Abstract
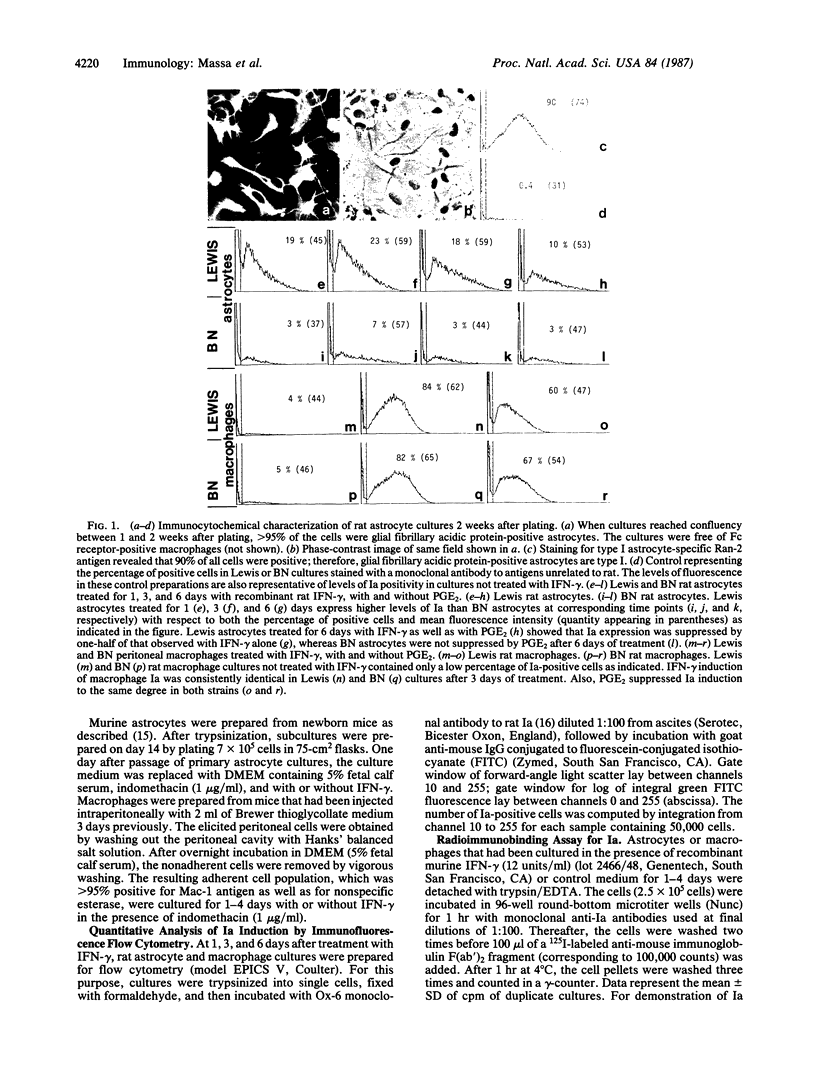
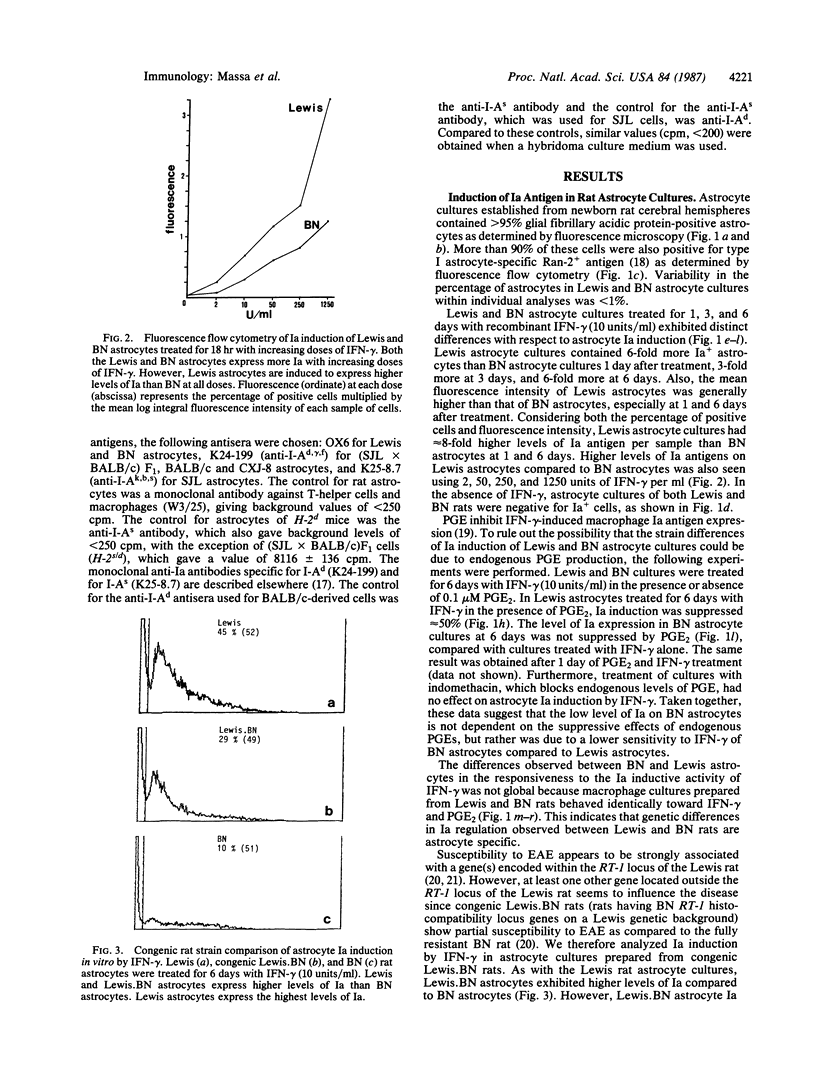
In search of a phenotypic marker determining genetically controlled susceptibility to delayed-type hypersensitivity (DTH) reactions in the brain--in particular, experimental autoimmune encephalomyelitis (EAE)--we have compared the gamma-interferon (IFN-gamma) induction of Ia molecules on astrocytes and macrophages from rat and mouse strains that are susceptible or resistant to this disease. We focused on Ia expression because DTH reactions to self or foreign antigens are largely mediated by lymphocytes restricted by class II (Ia) antigens of the major histocompatibility complex (MHC). Our data demonstrate that Lewis (fully susceptible) and Brown Norway (BN) (fully resistant) rats are very different in that Lewis astrocytes express much higher levels of Ia than BN astrocytes. Similar data were obtained from an analysis of EAE-susceptible and -resistant mouse strains (SJL and BALB/c, respectively), which suggests that this phenomenon may be universal and not limited to only one mammalian species. At least one gene responsible for Ia hyperinduction is located outside the rat RT-1 or the mouse MHC locus. Animals congenic at the RT-1 or MHC locus of the resistant strain but with background genes of the susceptible strain exhibit intermediate levels of Ia compared to fully resistant and susceptible rodents, which fits well with the reduced EAE susceptibility of these congenic animals. Furthermore, hyperinduction of Ia is astrocyte specific, since peritoneal macrophages of susceptible and resistant strains exhibit identical profiles of Ia induction. Thus, astrocyte Ia hyperinducibility may be a major strain- and tissue-specific factor that contributes to Ia-restricted DTH reactions in the brain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Bartlett P. F., Noble M. D., Pruss R. M., Raff M. C., Rattray S., Williams C. A. Rat neural antigen-2 (RAN-2): a cell surface antigen on astrocytes, ependymal cells, Müller cells and lepto-meninges defined by a monoclonal antibody. Brain Res. 1981 Jan 12;204(2):339–351. doi: 10.1016/0006-8993(81)90593-x. [DOI] [PubMed] [Google Scholar]
- Ben-Nun A., Wekerle H., Cohen I. R. The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur J Immunol. 1981 Mar;11(3):195–199. doi: 10.1002/eji.1830110307. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Pujol-Borrell R., Hanafusa T., Feldmann M. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet. 1983 Nov 12;2(8359):1115–1119. doi: 10.1016/s0140-6736(83)90629-3. [DOI] [PubMed] [Google Scholar]
- Fierz W., Endler B., Reske K., Wekerle H., Fontana A. Astrocytes as antigen-presenting cells. I. Induction of Ia antigen expression on astrocytes by T cells via immune interferon and its effect on antigen presentation. J Immunol. 1985 Jun;134(6):3785–3793. [PubMed] [Google Scholar]
- Fontana A., Fierz W., Wekerle H. Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature. 1984 Jan 19;307(5948):273–276. doi: 10.1038/307273a0. [DOI] [PubMed] [Google Scholar]
- Fontana A., Kristensen F., Dubs R., Gemsa D., Weber E. Production of prostaglandin E and an interleukin-1 like factor by cultured astrocytes and C6 glioma cells. J Immunol. 1982 Dec;129(6):2413–2419. [PubMed] [Google Scholar]
- Frei K., Bodmer S., Schwerdel C., Fontana A. Astrocytes of the brain synthesize interleukin 3-like factors. J Immunol. 1985 Dec;135(6):4044–4047. [PubMed] [Google Scholar]
- Gasser D. L., Newlin C. M., Palm J., Gonatas N. K. Genetic control of susceptibility to experimental allergic encephalomyelitis in rats. Science. 1973 Aug 31;181(4102):872–873. doi: 10.1126/science.181.4102.872. [DOI] [PubMed] [Google Scholar]
- Gillies S. D., Folsom V., Tonegawa S. Cell type-specific enhancer element associated with a mouse MHC gene, E beta. Nature. 1984 Aug 16;310(5978):594–597. doi: 10.1038/310594a0. [DOI] [PubMed] [Google Scholar]
- Gonatas N. K., Howard J. C. Inhibition of experimental allergic encephalomyelitis in rats severely depleted of T cells. Science. 1974 Nov 29;186(4166):839–841. doi: 10.1126/science.186.4166.839. [DOI] [PubMed] [Google Scholar]
- Günther E., Odenthal H., Wechsler W. Association between susceptibility to experimental allergic encephalomyelitis and the major histocompatibility system in congenic rat strains. Clin Exp Immunol. 1978 Jun;32(3):429–434. [PMC free article] [PubMed] [Google Scholar]
- Hickey W. F., Gonatas N. K., Kimura H., Wilson D. B. Identification and quantitation of T lymphocyte subsets found in the spinal cord of the Lewis rat during acute experimental allergic encephalomyelitis. J Immunol. 1983 Dec;131(6):2805–2809. [PubMed] [Google Scholar]
- Hirsch M. R., Wietzerbin J., Pierres M., Goridis C. Expression of Ia antigens by cultured astrocytes treated with gamma-interferon. Neurosci Lett. 1983 Oct 31;41(1-2):199–204. doi: 10.1016/0304-3940(83)90247-1. [DOI] [PubMed] [Google Scholar]
- Knobler R. L., Linthicum D. S., Cohn M. Host genetic regulation of acute MHV-4 viral encephalomyelitis and acute experimental autoimmune encephalomyelitis in (BALB/cKe x SJL/J) recombinant-inbred mice. J Neuroimmunol. 1985 Apr;8(1):15–28. doi: 10.1016/S0165-5728(85)80044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch N., Hämmerling G. J., Tada N., Kimura S., Hämmerling U. Cross-blocking studies with monoclonal antibodies against I-A molecules of haplotypes b, d and k. Eur J Immunol. 1982 Nov;12(11):909–914. doi: 10.1002/eji.1830121103. [DOI] [PubMed] [Google Scholar]
- Kong Y. C., Audibert F., Giraldo A. A., Rose N. R., Chedid L. Effects of natural or synthetic microbial adjuvants on induction of autoimmune thyroiditis. Infect Immun. 1985 Jul;49(1):40–45. doi: 10.1128/iai.49.1.40-45.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S., Sowinski R. Allergic encephalomyelitis in the reputedly resistant Brown Norway strain of rats. J Immunol. 1975 Feb;114(2 Pt 1):597–601. [PubMed] [Google Scholar]
- Levine S., Sowinski R. Experimental allergic encephalomyelitis in inbred and outbred mice. J Immunol. 1973 Jan;110(1):139–143. [PubMed] [Google Scholar]
- Mason D. W., Dallman M., Barclay A. N. Graft-versus-host disease induces expression of Ia antigen in rat epidermal cells and gut epithelium. Nature. 1981 Sep 10;293(5828):150–151. doi: 10.1038/293150a0. [DOI] [PubMed] [Google Scholar]
- Massa P. T., Mugnaini E. Cell junctions and intramembrane particles of astrocytes and oligodendrocytes: a freeze-fracture study. Neuroscience. 1982 Feb;7(2):523–538. doi: 10.1016/0306-4522(82)90285-8. [DOI] [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979 Jun;9(6):426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- Montgomery I. N., Rauch H. C. Experimental allergic encephalomyelitis (EAE) in mice: primary control of EAE susceptibility is outside the H-2 complex. J Immunol. 1982 Jan;128(1):421–425. [PubMed] [Google Scholar]
- Pettinelli C. B., McFarlin D. E. Adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice after in vitro activation of lymph node cells by myelin basic protein: requirement for Lyt 1+ 2- T lymphocytes. J Immunol. 1981 Oct;127(4):1420–1423. [PubMed] [Google Scholar]
- Sakai K., Tabira T., Endoh M., Steinman L. Ia expression in chronic relapsing experimental allergic encephalomyelitis induced by long-term cultured T cell lines in mice. Lab Invest. 1986 Mar;54(3):345–352. [PubMed] [Google Scholar]
- Snyder D. S., Beller D. I., Unanue E. R. Prostaglandins modulate macrophage Ia expression. Nature. 1982 Sep 9;299(5879):163–165. doi: 10.1038/299163a0. [DOI] [PubMed] [Google Scholar]
- Sriram S., Steinman L. Anti I-A antibody suppresses active encephalomyelitis: treatment model for diseases linked to IR genes. J Exp Med. 1983 Oct 1;158(4):1362–1367. doi: 10.1084/jem.158.4.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D., Wekerle H. Ia-restricted encephalitogenic T lymphocytes mediating EAE lyse autoantigen-presenting astrocytes. Nature. 1986 Mar 6;320(6057):70–72. doi: 10.1038/320070a0. [DOI] [PubMed] [Google Scholar]
- Traugott U., Scheinberg L. C., Raine C. S. On the presence of Ia-positive endothelial cells and astrocytes in multiple sclerosis lesions and its relevance to antigen presentation. J Neuroimmunol. 1985 Apr;8(1):1–14. doi: 10.1016/s0165-5728(85)80043-6. [DOI] [PubMed] [Google Scholar]
- Trotter J., Sriram S., Rassenti L., Chou C. H., Fritz R. B., Steinman L. Characterization of T cell lines and clones from SJL/J and (BALB/c x SJL/J)F1 mice specific for myelin basic protein. J Immunol. 1985 Apr;134(4):2322–2327. [PubMed] [Google Scholar]
- Wong G. H., Bartlett P. F., Clark-Lewis I., Battye F., Schrader J. W. Inducible expression of H-2 and Ia antigens on brain cells. Nature. 1984 Aug 23;310(5979):688–691. doi: 10.1038/310688a0. [DOI] [PubMed] [Google Scholar]
- de Préval C., Lisowska-Grospierre B., Loche M., Griscelli C., Mach B. A trans-acting class II regulatory gene unlinked to the MHC controls expression of HLA class II genes. Nature. 1985 Nov 21;318(6043):291–293. doi: 10.1038/318291a0. [DOI] [PubMed] [Google Scholar]




