B, Kessler U, Krull M, Hammerschmidt S, Hippenstiel S, Schutte H, Suttorp N, Rosseau S. Cell-specific interleukin-15 and interleukin-15 receptor subunit expression and regulation in pneumococcal pneumonia--comparison to chlamydial lung infection. Cytokine. 2007;38:61–73. doi: 10.1016/j.cyto.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Pruessmeyer J, Martin C, Hess FM, Schwarz N, Schmidt S, Kogel T, Hoettecke N, Schmidt B, Sechi A, Uhlig S, Ludwig A. A disintegrin and metalloproteinase 17 (ADAM17) mediates inflammation-induced shedding of syndecan-1 and -4 by lung epithelial cells. J Biol Chem. 2010;285:555–564. doi: 10.1074/jbc.M109.059394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 42.Budagian V, Bulanova E, Orinska Z, Thon L, Mamat U, Bellosta P, Basilico C, Adam D, Paus R, Bulfone-Paus S. A promiscuous liaison between IL-15 receptor and Axl receptor tyrosine kinase in cell death control. EMBO J. 2005;24:4260–4270. doi: 10.1038/sj.emboj.7600874. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perera LP, Goldman CK, Waldmann TA. IL-15 induces the expression of chemokines and their receptors in T lymphocytes. J Immunol. 1999;162:2606–2612. [PubMed] [Google Scholar]
- 45.Nieto M, del Pozo MA, Sanchez-Madrid F. Interleukin-15 induces adhesion receptor redistribution in T lymphocytes. Eur J Immunol. 1996;26:1302–1307. doi: 10.1002/eji.1830260619. [DOI] [PubMed] [Google Scholar]
- 46.Oh S, Berzofsky JA, Burke DS, Waldmann TA, Perera LP. Coadministration of HIV vaccine vectors with vaccinia viruses expressing IL-15 but not IL-2 induces long-lasting cellular immunity. Proc Natl Acad Sci U S A. 2003;100:3392–3397. doi: 10.1073/pnas.0630592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaplan G, Cohn ZA, Smith KA. Rational Immunotherapy with Interleukin 2. Nat Biotech. 1992;10:157–162. doi: 10.1038/nbt0292-157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.