Abstract
Dilated cardiomyopathy is a devastating disease associated with poor outcomes. Although the etiology of this disease remains largely unknown, so-called “idiopathic” dilated cardiomyopathy (iDCM) is associated with evidence of an autoimmune process that may be contributing to the pathophysiology of this disease. Indeed, iDCM shares many characteristics with other autoimmune diseases, including an association with systemic and organ-specific inflammation, an association with viral infections, a genetic predisposition, and a correlation with specific human leukocyte antigen subtypes.
Additionally, numerous pathologic cardiac-specific autoantibodies have been associated with iDCM, including those against α-myosin, the β1-adrenoceptor, and cardiac troponin I.
This review highlights the emerging evidence regarding autoimmune characteristics of iDCM, and summarizes the data of specific immunomodulatory therapies used to target autoimmune mechanisms in the treatment of patients with this devastating disease.
Keywords: Autoimmune, heart failure, immunoadsorption, intravenous immunoglobulin, inflammation
Within the broad category of heart failure, dilated cardiomyopathy is the most common nonischemic etiology.1 Despite the immense impact of this disease, the majority (65%e80%) of cases are idiopathic in nature.1 Without an understanding of the etiology of this disease, management focuses on restoring neurohormonal balance rather than targeting the primary cause. Consequently, the incidence, morbidity, and mortality of this disease remain unacceptably high. This review aims to summarize the contemporary understanding of autoimmune characteristics of idiopathic dilated cardiomyopathy (iDCM) in humans, the proposed pathophysiologic mechanisms, as well as the ongoing clinical investigations that have focused on autoimmunity as a target for therapy.
Immunologic Pathogenesis of Idiopathic Dilated Cardiomyopathy
Despite the designation of idiopathic, the inflammatory nature of iDCM has long been recognized, with the hypothesis that many originated from an extended process of myocarditis. Nonspecific markers of inflammation such as C-reactive protein, C3 and C4 complement fractions, soluble intercellular adhesion molecule-1, and soluble endothelial leukocyte adhesion molecule-1 have been found to be elevated in iDCM.2 Also, in a study of more than 150 individuals with iDCM undergoing endomyocardial biopsy, 48% demonstrated evidence of significant lymphocyte infiltration, 89% showed interstitial and endothelial immune activation, and 5% met the Dallas criteria for acute myocarditis.3
Many individuals with undiagnosed myocarditis may fall into the category of iDCM. Acute myocarditis is characterized by a clinical history of acute decompensated heart failure in a person with low cardiac risk or no other underlying cardiac dysfunction, as well as histologic findings on endomyocardial biopsy consistent with the Dallas Criteria.4 However, the inciting factor and clinical course of myocarditis are extremely varied, and in many cases difficult to distinguish from iDCM. Furthermore, iDCM shares many inflammatory and infectious characteristics with myocarditis. These shared characteristics include an association with systemic and organ-specific inflammation, a recently discovered correlation with chronic myocardial viral infections, and numerous cardiac-specific autoimmune antibodies. These discoveries have resulted in the development of 2 disparate potential explanatory pathophysiologic mechanisms for iDCM: 1) a primary cardiotoxicity resulting from viral infection of the myocardium and 2) an ongoing autoimmune insult triggered by a myocarditis-inducing pathogen.
Cardiotoxic Viral Infection
The first of these potential explanatory pathophysiologic mechanisms considers many cases of iDCM to be a sub-acute, chronic myocarditis in which the continued progression of the disease is attributed to a chronic deleterious viral infection. Traditionally, coxsackie viruses A and B have been considered the most common pathogens in viral myocarditis. These infections are known to result in iDCM in humans, and well-established animal models have been used to explore potential mechanisms of this progression.5 Recently, in a large series of patients with iDCM, 67% of patients showed evidence of viral genomes within endomyocardial biopsy samples. Unexpectedly, the majority (73%) of patients were found to have parvovirus B19 (PV) and human herpes virus-6 (HHV6), instead of coxsackievirus. The same investigators demonstrated that the persistence of either virus in the myocardium was associated with a progressive impairment of cardiac function and spontaneous clearance with significant improvement.6 In addition, interferon-β therapy in virus-positive iDCM patients has been shown to clear the viruses and significantly improve left ventricular systolic function.7 Both parvovirus B19 (PV) and HHV6 are known to have cardiac tropism, and persistent infection with these agents may represent a primary causative factor in the pathogenesis of iDCM.
However, there are data suggesting that the association between viral persistence and iDCM may also be an epiphenomenon. PV and HHV6 infections are extremely prevalent in the population, with >70% of healthy adults being sero-positive (tested independently).8,9 Furthermore, only 1 of the many studies evaluating the prevalence of myocardial PV and HHV6 included a control population for comparison. In this study, 40% of the control population tested positive for PV.10 A recent study confirmed the finding that approximately 70% of iDCM patients having enterovirus, adenovirus, or PV genomes within their myocardium. However, unlike previous reports, the presence of a viral genome did not correlate with a decreased improvement in ejection fraction over the approximately 18-month follow-up.11 Additionally, the presence of enteroviral RNA replication (identified by detection of enteroviral minus-strand RNA) was not associated with a deterioration of left ventricular function when compared with the virus-negative group. Hence the clinical significance of viral persistence as the primary pathophysiologic process remains debated.
Autoimmune Response
The second potential explanatory pathophysiologic mechanism considers many cases of iDCM to be a subacute, chronic myocarditis, but one that is propagated by an underlying autoimmune etiology (possibly triggered by an initial viral insult). Recent work evaluating the etiology of iDCM suggests that an autoimmune process may be contributing to the disease progression. Autoimmune diseases are a group of heterogeneous disorders with almost endless etiologies and clinical presentations. However, despite this diversity, this group of diseases shares many common characteristics. These include an association with systemic and organ-specific inflammation, an association with viral infections, a genetic predisposition, and a correlation with specific human leukocyte antigen (HLA) subtypes. These traits are also characteristic of iDCM.
Genetic Predisposition
Genetic polymorphisms that confer susceptibility to development of an autoimmune disease can be located in the genes of proteins that regulate the immune response (eg, major histocompatibility complex proteins, immunoglobulins, T-cell receptor genes). This is also true of proteins involved with the metabolism of drugs, chemicals, or antigens, and within structural proteins themselves. Although the exact genetic changes that predispose to an autoimmune disease are yet to be identified, a comparison between the concordance rates of autoimmune diseases in monozygotic twins and the rates of these diseases in the general population, indicates that genetic factors play a significant role in the development of these diseases.12,13 Genetic predisposition appears to play a strong role in iDCM as well.14 Although some of the predisposing inheritance involves unidentified mutations of sarcolemmal structural proteins such as lamin A/C, there also appears to be a genetically derived propensity for autoimmune disease. Patients with iDCM are more likely to have another autoimmune disease than the general population, and there is an increased incidence of autoimmune diseases such as psoriasis, thyroid disease, rheumatoid arthritis, and hemolytic anemia in the family members of patients with iDCM.15
HLA Specificity
There have been reports of associations between iDCM and the HLA-DR4 and HLA-DQ4/6 subtypes. The prevalence of these HLA subtypes is 27% to 50% in iDCM as compared with 6% to 21% in controls.16,17 Both the HLA-DR and HLA-DQ subtypes are major histocompatibility complex (MHC) class II proteins. After processing, MHC class II proteins interact exclusively with CD4+ T-helper cells, whose primary mechanism of immunologic retaliation is the cytokine-dependent activation of B lymphocytes. Numerous cardiac-specific autoantibodies are also associated with iDCM.
Cardiac-Specific Autoantibodies
In patients with iDCM, autoantibodies to contractile proteins, structural proteins, proteins of energy metabolism/transfer, ion channels, and sarcolemmal receptors have been identified.18–21 The average prevalence of these autoantibodies in patients with iDCM is between 65% and 70% (range from 20%–95%, depending on the screening techniques and the antibody in question).19–23 Several of these cardiac-specific autoantibodies have demonstrated signifi-cant pathologic potential.
α-Myosin Autoantibodies
Autoantibodies specific for α-myosin have been identified since the mid-1980s and have been found to cross-react with bacterial (group A streptococcus) and viral (Coxsackievirus B3) proteins.24,25 However, the formation of α-myosin autoantibodies is not limited to the molecular mimicry of infectious agents. These autoantibodies have also been found in animal models of myocarditis created by immunizing mice with α-myosin; a process that mimics the necrotic release of this protein. Through this work, the S2 segment (amino acids 596-1295), corresponding to the rod region of the protein, has been found to contain the epitopes responsible for producing the inflammatory response in this model of myocarditis.26
α-Myosin autoantibodies have been reported in 23% to 66% of patients with iDCM (0.0%–2.5% of healthy controls; 4%–21% of patients with ischemic cardiomyopathy), and their presence correlated with worsening left ventricular systolic function and increased diastolic stiffness.19,22,27 Additionally, the persistence of α-myosin autoantibodies has been associated with an elevation of blood levels of cardiac troponin, suggesting persistent myocardial injury.28 However, α-myosin is normally immunologically sequestered within cells, and is not expressed on or within the plasma membrane, making it unclear how these autoantibodies would initiate or propagate cardiomyocyte damage. Recent work has demonstrated that autoantibodies specific for α-myosin cross-react with the cell surface β1 adrenoreceptor (β1AR), and induce cyclic adenosine monophosphate–dependent phosphokinase-A (PKA) activity.29 Antibody-mediated activation of β1AR is known to induce cardiomyocyte apoptosis.30
β1 Adrenoreceptor Autoantibodies
Autoantibodies specific to the β1AR have also been recognized for almost 2 decades. As with a subset of the α-myosin autoantibodies, β1AR antibodies have been shown to form through molecular mimicry of an infectious pathogen. Specifically, antibodies to a ribosomal protein of Trypanosoma cruzi (Chagas’ disease) cross-react with the second extracellular domain of the β1AR.31 However, the majority of individuals with these autoantibodies have not been infected with this pathogen, suggesting other mechanisms may underlie the production of these autoantibodies.
The β1AR is a G protein–coupled receptor that triggers a signaling cascade through adenylate cyclase, cyclic adenosine monophosphate, and PKA. These signaling molecules regulate the sarcoplasmic calcium concentration and increase myocyte inotropy, chronotropy, and lusitropy. Autoantibodies to the β1AR do not bind to the physiologic receptor site, but instead bind to the second extracellular loop.32,33 Despite this nonphysiologic interaction, these autoantibodies have been shown to increase cyclic adenosine monophosphate production, l-type Ca2+ currents, the action potential duration, and cardiomyocyte fractional shortening (Fig. 1).33–35 Additionally, these autoantibodies induce a dose-dependent increase in the rate of cardiomyocyte apoptosis, a response that is attenuated by the PKA inhibitor Rp-adenosine-3′,5′-cyclic monophosphothioate and the β1-selective antagonist metoprolol.30 In a landmark study, mice immunized with a peptide corresponding to the second extracellular loop of the β1AR developed stimulating antibodies to the receptor and, subsequently, iDCM.35 When serum from these mice was transferred to healthy animals, they too developed iDCM.
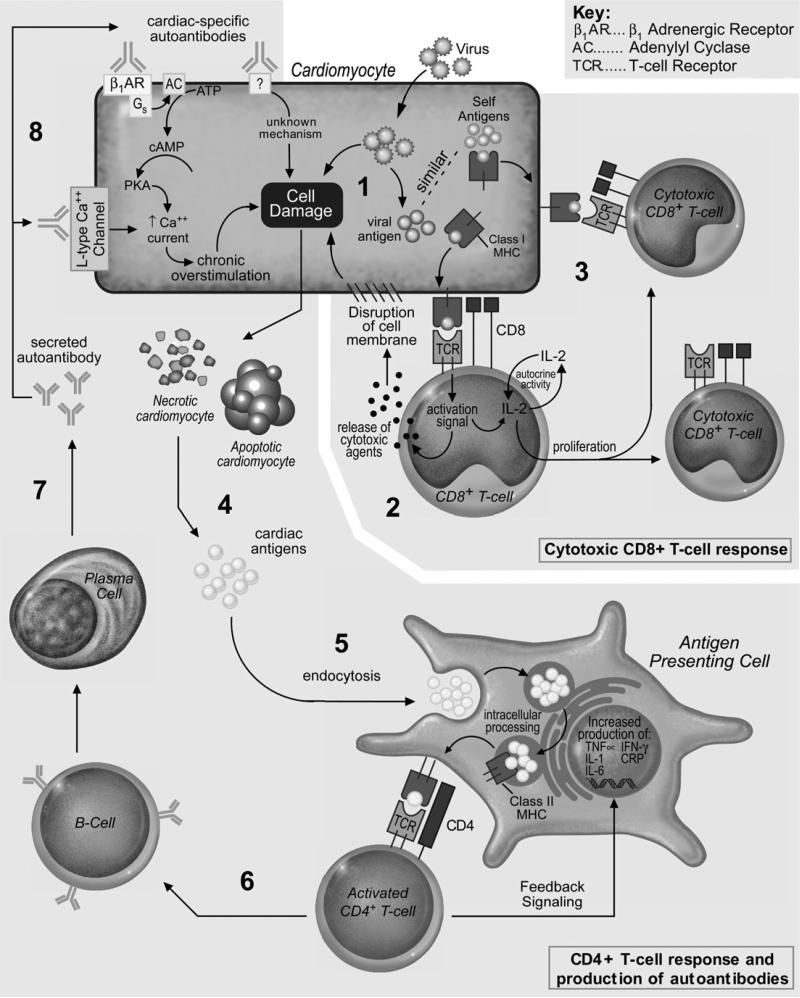
Fig. 1.
Autoimmune mechanisms potentially operative in the pathogenesis of idiopathic dilated cardiomyopathy. (1) Viral infection leading to cell damage and viral antigen presentation; (2) CD8+ T-cell response to viral antigen presentation, and clonal expansion of CD8+ cytotoxic T cells; (3) molecular mimicry of viral antigens; (4) release of cardiac antigens from apoptotic/necrotic cardiomyocytes; (5) endocytosis and cardiac-antigen presentation to CD4+ T cell; (6) CD4+ T-cell activation of B cell; (7) release of cardiac-specific autoimmune antibodies; (8) cardiac-specific autoimmune antibody disruption of cellular homeostasis.
β1AR autoantibodies have been reported in approximately 26% to 46% of patients with iDCM (1%–10% of healthy controls; 10%–13% of patients with ischemic cardiomyopathy), and the presence of these autoantibodies has been found to be associated with cardiac-specific morbidity and mortality.20,36–38 Over a 10-year period, the presence of β1AR autoantibodies independently predicts an increased risk of all-cause and cardiovascular mortality.20 β1AR autoantibodies have also been linked to depressed myocardial function, a higher incidence of severe ventricular arrhythmia, and a higher incidence of sudden cardiac death.23,39 An interaction between the presence of β1AR autoantibodies and metoprolol has been evaluated, showing that autoantibody-positive patients experienced a significantly greater decrease in left ventricular end-diastolic dimension, and a significantly greater increase in left ventricular ejection fraction after 1 year of metoprolol therapy when compared with autoantibody-negative patients.38 Further research is needed to determine the efficacy with which β-blockers inhibit β1AR autoantibody toxicity and to determine if the development of specific anti-β1AR autoantibody therapy is warranted.
Cardiac Troponin I Autoantibodies
As many as 50% of patients with severe iDCM have elevated levels of troponin.40 It is theorized that the large-scale release of this normally immunologically sequestered protein can induce the formation of cardiac-specific autoantibodies. Animal studies have shown that the immunization of mice with cardiac troponin-I (cTnI) induced the formation of autoantibodies, and the introduction of these autoantibodies into healthy animals resulted in cardiomyopathy.41 However, these findings cannot be reproduced with cardiac troponin T. It is speculated that the discrepancy is due to the cellular location of these proteins–cardiac troponin T is contained within cardiac myocytes, whereas cTnI is also found on the outer cell membrane where it can interact with circulating autoantibodies or lymphocytes.41,42
To evaluate the mechanism of the cTnI autoantibody induced cardiac damage, a Japanese group performed an elegant study in which they found that cTnI autoantibodies chronically increase the L-type Ca2+ current via a PKA-independent mechanism (Fig. 1).42 However, when cTnI autoantibodies were isolated from humans and applied to cultured rat myocytes, there was no effect on Ca2+ currents and no binding to the cell surface.43 At this point, it remains unclear whether the human cTnI autoantibodies are specific for an epitope not contained in the rat l-type Ca2+ channel proteins, and further work will be needed to confirm the role of cTnI autoantibodies in humans.
Other Cardiac-Specific Autoantibodies
Autoantibodies specific for the sarcolemmal Na-K-ATPase were shown to be independent predictors of poor systolic function, ventricular tachycardia, and sudden cardiac death.18 Also, autoantibodies specific for the M2-muscarinic acetylcholine receptor have been correlated with a greater incidence of atrial fibrillation.21 However, in both of these studies, the number of patients with these autoantibodies and the number of reported events were extremely small, and further work is required to fully evaluate their pathologic potential.
Cellular Autoimmunity
Despite the preponderance of research on humoral autoimmunity in iDCM, there is a paucity of information about the cellular autoimmune response. However, a CD4+ T-lymphocyte response is an essential component in the production of antibodies and, if this disease is related to a viral infection, CD8+ T-lymphocytes are required for the clearance of viral pathogens. Although there is no definitive evidence, there is indirect evidence that both CD4+ and CD8+ T cells are involved in a pathologic role in iDCM. Histologic evaluation of endomyocardial biopsies of patients with iDCM consistently show both CD4+ and CD8+ T-cell infiltrates.3,44 Also, when lymphocytes from patients with iDCM are transferred into mice, the mice develop myocardial fibrosis and an increased left-ventricular end-diastolic dimension: characteristics similar to iDCM.45
Potential Mechanisms of Autoimmune Initiation and Progression
Initiation of any autoimmune disease requires a breakdown in self-tolerance within a genetically susceptible environment. In iDCM, this breakdown in self-tolerance may be triggered by idiopathic mechanisms, a disease-associated necrotic release of immunologically sequestered cardiomyocyte proteins, or by a viral infection. Infections have been shown to result in the breakdown of self-tolerance in several disease states.46,47 This is thought to occur via: 1) modification or release of immunologically sequestered proteins, 2) polyclonal activation of lymphocytes, 3) activation of resting T cells through the release of cytokines, or 4) through molecular mimicry.
A breakdown in self-tolerance can produce both a T-lymphocyte mediated cellular autoimmune response and a B-lymphocyte mediated humoral autoimmune response.46,47 In a T-lymphocyte mediated autoimmune response, such as autoimmune hepatitis, cytotoxic CD8+ T-lymphocytes recognize an MHC class I-autoantigen complex on the hepatocyte surface, which triggers the release of cytotoxic agents (granzyme B and perforin), and subsequently induces apoptosis (Fig. 1).48 Additionally, these activated cytotoxic CD8+ T-cells produce proliferative cytokines such as interleukin-2, resulting in expansion of the autoreactive clone and propagation of the autoimmune response. In contrast, in a B-lymphocyte–mediated autoimmune response, such as Guillain-Barré and Grave's disease, cellular antigens are processed by antigen presenting cells (macrophages, dendritic cells) and presented to CD4+ T cells, which in turn activate B-lymphocytes and induce the production of autoantibodies specific to the self-antigen (Fig. 1).49 These autoantibodies may be an inconsequential side-product of a cell-mediated autoimmune response (as in scleroderma), or they may have direct deleterious effects on the cell of origin, as is theorized in iDCM. In this latter instance, the autoantibody response would also be responsible for propagation of the autoimmune response.
Targeting Autoimmune Mechanisms in iDCM
For almost as long as inflammation has been recognized as being a part of iDCM, there have been attempts to treat this disease with immunomodulatory therapies. These therapies have ranged from generalized immune suppression to specific anti-inflammatory cytokine therapies.50–56 Table 1 lists the various immunomodulatory therapies that have been tested in the iDCM population and their mechanisms of action. However, thus far, no therapy has emerged as being truly efficacious in the treatment of iDCM, and few specifically target autoimmune mechanisms. Perhaps this shortcoming is due to the lack of target specificity and the delicate balance between the risks and benefits of broad immunosuppression. Prednisone, thalidomide, and azathioprine, although partially successful in selected reports, often resulted in significant adverse side effects.51,52,56 Two large trials using anti–tumor necrosis factor-α therapies were also not successful in the broad heart failure population. Specifically, the anti-TNF Therapy Against Congestive Heart Failure (ie, ATTACH) trial tested 2 doses of infliximab in 150 patients with stable New York Heart Association Class III/IV heart failure and left ventricular ejection fraction ≤35%. This trial did not achieve the primary end point of improved clinical status at 14 weeks, and the combined risk of death from any cause or hospitalization for heart failure through 28 weeks was significantly increased in the patients randomized to the higher dose.54 The Randomized Etanercept Worldwide Evaluation (ie, RENEWAL) trial evaluated the efficacy of different regimes of etanercept in a cohort of >1500 patients with New York Heart Association Class II-IV heart failure and a left ventricular ejection fraction of ≤30%. Etanercept had no effect on clinical status, heart failure hospitalizations, or death, and both trials were stopped early because of a lack of benefit.50 Pentoxifylline, despite being an inhibitor of tumor necrosis factor-α, showed some therapeutic potential; however, its benefits are unclear and are thought to be mediated by an as yet unknown non–anti-inflammatory action.55 A preliminary report on the broad use of immunomodulatory therapy (Celecade) also failed to significantly improve overall morbidity and mortality except in subgroups.57 Statin therapy has shown some promise in mechanistic studies and post-hoc analyses, but a recent large-scale randomized clinical trial on elderly patients with ischemic cardiomyopathy did not find significant improvements in mortality.58
Table 1.
Immunomodulatory Therapies Tested in the Treatment of Idiopathic Dilated Cardiomyopathy
| Immunomodulatory Therapy | Mechanism of Action |
|---|---|
| Prednisone | Suppresses migration of leukocytes and reverses increased capillary permeability. Decreases inflammatory mediators by inhibiting phospholipase A2 as well the expression of COX-2 (but not COX-1). Although cellular immunity is affected more than humoral immunity, the primary antibody response can also be diminished. |
| Azathioprine | Chemotherapeutic pro-drug, converted in the body to the active metabolites 6-mercaptopurine and 6-thioinosinic acid, which inhibit leukocyte and lymphocyte proliferation. |
| Infliximab | Chimeric monoclonal antibody that binds to human TNF-α, interfering with endogenous activity. Biologic activities of TNF-α include the induction of pro-inflammatory cytokines, enhancement of leukocyte migration, activation of neutrophils and eosinophils, and the induction of acute phase reactants and tissue degrading enzymes. |
| Etanercept | Recombinant protein composed of TNF-α receptor linked to the Fc portion of human IgG1 that binds TNF-α and blocks its interaction with cell surface receptors. |
| Thalidomide | Sedative-hypnotic drug that is also known to have anti-oncogenic and anti-inflammatory properties; including the ability to inhibit angiogenesis and decrease TNF-α levels. |
| Pentoxifylline | A xanthine-derived agent known to inhibit the production of TNF-α, to inhibit neutrophil adhesion and activation, and to inhibit PDE4 activity. |
| Statin medications | Decrease both acute and chronic inflammatory processes by mechanisms thought to act by reduction of oxidized LDL, modulation of endothelial cell nitric oxide synthase, or decreased leukocyte adhesion. |
| Immunoadsorption | Extracorporeal blood is passed through a column with highly purified protein A (isolated from Staphylococcus aureus) bonded to a silica matrix. Circulating immune complexes and IgG bind to the protein A and are selectively removed from the plasma. The plasma is then returned to the patient. |
| Intravenous immunoglobulin (IVIg) | A conglomerate of immunoglobulins extracted from the plasma of thousands of individuals that is thought to either stimulate the host's complement system to enhance the removal of all antibodies, or block the antibody receptors on immune cells (eg, macrophages), thus reducing inflammatory tissue damage. |
COX, cyclooxygenase; Ig, immunoglobulin; LDL, low-density lipoprotein; PDE, phosphodiesterase; TNF-α, tumor necrosis factor-alpha.
Although many of the aforementioned therapies dampen the overall immune response, none effectively targets antibody expression (especially with autoantibodies). Despite the discovery of cardiac-specific autoantibodies and their pathogenic potentials, thorough examination of this therapeutic approach against autoimmune mechanisms has yet to be widely studied in the clinical setting. We will discuss 2 such targeted strategies that have been investigated: immunoadsorption (IA) and intravenous immunoglobulin (IVIg).
Immunoadsorption
Immunoadsorption is a method of extracting immunoglobulins from the plasma by passing extracorporeal blood through an affinity column to enhance the clearance of cardiac-specific autoantibodies. This is the most direct way of eliminating the presence of autoantibodies in the circulation. Clinical trials using IA have shown successes (Table 2).23,36,59–66 Also, removal of antibodies by IA normalized the abnormal myocardial gene expression of desmin found in iDCM patients.67 However, many of these studies have very small sample sizes, often restricted to single-center experiences, and many lack proper controls or blinding. Patient selection criteria can also vary widely among IA studies, and the invasive and complicated aspects of the IA process have limited its broad clinical adoption.
Table 2.
Summary of Trials Evaluating the Efficacy of Immunoadsorption in Idiopathic Dilated Cardiomyopathy
| Trial | Study Design | Intervention | Patient # (Treated/Controls) | Baseline Characteristics | Follow-up | Results |
|---|---|---|---|---|---|---|
| Dorffel, 199759 | CS | IA for 5 days | 9/0 | NYHA III/IV, LVEF <25%, positive for B1AR autoantibodies | 5 days | Improved CO (3.7 ± 0.8 to 5.5 ± 1.8 L/min, P < .01), and decreased MAP (76.0 ± 9.9 to 65.0 ± 11.2 mm Hg, P < .05). |
| Muller, 200023 | CC | IA for 5 days | 17/17 | NYHA II-IV, LVEF <30%, positive for B1AR autoantibodies | 12 months | Improved LVEF in treatment group (22.3 ± 3.3% to 37.9 ± 7.9%; P = .0001). No change in control group. Levels of B1AR autoantibodies decreased with IA and did not return during follow-up. |
| Felix, 200060 | RCT | IA for 3 days with 0.5 g/kg of IgG substitution on day 3, followed by 2 sessions once per month for 3 months | 9/9 | NYHA III/IV, LVEF <30% | 3 months | Increased CI in the treatment group (2.3 ± 0.1 L/min/m2 to 3.0 ± 0.3 L/min/m2; P = .01 vs. baseline, P = .05 vs. controls). |
| Wallukat, 200262 | CS | Selective removal of B1AR antibodies over 5 days | 8/0 | LVEF <35%, positive for B1AR autoantibodies | 12 months | Improved LVEF (28.5 ± 6.1 to 36.6. ± 10.7; P = .002). Levels of B1AR autoantibodies decreased with IA and did not return during follow-up. |
| Felix,200261 | CC | IA for 3 days | 11/9 | NYHA III/IV, LVEF <30% vs. healthy controls | 3 days | Improved CI (2.2 ± 0.1 to 2.7 ± 0.2 L/min/m2; P < .01). |
| Mobini, 200336 | CS | IA for 3 days with 0.5 g/kg of IgG substitution on day 3, followed by 2 sessions once per month for 3 months | 22/0 | NYHA III-IV, LVEF <30% | 3 months | No difference in CI and LVEF between B1AR antibody–positive and B1AR antibody–negative patients. |
| Staudt, 200564 | CC | IA with improved IgG3 removal vs. normal IA | 9/9 | NYHA III/IV, LVEF <35% | 3 months | Greater improvement in LVEF using IA with improved IgG3 removal (24.3 ± 2 to 34.7 ± 4% vs. 21.6 ± 2% to 24.4 ± 2%; P < .05). |
| Schimke, 200163 | CS | Selective removal of B1AR antibodies | 8/0 | Not reported | 12 months | Significant decrease in oxidative stress and an increase in wall motion velocity and LVEF. |
| Staudt, 200666 | RCT | One session of IA vs. 1 session per month for 3 months | 11/11 | NYHA III/IV, LVEF <35% | 6 months | Improved LVEF (P < .01), but no difference between groups. |
| Cooper, 2007 (65) | CS | IA for 5 days | 4/0 | NYHA II/III, mean LVEF 34.6 ± 12.3% | 6 months | Decrease in total IgG and IgG3 (P < .05), but no change in LVEF. |
B1AR, beta-1 adrenoreceptor; CC, case control; CI, cardiac index; CO, cardiac output; CS, case series; IA, immunoadsorption; LVEF, left ventricular ejection fraction; MAP, mean arterial pressure; NYHA, New York Heart Association class; RCT, randomized controlled trial; Ig, immunoglobulin.
Intravenous Immunoglobulin (IVIg)
In other autoimmune diseases with pathologic autoantibodies, such as Guillain-Barré syndrome, the use of IVIg has been shown to provide equivalent therapeutic efficacy to IA with a less elaborate setup and fewer side effects.68 Trials in which IVIg has been used in patients with iDCM are outlined in Table 3.69–73 This therapy has been shown to be effective in several small trials, and may be a safer alternative to IA. In 1 of the first studies with IVIg, pediatric patients who presented with acute myocarditis were treated with high-dose IVIg over 24 hours. After this single treatment, patients were more likely to achieve normal ejection fraction at 1 year when compared with controls.73 However, as is highlighted by the Intervention in Myocarditis and Acute Cardiomyopathy (IMAC) trial, the appropriate timing and patient selection criteria for IVIg therapy is still debatable. In the IMAC trial, patients with recent-onset iDCM (<6 months of symptoms) were randomized to IVIg therapy or placebo. During follow-up, no significant benefit to the IVIg therapy was observed. However, both the treatment and control groups experienced significant improvement in cardiac function (25 ± 0.08% to 42 ± 14%; P < .001).70
Table 3.
Summary of Trials Evaluating the Efficacy of Intravenous Immunoglobulin in Idiopathic Dilated Cardiomyopathy
| Study | Design | Intervention | Patient No. (treated/controls) | Baseline Characteristics | Follow-up | Results |
|---|---|---|---|---|---|---|
| Drucker 199473 | CC | 2 g/kg IVIg over 24 h | 21/25 | Pediatric patients presenting with acute myocarditis and historical controls | 12 months | The treated group had a greater likelihood of achieving normal LVEF at 1 y (P = .03), and the probability of survival tended to be higher among IVIg-treated patients (0.84 vs. 0.60, P = .069). |
| McNamara, 199771 | CS | 2 g/kg IVIg over 2–4 days | 10/0 | Symptoms <6 months, NYHA III-IV, LVEF <40% | 12 months | Improved LVEF (24 ± 2% to 41 ± 4%; P = .003). |
| Gullestad, 200169 | RCT | 0.4 g/kg IVIg for 5 days followed by 0.4 g/kg monthly for 5 months | ICM 11/12 iDCM 9/8 | NYHA II/III, LVEF <40% | 6.5 months | Improved LVEF in the treatment group (26 ± 2% to 31 ± 3%; P < .01); significant decrease in anti-inflammatory markers and BNP (P < .001). |
| McNamara, 200170 | RCT | 2 g/kg IVIg over 2 days | 62/31 | Symptoms <6 mo, NYHA II/III LVEF <40% | 12 months | Improved LVEF when compared to baseline (25 ± 0.08% with 42 ± 14%; P < .001), but no difference between treatment and placebo. |
| Kishimoto, 200372 | CS | 1-2 g/kg IVIg over 2 days | 9/0 | Symptoms <6 months, NYHA III-IV, LVEF <40% | 12 days | Improved LVEF (19 ± 7.5% to 35 ± 9.1%; P < .01). |
CS, case series; CC, case control; ICM, ischemic cardiomyopathy; iDCM, Idiopathic dilated cardiomyopathy; IVIg, intravenous immunoglobulin; LVEF, left ventricular ejection fraction; RCT, randomized controlled trial; NYHA, New York Heart Association class.
Based on published clinical studies, the discrepancies between effective and neutral studies may be linked to study design, sample sizes, and publication biases. It is also important to note that those individuals who experienced significant benefits from IA (or even IVIg in smaller reports) often have chronic iDCM already treated on standard therapy. This may suggest that like other neurohormonal mechanisms, the role of autoimmunity in iDCM may not be in the initiation of the disease but in facilitating disease progression after initial stabilization or in later stages when immune mechanism are more pronounced. The timing of the intervention may also affect the effects of the interventions. In particular, treatment effects of IVIg may differ when targeting the acute vs. chronic phases of the condition. However, the biggest limitation remains the difficulty in phenotyping a specific “autoimmune” characterization for patients presenting with a clinical (or histologic) diagnosis of myocarditis or iDCM. Studies are under way to determine the synergistic effects of combining these strategies, and further well-conducted clinical research is needed to clarify these points.
Clinical Perspectives
The incorporation of autoimmunity in the paradigm of iDCM pathogenesis is not a new concept. However, decades of research continue to be challenged by limitations in methodology, therapeutic options, and lack of reliable phenotyping. The lack of safe and direct access to failing heart tissue has impeded ongoing research efforts to understand the role of autoimmunity in the progression or resolution of iDCM. Subsequently, there remains a lack of specific human data on the underlying immune mechanisms or the specific antigens involved. Cardiac-specific autoantibodies were shown to be associated with a variety of deleterious processes and outcomes. However, antibodies represent only 1 small part of the immune response, and further research is needed to elucidate the role of the cellular immune response in iDCM. Without this information, it remains unclear whether the presence of autoimmune antibodies represents the primary culprit, a secondary response to ongoing myocardial damage caused by other processes, an epiphenomenon, or a predisposition to developing iDCM. For example, autoantibodies predict the onset of disease in healthy adults, and it is possible that many of us develop “myocarditis” during a viral infection, but only those with preexisting cardiac autoantibodies go on to develop iDCM.74 Future studies are needed to identify the role of cellular immunity and to track the prevalence and burden of autoimmune antibodies through the disease course.
Trials using IA and IVIg therapies to target specific autoimmune mechanisms have demonstrated therapeutic potential, and the background literature support further investigations. However, trials thus far have been underpowered and have not focused on a specific population within iDCM. It is unlikely that all patients with iDCM will benefit from this form of therapy. Before larger therapeutic trials are undertaken to support or refute autoimmunity as a potential therapeutic target, steps to identify which patients with iDCM have an underlying autoimmune etiology must be constructed. Also, the point in the disease course at which this autoimmune process is most burdensome must be identified.
Acknowledgments
Supported in part by the National Institutes of Health, National Center for Research Resources, CTSA 1UL1RR024989, Cleveland, Ohio. Dr. Tang also received research support from Amgen Inc and Abbott Diagnostics, Inc.
References
- 1.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807–16. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 2.Sampietro T, Neglia D, Bionda A, Dal Pino B, Bigazzi F, Puntoni M, et al. Inflammatory markers and serum lipids in idiopathic dilated cardiomyopathy. Am J Cardiol. 2005;96:1718–20. doi: 10.1016/j.amjcard.2005.07.093. [DOI] [PubMed] [Google Scholar]
- 3.Kuhl U, Noutsias M, Seeberg B, Schultheiss HP. Immunohistological evidence for a chronic intramyocardial inflammatory process in dilated cardiomyopathy. Heart. 1996;75:295–300. doi: 10.1136/hrt.75.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aretz HT. Myocarditis: the Dallas criteria. Hum Pathol. 1987;18:619–24. doi: 10.1016/s0046-8177(87)80363-5. [DOI] [PubMed] [Google Scholar]
- 5.Liu PP, Mason JW. Advances in the understanding of myocarditis. Circulation. 2001;104:1076–82. doi: 10.1161/hc3401.095198. [DOI] [PubMed] [Google Scholar]
- 6.Kuhl U, Pauschinger M, Seeberg B, Lassner D, Noutsias M, Poller W, et al. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation. 2005;112:1965–70. doi: 10.1161/CIRCULATIONAHA.105.548156. [DOI] [PubMed] [Google Scholar]
- 7.Kuhl U, Pauschinger M, Schwimmbeck PL, Seeberg B, Lober C, Noutsias M, et al. Interferon-beta treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation. 2003;107:2793–8. doi: 10.1161/01.CIR.0000072766.67150.51. [DOI] [PubMed] [Google Scholar]
- 8.Woolf AD, Campion GV, Chishick A, Wise S, Cohen BJ, Klouda PT, et al. Clinical manifestations of human parvovirus B19 in adults. Arch Intern Med. 1989;149:1153–6. [PubMed] [Google Scholar]
- 9.Okuno T, Takahashi K, Balachandra K, Shiraki K, Yamanishi K, Takahashi M, et al. Seroepidemiology of human herpesvirus 6 infection in normal children and adults. J Clin Microbiol. 1989;27:651–3. doi: 10.1128/jcm.27.4.651-653.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lotze U, Egerer R, Tresselt C, Gluck B, Dannberg G, Stelzner A, et al. Frequent detection of parvovirus B19 genome in the myocardium of adult patients with idiopathic dilated cardiomyopathy. Med Microbiol Immunol. 2004;193:75–82. doi: 10.1007/s00430-003-0211-0. [DOI] [PubMed] [Google Scholar]
- 11.Kuethe F, Sigusch HH, Hilbig K, Tresselt C, Gluck B, Egerer R, et al. Detection of viral genome in the myocardium: lack of prognostic and functional relevance in patients with acute dilated cardiomyopathy. Am Heart J. 2007;153:850–8. doi: 10.1016/j.ahj.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Kyvik KO, Green A, Beck-Nielsen H. Concordance rates of insulin dependent diabetes mellitus: a population based study of young Danish twins. BMJ. 1995;311:913–7. doi: 10.1136/bmj.311.7010.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willer CJ, Dyment DA, Risch NJ, Sadovnick AD, Ebers GC. Twin concordance and sibling recurrence rates in multiple sclerosis. Proc Natl Acad Sci U S A. 2003;100:12877–82. doi: 10.1073/pnas.1932604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michels VV, Moll PP, Miller FA, Tajik AJ, Chu JS, Driscoll DJ, et al. The frequency of familial dilated cardiomyopathy in a series of patients with idiopathic dilated cardiomyopathy. N Engl J Med. 1992;326:77–82. doi: 10.1056/NEJM199201093260201. [DOI] [PubMed] [Google Scholar]
- 15.Limas CJ, Iakovis P, Anyfantakis A, Kroupis C, Cokkinos DV. Familial clustering of autoimmune diseases in patients with dilated cardiomyopathy. Am J Cardiol. 2004;93:1189–91. doi: 10.1016/j.amjcard.2004.01.060. [DOI] [PubMed] [Google Scholar]
- 16.Carlquist JF, Menlove RL, Murray MB, O'Connell JB, Anderson JL. HLA class II (DR and DQ) antigen associations in idiopathic dilated cardiomyopathy. Validation study and meta-analysis of published HLA association studies. Circulation. 1991;83:515–22. doi: 10.1161/01.cir.83.2.515. [DOI] [PubMed] [Google Scholar]
- 17.McKenna CJ, Codd MB, McCann HA, Sugrue DD. Idiopathic dilated cardiomyopathy: familial prevalence and HLA distribution. Heart. 1997;77:549–52. doi: 10.1136/hrt.77.6.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baba A, Yoshikawa T, Ogawa S. Autoantibodies produced against sarcolemmal Na-K-ATPase: possible upstream targets of arrhythmias and sudden death in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:1153–9. doi: 10.1016/s0735-1097(02)02075-2. [DOI] [PubMed] [Google Scholar]
- 19.Konstadoulakis MM, Kroumbouzou H, Tsiamis E, Trikas A, Toutouzas P. Clinical significance of antibodies against tropomyosin, actin and myosin in patients with dilated cardiomyopathy. J Clin Lab Immunol. 1993;40:61–7. [PubMed] [Google Scholar]
- 20.Stork S, Boivin V, Horf R, Hein L, Lohse MJ, Angermann CE, et al. Stimulating autoantibodies directed against the cardiac beta1-adrenergic receptor predict increased mortality in idiopathic cardiomyopathy. Am Heart J. 2006;152:697–704. doi: 10.1016/j.ahj.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Baba A, Yoshikawa T, Fukuda Y, Sugiyama T, Shimada M, Akaishi M, et al. Autoantibodies against M2-muscarinic acetylcholine receptors: new upstream targets in atrial fibrillation in patients with dilated cardiomyopathy. Eur Heart J. 2004;25:1108–15. doi: 10.1016/j.ehj.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Lauer B, Schannwell M, Kuhl U, Strauer BE, Schultheiss HP. Anti-myosin autoantibodies are associated with deterioration of systolic and diastolic left ventricular function in patients with chronic myocarditis. J Am Coll Cardiol. 2000;35:11–8. doi: 10.1016/s0735-1097(99)00485-4. [DOI] [PubMed] [Google Scholar]
- 23.Muller J, Wallukat G, Dandel M, Bieda H, Brandes K, Spiegelsberger S, et al. Immunoglobulin adsorption in patients with idiopathic dilated cardiomyopathy. Circulation. 2000;101:385–91. doi: 10.1161/01.cir.101.4.385. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham MW, Hall NK, Krisher KK, Spanier AM. A study of anti-group A streptococcal monoclonal antibodies cross-reactive with myosin. J Immunol. 1986;136:293–8. [PubMed] [Google Scholar]
- 25.Alvarez FL, Neu N, Rose NR, Craig SW, Beisel KW. Heart-specific autoantibodies induced by Coxsackievirus B3: identification of heart autoantigens. Clin Immunol Immunopathol. 1987;43:129–39. doi: 10.1016/0090-1229(87)90164-4. [DOI] [PubMed] [Google Scholar]
- 26.Galvin JE, Hemric ME, Kosanke SD, Factor SM, Quinn A, Cunningham MW. Induction of myocarditis and valvulitis in lewis rats by different epitopes of cardiac myosin and its implications in rheumatic carditis. Am J Pathol. 2002;160:297–306. doi: 10.1016/S0002-9440(10)64373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caforio AL, Grazzini M, Mann JM, Keeling PJ, Bottazzo GF, McKenna WJ, et al. Identification of alpha- and beta-cardiac myosin heavy chain isoforms as major autoantigens in dilated cardiomyopathy. Circulation. 1992;85:1734–42. doi: 10.1161/01.cir.85.5.1734. [DOI] [PubMed] [Google Scholar]
- 28.Dangas G, Konstadoulakis MM, Epstein SE, Stefanadis CI, Kymionis GD, Toutouza MG, et al. Prevalence of autoantibodies against contractile proteins in coronary artery disease and their clinical implications. Am J Cardiol. 2000;85:870–2. A6, A9. doi: 10.1016/s0002-9149(99)00883-8. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Heuser JS, Cunningham LC, Kosanke SD, Cunningham MW. Mimicry and antibody-mediated cell signaling in autoimmune myocarditis. J Immunol. 2006;177:8234–40. doi: 10.4049/jimmunol.177.11.8234. [DOI] [PubMed] [Google Scholar]
- 30.Staudt Y, Mobini R, Fu M, Felix SB, Kuhn JP, Staudt A. Beta1-adrenoceptor antibodies induce apoptosis in adult isolated cardiomyocytes. Eur J Pharmacol. 2003;466:1–6. doi: 10.1016/s0014-2999(03)01431-6. [DOI] [PubMed] [Google Scholar]
- 31.Ferrari I, Levin MJ, Wallukat G, Elies R, Lebesgue D, Chiale P, et al. Molecular mimicry between the immunodominant ribosomal protein P0 of Trypanosoma cruzi and a functional epitope on the human beta 1-adrenergic receptor. J Exp Med. 1995;182:59–65. doi: 10.1084/jem.182.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jahns R, Boivin V, Krapf T, Wallukat G, Boege F, Lohse MJ. Modulation of beta1-adrenoceptor activity by domain-specific antibodies and heart failure-associated autoantibodies. J Am Coll Cardiol. 2000;36:1280–7. doi: 10.1016/s0735-1097(00)00881-0. [DOI] [PubMed] [Google Scholar]
- 33.Wallukat G, Wollenberger A, Morwinski R, Pitschner HF. Anti-beta 1-adrenoceptor autoantibodies with chronotropic activity from the serum of patients with dilated cardiomyopathy: mapping of epitopes in the first and second extracellular loops. J Mol Cell Cardiol. 1995;27:397–406. doi: 10.1016/s0022-2828(08)80036-3. [DOI] [PubMed] [Google Scholar]
- 34.Christ T, Wettwer E, Dobrev D, Adolph E, Knaut M, Wallukat G, et al. Autoantibodies against the beta1 adrenoceptor from patients with dilated cardiomyopathy prolong action potential duration and enhance contractility in isolated cardiomyocytes. J Mol Cell Cardiol. 2001;33:1515–25. doi: 10.1006/jmcc.2001.1414. [DOI] [PubMed] [Google Scholar]
- 35.Jahns R, Boivin V, Hein L, Triebel S, Angermann CE, Ertl G, et al. Direct evidence for a beta 1-adrenergic receptor-directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J Clin Invest. 2004;113:1419–29. doi: 10.1172/JCI20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mobini R, Staudt A, Felix SB, Baumann G, Wallukat G, Deinum J, et al. Hemodynamic improvement and removal of autoantibodies against beta1-adrenergic receptor by immunoadsorption therapy in dilated cardiomyopathy. J Autoimmun. 2003;20:345–50. doi: 10.1016/s0896-8411(03)00042-8. [DOI] [PubMed] [Google Scholar]
- 37.Jahns R, Boivin V, Siegmund C, Inselmann G, Lohse MJ, Boege F. Autoantibodies activating human beta1-adrenergic receptors are associated with reduced cardiac function in chronic heart failure. Circulation. 1999;99:649–54. doi: 10.1161/01.cir.99.5.649. [DOI] [PubMed] [Google Scholar]
- 38.Miao GB, Liu JC, Liu MB, Wu JL, Zhang G, Chang J, et al. Autoantibody against beta1-adrenergic receptor and left ventricular remodeling changes in response to metoprolol treatment. Eur J Clin Invest. 2006;36:614–20. doi: 10.1111/j.1365-2362.2006.01705.x. [DOI] [PubMed] [Google Scholar]
- 39.Iwata M, Yoshikawa T, Baba A, Anzai T, Mitamura H, Ogawa S. Autoantibodies against the second extracellular loop of beta1-adrenergic receptors predict ventricular tachycardia and sudden death in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2001;37:418–24. doi: 10.1016/s0735-1097(00)01109-8. [DOI] [PubMed] [Google Scholar]
- 40.Horwich TB, Patel J, MacLellan WR, Fonarow GC. Cardiac troponin I is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation. 2003;108:833–8. doi: 10.1161/01.CIR.0000084543.79097.34. [DOI] [PubMed] [Google Scholar]
- 41.Goser S, Andrassy M, Buss SJ, Leuschner F, Volz CH, Ottl R, et al. Cardiac troponin I but not cardiac troponin T induces severe autoimmune inflammation in the myocardium. Circulation. 2006;114:1693–702. doi: 10.1161/CIRCULATIONAHA.106.635664. [DOI] [PubMed] [Google Scholar]
- 42.Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9:1477–83. doi: 10.1038/nm955. [DOI] [PubMed] [Google Scholar]
- 43.Shmilovich H, Danon A, Binah O, Roth A, Chen G, Wexler D, et al. Autoantibodies to cardiac troponin I in patients with idiopathic dilated and ischemic cardiomyopathy. Int J Cardiol. 2007;117:198–203. doi: 10.1016/j.ijcard.2006.04.077. [DOI] [PubMed] [Google Scholar]
- 44.Noutsias M, Pauschinger M, Schultheiss H, Kh U. Phenotypic characterization of infiltrates in dilated cardiomyopathy—diagnostic significance of T-lymphocytes and macrophages in inflammatory cardiomyopathy. Med Sci Monit. 2002;8:CR478–87. [PubMed] [Google Scholar]
- 45.Omerovic E, Bollano E, Andersson B, Kujacic V, Schulze W, Hjalmarson A, et al. Induction of cardiomyopathy in severe combined immunodeficiency mice by transfer of lymphocytes from patients with idiopathic dilated cardiomyopathy. Autoimmunity. 2000;32:271–80. doi: 10.3109/08916930008994101. [DOI] [PubMed] [Google Scholar]
- 46.Lunardi C, Tiso M, Borgato L, Nanni L, Millo R, De Sandre G, et al. Chronic parvovirus B19 infection induces the production of anti-virus antibodies with autoantigen binding properties. Eur J Immunol. 1998;28:936–48. doi: 10.1002/(SICI)1521-4141(199803)28:03<936::AID-IMMU936>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 47.Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lapierre P, Beland K, Alvarez F. Pathogenesis of autoimmune hepatitis: from break of tolerance to immune-mediated hepatocyte apoptosis. Transl Res. 2007;149:107–13. doi: 10.1016/j.trsl.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 49.Hughes RA, Allen D, Makowska A, Gregson NA. Pathogenesis of chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 2006;11:30–46. doi: 10.1111/j.1085-9489.2006.00061.x. [DOI] [PubMed] [Google Scholar]
- 50.Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594–602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 51.Parrillo JE, Cunnion RE, Epstein SE, Parker MM, Suffredini AF, Brenner M, et al. A prospective, randomized, controlled trial of prednisone for dilated cardiomyopathy. N Engl J Med. 1989;321:1061–8. doi: 10.1056/NEJM198910193211601. [DOI] [PubMed] [Google Scholar]
- 52.Wojnicz R, Nowalany-Kozielska E, Wojciechowska C, Glanowska G, Wilczewski P, Niklewski T, et al. Randomized, placebo-controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy: two-year follow-up results. Circulation. 2001;104:39–45. doi: 10.1161/01.cir.104.1.39. [DOI] [PubMed] [Google Scholar]
- 53.Gullestad L, Ueland T, Fjeld JG, Holt E, Gundersen T, Breivik K, et al. Effect of thalidomide on cardiac remodeling in chronic heart failure: results of a double-blind, placebo-controlled study. Circulation. 2005;112:3408–14. doi: 10.1161/CIRCULATIONAHA.105.564971. [DOI] [PubMed] [Google Scholar]
- 54.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–40. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 55.Skudicky D, Bergemann A, Sliwa K, Candy G, Sareli P. Beneficial effects of pentoxifylline in patients with idiopathic dilated cardiomyopathy treated with angiotensin-converting enzyme inhibitors and carvedilol: results of a randomized study. Circulation. 2001;103:1083–8. doi: 10.1161/01.cir.103.8.1083. [DOI] [PubMed] [Google Scholar]
- 56.Gullestad L, Semb AG, Holt E, Skardal R, Ueland T, Yndestad A, et al. Effect of thalidomide in patients with chronic heart failure. Am Heart J. 2002;144:847–50. doi: 10.1067/mhj.2002.125628. [DOI] [PubMed] [Google Scholar]
- 57.Torre-Amione G, Bourge RC, Colucci WS, Greenberg B, Pratt C, Rouleau JL, et al. A study to assess the effects of a broad-spectrum immune modulatory therapy on mortality and morbidity in patients with chronic heart failure: the ACCLAIM trial rationale and design. Can J Cardiol. 2007;23:369–76. doi: 10.1016/s0828-282x(07)70770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kjekshus J, Apetrei E, Barrios V, Bohm M, Cleland JG, Cornel JH, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–61. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 59.Dorffel WV, Felix SB, Wallukat G, Brehme S, Bestvater K, Hofmann T, et al. Short-term hemodynamic effects of immunoadsorption in dilated cardiomyopathy. Circulation. 1997;95:1994–7. doi: 10.1161/01.cir.95.8.1994. [DOI] [PubMed] [Google Scholar]
- 60.Felix SB, Staudt A, Dorffel WV, Stangl V, Merkel K, Pohl M, et al. Hemodynamic effects of immunoadsorption and subsequent immunoglobulin substitution in dilated cardiomyopathy: three-month results from a randomized study. J Am Coll Cardiol. 2000;35:1590–8. doi: 10.1016/s0735-1097(00)00568-4. [DOI] [PubMed] [Google Scholar]
- 61.Felix SB, Staudt A, Landsberger M, Grosse Y, Stangl V, Spielhagen T, et al. Removal of cardiodepressant antibodies in dilated cardiomyopathy by immunoadsorption. J Am Coll Cardiol. 2002;39:646–52. doi: 10.1016/s0735-1097(01)01794-6. [DOI] [PubMed] [Google Scholar]
- 62.Wallukat G, Muller J, Hetzer R. Specific removal of beta1-adrenergic autoantibodies from patients with idiopathic dilated cardiomyopathy. N Engl J Med. 2002;347:1806. doi: 10.1056/NEJM200211283472220. [DOI] [PubMed] [Google Scholar]
- 63.Schimke I, Muller J, Priem F, Kruse I, Schon B, Stein J, et al. Decreased oxidative stress in patients with idiopathic dilated cardiomyopathy one year after immunoglobulin adsorption. J Am Coll Cardiol. 2001;38:178–83. doi: 10.1016/s0735-1097(01)01309-2. [DOI] [PubMed] [Google Scholar]
- 64.Staudt A, Dorr M, Staudt Y, Bohm M, Probst M, Empen K, et al. Role of immunoglobulin G3 subclass in dilated cardiomyopathy: results from protein A immunoadsorption. Am Heart J. 2005;150:729–36. doi: 10.1016/j.ahj.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Cooper LT, Belohlavek M, Korinek J, Yoshifuku S, Sengupta PP, Burgstaler EA, et al. A pilot study to assess the use of protein a immunoadsorption for chronic dilated cardiomyopathy. J Clin Apher. 2007;22:210–4. doi: 10.1002/jca.20130. [DOI] [PubMed] [Google Scholar]
- 66.Staudt A, Hummel A, Ruppert J, Dorr M, Trimpert C, Birkenmeier K, et al. Immunoadsorption in dilated cardiomyopathy: 6-month results from a randomized study. Am Heart J. 2006;152:712.e1–6. doi: 10.1016/j.ahj.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 67.Dorner A, Kallwellis-Opara A, Pauschinger M, Kuhl U, Schultheiss H. Cardiac autoantibodies in viral myocarditis. Heart Fail Clin. 2005;1:333–43. doi: 10.1016/j.hfc.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 68.van der Meche FG, Schmitz PI. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain-Barre syndrome. Dutch Guillain-Barre Study Group. N Engl J Med. 1992;326:1123–9. doi: 10.1056/NEJM199204233261705. [DOI] [PubMed] [Google Scholar]
- 69.Gullestad L, Aass H, Fjeld JG, Wikeby L, Andreassen AK, Ihlen H, et al. Immunomodulating therapy with intravenous immunoglobulin in patients with chronic heart failure. Circulation. 2001;103:220–5. doi: 10.1161/01.cir.103.2.220. [DOI] [PubMed] [Google Scholar]
- 70.McNamara DM, Holubkov R, Starling RC, Dec GW, Loh E, Torre-Amione G, et al. Controlled trial of intravenous immune globulin in recent-onset dilated cardiomyopathy. Circulation. 2001;103:2254–9. doi: 10.1161/01.cir.103.18.2254. [DOI] [PubMed] [Google Scholar]
- 71.McNamara DM, Rosenblum WD, Janosko KM, Trost MK, Villaneuva FS, Demetris AJ, et al. Intravenous immune globulin in the therapy of myocarditis and acute cardiomyopathy. Circulation. 1997;95:2476–8. doi: 10.1161/01.cir.95.11.2476. [DOI] [PubMed] [Google Scholar]
- 72.Kishimoto C, Shioji K, Kinoshita M, Iwase T, Tamaki S, Fujii M, et al. Treatment of acute inflammatory cardiomyopathy with intravenous immunoglobulin ameliorates left ventricular function associated with suppression of inflammatory cytokines and decreased oxidative stress. Int J Cardiol. 2003;91:173–8. doi: 10.1016/s0167-5273(03)00002-0. [DOI] [PubMed] [Google Scholar]
- 73.Drucker NA, Colan SD, Lewis AB, Beiser AS, Wessel DL, Takahashi M, et al. Gamma-globulin treatment of acute myocarditis in the pediatric population. Circulation. 1994;89:252–7. doi: 10.1161/01.cir.89.1.252. [DOI] [PubMed] [Google Scholar]
- 74.Caforio AL, Mahon NG, Baig MK, Tona F, Murphy RT, Elliott PM, et al. Prospective familial assessment in dilated cardiomyopathy: cardiac autoantibodies predict disease development in asymptomatic relatives. Circulation. 2007;115:76–83. doi: 10.1161/CIRCULATIONAHA.106.641472. [DOI] [PubMed] [Google Scholar]