Abstract
Primary objectives
Children with a history of cancer are at increased risk for cognitive impairments, particularly in executive and memory domains. Traditional, in-person cognitive rehabilitation strategies may be unavailable and/or impractical for many of these children given difficulties related to resources and health status. The feasibility and efficacy of implementing a computerized, home-based cognitive rehabilitation curriculum designed to improve executive function skills was examined in these children.
Methods
A one-arm open trial pilot study of an original executive function cognitive rehabilitation curriculum was conducted with 23 paediatric cancer survivors aged 7–19.
Results
Compliance with the cognitive rehabilitation program was 83%, similar to that of many traditional programs. Following the cognitive intervention, participants showed significantly increased processing speed, cognitive flexibility, verbal and visual declarative memory scores as well as significantly increased pre-frontal cortex activation compared to baseline.
Conclusions
These results suggest that a program of computerized cognitive exercises can be successfully implemented at home in young children with cancer. These exercises may be effective for improving executive and memory skills in this group, with concurrent changes in neurobiologic status.
Keywords: Leukaemia, brain tumour, children, cancer, cognitive rehabilitation, functional MRI
Introduction
Survivors of paediatric leukaemia and brain tumour are at significantly increased risk for long-term cognitive impairments including executive function, memory, motor, visual-spatial and language deficits [1–3]. Human neuroimaging studies as well as animal models suggest that these cancers may be associated with brain injury related to chemotherapy, radiation and/or surgery [4–9]. In fact, neuroimaging studies of these children have revealed reduced brain volumes, impaired brain function and altered neurochemistry, even into adulthood [5, 10–19]. Increasing survival rates contribute to a large and growing cohort of cognitively affected children. The prospect of intervening to change this trajectory thus has tremendous significance—potentially affecting school services, family dynamics, vocational readiness and social services support.
Cognitive rehabilitation is a method of treatment for cognitive deficits that involves restoring impaired function and/or increased compensation for the area of deficit through strategy training and/or repeated skills practice [20]. It has been implemented successfully in children with other forms of brain injury including traumatic brain injury (TBI) [21] as well as other brain-based disorders such as attention-deficits [22] and dyslexia [23]. Butler et al. [24] conducted a successful remediation program for attention deficits in young survivors of leukaemia and brain tumour involving multiple, in-person sessions. These results suggest that traditional, in-person cognitive rehabilitation is a promising treatment method for children with cancer; however, these programs tend to be costly, impractical and/or unavailable to the majority of patients and standardization across clinicians is difficult.
Children with leukaemia or brain tumour show deficits in executive function areas in addition to attention, including impairments in cognitive flexibility and working memory [25, 26]. Similar to attention, cognitive flexibility and working memory are executive function skills critical for goal-directed behaviour [27]. Cognitive flexibility is the ability to generate alternate solutions to problems and fluidly shift between ideas and actions [28]. Working memory refers to the manipulation of information in temporary storage [29]. These skills are vulnerable to a variety of neuropsychiatric, developmental and medical conditions and tend to have diffuse effects on behaviour and quality-of-life, but are often the most difficult to assess and treat [27, 30–32]. Improving these skills can have positive downstream effects on other deficits including memory, social cognition, visual-spatial skills and expressive language [33, 34]. Thus, treatment for these skills may be the most likely means for having the greatest impact on cognitive outcome in paediatric cancer.
Computer-based cognitive intervention programs for children have become increasingly popular during the past several years [22, 35, 36] and have many advantages over traditional approaches including immediate feedback, the ability to systematize delivery of the intervention and modifications to difficulty level, the ability to quantify progress and the provision of an entertaining and engaging interface. Studies of computer-based cognitive skills training programs demonstrate that they are at least equally effective as compared to traditional approaches [37]. Computerized programs also allow for home practice, making them more accessible and practical for a wider range of participants. This may be particularly important for populations such as paediatric cancer patients whose condition may require isolation precautions during certain disease and treatment stages due to lowered immune function, for example.
However, the lack of direct clinician/researcher supervision may make home training more practical, but may also reduce motivation and compliance. These potential problems are increased in children and adolescents given that they often require more supervision and structure than adults. This may be particularly true of children and adolescents who have executive function impairments that reduce behavioural regulation. Children with executive functions who also have significant health problems may have even more difficulty with a home-based, computerized program. Paediatric cancer survivors tend to experience long-term fatigue, health issues and/or psychological difficulties [38, 39]. As a result, they may require more intensive clinical support and supervision during cognitive interventions. Therefore, one of the main goals of this pilot study was to determine the feasibility of implementing a computerized, home-based cognitive skills training program in a sample of children with cancer. This goal is important not only for evaluating executive function remediation specifically in paediatric cancer, but also to increase understanding regarding potential remediation alternatives for children in general.
A cognitive rehabilitation program for executive function was designed, including attention, cognitive flexibility and working memory skills remediation. A computer-based delivery of the intervention was chosen to significantly reduce participant and clinician burden and to provide a highly consistent intervention implementation. A one-armed, open, pilot trial of this program was conducted to test its feasibility and efficacy in children with paediatric cancer. Children with a history of either acute lymphoblastic leukaemia (ALL) or posterior fossa brain tumour were included as these are the diagnoses included in existing studies of cognitive and neurodevelopmental outcome in paediatric cancer. Additionally, these were the diagnostic categories included in previous paediatric cancer remediation studies [24, 40]. Furthermore, the authors did not want the results of the trial to be restricted to just one paediatric cancer type. While it was not feasible due to resource limitations to include every paediatric cancer in this trial, ALL and posterior fossa brain tumour are the most common paediatric cancers [41] and thus it was believed that these categories would be the most representative of cancer-related brain injury in children for this initial, preliminary investigation.
Deficits in attention, cognitive flexibility and working memory, as well as other executive functions, tend to be associated with abnormalities in the prefrontal cortex in a variety of paediatric conditions [27], including leukaemia and brain tumour [14, 26]. Functional MRI (fMRI) provides noninvasive, in vivo assessment of cognitive rehabilitation treatment efficacy in terms of the intervention’s putative ability to result in neurobiologic changes that represent improved cognitive performance [42]. Previous studies have shown significantly increased brain activation associated with various cognitive training programmes, irrespective of treatment length (some interventions as short as 30 minutes) [43–48]. Thus, it was hypothesized that a cognitive rehabilitation program targeting executive function skills would be associated with increased prefrontal function.
Methods
Participants
Twenty-five children and adolescents aged 7–19 years (mean = 12.6, SD = 4.1) with a history of malignancy (acute lymphoblastic leukaemia, n = 14, and posterior fossa brain tumours: medulloblastoma, 4th ventricle ependymoma and pineal tumor, n = 9) that involved radiation and/or chemotherapy treatment were screened for inclusion in this study. Participants were included if they had completed all anti-cancer treatments including chemotherapy, radiation and surgery for at least 6 months (mean = 37, SD = 30; range = 6–126 months). A minimum age of 7 years was selected to ensure adequate reading level for the intervention program as well as compliance with the behavioural demands of functional neuroimaging.
Participants were enrolled only if they demonstrated ‘deficient’ executive function defined as two or more executive function test scores that were 1 SD or more below the test normative mean or their own Full Scale IQ score (mean number of ‘deficient’ test scores = 3.8, SD = 1.8, range = 2–8). One standard deviation below the normative mean is a commonly used marker of clinical significance, tends to generally demarcate clinical classification categories and is a conservative standard for indicating if the difference between two test scores is significant [49]. The requirement of two or more low scores to indicate deficient function was largely arbitrary but, given that this constituted 40% of the executive function tests, it was judged to be a reasonably conservative but not overly restrictive criterion. Scores that were lower than the child’s own Full Scale IQ score were included in the deficit definition to be consistent with standard clinical practice. Specifically, ability-achievement discrepancy analysis of children’s testing performance reflects the federal mandate regarding the definition of learning difficulties [50].
Participants were excluded for any magnetic resonance imaging (MRI) contraindications, major sensory deficit (hearing loss, blindness, significant motor dysfunction) and any history of neurologic, psychiatric or major medical conditions unrelated to the cancer diagnosis that are known to affect cognitive function (e.g. pre-term birth, seizure disorder). Participation required home internet access, but this was not an issue for any of the individuals screened for the study. This study was approved by the Stanford University Institutional Review Board and all participants of 18 years and older or the parents of participants under 18 years provided written informed consent prior to participation.
Neurocognitive assessment
All participants were administered standardized tests, detailed in Table I, both before and after the cognitive rehabilitation program. The testing battery was designed to assess cognitive domains previously shown to be disrupted in ALL or brain tumour including intellectual function (IQ), memory, language and visual-spatial processing with an emphasis on the executive functions to be remediated in the intervention. Selected measures were chosen for having strong psychometric properties (e.g. test–re-test reliability) and specific validation in children. The battery also was designed to be relatively brief (1.5–2 hours) to increase compliance and reduce participant fatigue considering that children with cancer tend to have increased levels of fatigue [51]. Participants were given scheduled rest breaks as well as any additional requested breaks. Examiners were blinded to assessment status (pre- vs post-intervention). Participants were administered the testing battery on the same day as their MRI scans.
Table I.
Neurocognitive assessment battery.
| Test name | Domain measured | Reference |
|---|---|---|
| Wechsler Intelligence Scale for Children 4th Edition (WISC-IV)–age 7–16 or Wechsler Adult Intelligence Scale 3rd Edition (WAIS-III)–age 17–19 | Intellectual function (including Full Scale IQ, Verbal Comprehension, Perceptual Reasoning, Working Memory and Processing Speed Indices) | [47, 88] |
| Wide Range Assessment of Learning and Memory 2nd Edition (WRAML2) List Memory and Picture Memory | Verbal and visual memory | [89] |
| NEPSY II Animal Sort (age 7–16) or Delis Kaplin Executive System (DKEFS) Sorting Test (age 17–19) | Executive function: cognitive flexibility | [90, 91] |
| Woodcock-Johnson 3rd Edition (WJ-III) Cancellation Test | Executive function: attention and processing speed | [92] |
| Motor Free Test of Visual Perception 3rd Edition (MVPT-3) | Spatial relationships, visual discrimination, visual memory | [93] |
Neuroimaging assessment
Functional MRI (fMRI) scans were obtained using a T2* weighted [52] gradient echo spiral pulse sequence: TR = 2000 msec, TE = 30 msec, flip angle = 89° and 1 interleave, FOV = 200, matrix = 64 × 64, in-plane resolution = 3.125 with a 3T GE Signa HDx whole body scanner (GE Medical Systems, Milwaukee, WI). Executive prefrontal activation during the fMRI scan was measured using a card sorting task. Participants completed fMRI scans before and after the cognitive rehabilitation program. All participants completed MRI scans and cognitive testing on the same day.
The card sorting task was based on the Wisconsin Card Sorting Test, which has been used to assess executive function in children [53] and to activate the executive prefrontal regions [54, 55]. The present adaptation of this task for fMRI included an experimental and a control condition, both comprised of four, 12 trial blocks. Stimuli for each trial consisted of three target cards at the top of the screen and one variable card at the bottom. The target cards were labelled 1, 2 or 3 and were the same across all trials in both conditions: card 1 showed three blue circles, card 2 showed one red triangle and card 3 showed four green stars. The variable card changed with each trial and, in the control condition, exactly matched one of the three target cards but not in the experimental condition. There were 24 unique variable cards, each used twice across the experimental condition, but never within the same block. The object of the experimental condition was to determine via trial and error the ‘rule’ for sorting the variable card to one of the target cards via button press. The rule remained the same throughout each experimental block. Stimuli were shown for 3500 milliseconds (msec) followed by a 450 msec feedback screen, which informed the participant whether his/her response was correct or incorrect. There was a 50 msec interstimulus interval between the feedback screen and the next trial.
During the control condition, the participant was required to simply match the variable card to the identical target card via button press. Participants were cued as to which condition they were performing by a 6000 msec instruction screen presented at the beginning of each block that read either ‘sort’ or ‘match’. The card task required both cognitive flexibility (i.e. generating potential rules, adapting to feedback, switching rules) and working memory (i.e. retaining previous cards, response and feedback to determine next response) while controlling for visual attention, stimulation and discrimination as well as button pressing. Participants were randomized to one of three different versions of the card sort task to control for rule difficulty order. Version A presented the sort rules in the following order: colour, form, number, colour; Version B: form, number, colour, form; and Version C: number, colour, form, number. The variable cards were presented in the same order for all three versions. Participants completed a different version of the card sort task at post-intervention compared to baseline.
Cognitive rehabilitation curriculum (CRC)
The authors collaborated with Lumos Labs, Inc. (San Francisco, CA) to develop an 8 week, 40 session curriculum of cognitive exercises built in Flash (Adobe Systems, San Jose, CA) for web production. The authors have no financial relationships with or commercial interests in Lumos Labs or any other conflicts of interest related to the cognitive exercise curriculum. The development and validation of Lumos Labs cognitive exercises are described in detail in their white paper [56]. Only those exercises focusing on cognitive flexibility, attention and working memory (available in 2007 at the beginning of this study) were chosen for the CRC. The selected exercises were grouped into an online course that participants were automatically enrolled in at initial login. Each participant was provided with an anonymous user ID number and password to log into the encrypted website from their home computer. No personal information was stored. Participants and their parents were given verbal and written instructions as well as a live demonstration of how to do each exercise. After logging in, participants were automatically directed to their ‘trainer page’ which listed the exercises assigned that day.
Currently, no standards have been established regarding the number of sessions or duration of cognitive remediation programs for children. Significant variability exists among previous studies in terms of training duration. These have ranged from 5–8 weeks, 1–7 sessions per week, 25–45 minutes per session and 3–15 tasks per session [22, 35, 57–59]. For the present study, an 8 week, 5 sessions per week, 20 minutes per session program was utilized. The duration parameters used here were chosen in an effort to balance consistent, intensive practice with feasibility while remaining relatively consistent with previous studies. Research staff tracked participant progress and communicated with participants’ parents on a weekly basis to remind participants to complete their sessions and record any reasons for being off-schedule. Participants were given an incentive of $100 for completing the CRC.
Six tasks were presented during each session: (1) Spatial Speed Match (working memory): based on the classic n-back working memory paradigm used in previous studies [22, 35, 59], this task involved the presentation of a series of three circles in a pyramid formation with one circle being coloured blue. The participant had to indicate if the current stimulus matched the previous one in terms of the location of the coloured circle. The participant responded by clicking a red or a green button or by using the left or right arrow keys to indicate a no or yes, respectively. Correct responses were indicated with a green check mark and a sound; incorrect responses with a red ‘X’ and a different sound. As participants improved in reaction time, the number of trials increased, increasing difficulty level. (2) Monster Garden (working memory): participants were shown a garden grid. A creature or a beet would pop up briefly in some of the squares and then the participant had to guide a farmer to a flower on the grid by clicking a path of squares without clicking any squares that had creatures. Difficulty increased via increasing number of creatures and larger garden grid. (3) Lost in Migration (attention): based on the flanker paradigm, a well-known measure of selective attention [60], several birds were shown in various formations. The participant was required to use the arrow keys to indicate which direction the centre bird was facing. Surrounding birds faced the same or a different direction as the centre bird. As participants improved in reaction time, the number of trials increased, increasing difficulty level. (4) Bird Watching (attention): a bird and a letter were flashed briefly on a landscape background and participants were instructed to click on the location of the bird and simultaneously remember the letter. The participant was shown how close his/her click was to the actual location of the bird and points were given based on how closely the participant clicked to the centre of the bird. The participant was then asked to type in the letter that was shown. After several trials, the name of the bird was revealed via the letters. Difficulty was increased by increasing the spatial separation between the bird and the letter and by decreasing the amount of time the stimuli were shown. (5) By the Rules (cognitive flexibility): based on sorting tasks such as the Wisconsin Card Sorting Test [53] that require the generation of alternate solutions to ambiguous stimuli, participants were shown a single card depicting one or more geometric figures and asked to determine if the card followed ‘the rule’ or not. The rule had to be ascertained via trial and error. Participants responded by clicking a green or red button, dragging the figure to one of these buttons or using the left or right arrow keys to indicate no or yes, respectively. Correct responses were indicated with a green check mark and a sound; incorrect responses with a red ‘X’ and a different sound. Difficulty was increased by increasing the number of possible rules in play as well as rule subtlety or complexity. For example, colour was an easier rule, whereas card border was a more difficult rule. (6) Colour Match (cognitive flexibility): based on the Stroop task which measures set shifting [61], participants were shown two boxes side by side, the left labelled ‘meaning’ and the right labelled ‘colour’. Colour names were presented in each box and the participant had to indicate if the colour of the word on the right matched the meaning of the word on the left. Participants responded by clicking red or green buttons or using the left or right arrow keys to indicate no or yes, respectively. Correct responses were indicated with a green check mark and a sound; incorrect responses with a red ‘X’ and a different sound.
Each exercise of the CRC included a presentation of the rules and instructions for the exercise. Exercises were adaptively hierarchical; initial difficulty level was very low (e.g. simple stimuli, longer time limits, cued or scaffolded items, illustrations/explanations of correct responses) and then as the participant’s performance improved, difficulty level was increased (more complex stimuli, shorter time limits, no cuing or explanations). All levels provided immediate feedback regarding accuracy of responses.
FMRI pre-processing
Using Statistical Parametric Mapping 5 (SPM5) [62], images were realigned to correct for any head movement using least square minimization, normalized to a standardized template in order to directly compare brain activation across participants, stimulus types and experimental conditions; and smoothed to reduce the effects of noise. Images for each participant were then visually assessed for correct spatial normalization using in-house software that creates 3D renders and slice maps (axial, coronal, sagittal) of raw, normalized and statistical images for comparison with template examples of correctly aligned images. An in-house software program, ArtRepair [63], was utilized to automatically detect and repair motion and signal intensity outliers as well as other artifacts at the volume, slice and voxel level [64].
Statistical analyses
FMRI data analyses
Statistical analyses of whole brain fMRI data were performed in SPM5. Confounding effects of fluctuations in global mean were removed by proportional scaling. Low-frequency noise was removed with a high-pass filter (0.5 cycles/minute) applied to the fMRI time series at each voxel. A temporal smoothing function (Gaussian kernel corresponding to dispersion of 8 seconds) was applied to the fMRI time series to enhance the temporal signal-to-noise ratio. Individual contrast images were analysed using a general linear model to determine voxel-wise t-statistics. The experimental minus control contrast image was generated per participant. A one-sample t-test was then used to determine whole brain within-group activation. The statistics for analyses were normalized to Z-scores and significant clusters of activation were determined using height and extent thresholds of p < 0.05, controlling for multiple comparisons using false discovery rate [65]. Activation foci were superimposed on high-resolution T1-weighted images and their locations interpreted using known neuroanatomical landmarks.
Region of interest (ROI) analyses of whole brain fMRI data were then performed to allow for linear mixed modelling, to constrain analyses to regions corresponding to a priori hypotheses and to reduce the number of statistical tests performed. Automated Anatomic Labelling software [66] was used to create anatomic ROIs for bilateral middle, inferior and superior frontal gyri selected based on the a priori hypothesis regarding prefrontal cortex function. Whole brain analysis results, as shown in Table II, indicate that these prefrontal regions were areas of significant peak activation across participants at baseline (p < 0.05, corrected). The REX toolbox [67] was used to extract the mean intensity value across all voxels in the ROIs for each participant. The ROI values were scaled to the SPM5 default intracerebral mean of 100 to provide a metric of percentage signal change for the experimental minus control conditions of the card task.
Table II.
Whole brain results of fMRI analyses indicating significant brain activation during the card task (experimental minus control contrast).
| p-value (FDR corrected) | Cluster size | T-score | MNI coordinates | Location description |
|---|---|---|---|---|
| 0.002 | 28 358 | 11.55 | −34, −96, −12 | left inferior occipital gyrus extending into left inferior occipital gyrus, bilateral inferior and superior parietal lobe and bilateral fusiform gyrus |
| 0.002 | 26 785 | 8.31 | −38, 54, −4 | left middle frontal gyrus extending into left inferior and superior frontal gyri, left medial frontal gyrus, right superior, middle and inferior frontal gyri and bilateral striatum |
| 0.002 | 2 203 | 4.80 | −4, −18, 4 | left thalamus extending into left striatum, right thalamus and right striatum |
Height and extent threshold: p < 0.05, FDR (false discovery rate).
Changes in cognitive testing data between pre- and post-CRC were estimated using linear mixed modelling (e.g. [68–70]) with CRC duration, age and cancer type (1 = brain tumour, 2 = ALL) as covariates. All 23 cases with available data at Time 1 were included in the analysis assuming that data were missing at random at Time 2 [71]. Trained tests (resembles a CRC exercise) included the Sort Test and non-trained tests (measures the cognitive skill trained in a CRC exercise but does not resemble the CRC exercise) included the Working Memory Index, Processing Speed Index, Motor-free Visual Perception Test, Cancellation, List Memory and Picture Memory. The Visual Perception Test was included due to the highly visual nature of the CRC.
Changes in cognitive test scores associated with the CRC were also examined using the Reliable Change Index (RCI) [72, 73] to determine if statistically different scores were also clinically significant (i.e. recovered). The RCI is a well-known method used to determine if a change is reliable rather than simply due to test measurement error (i.e. practice effects), accounting for differences between pre- and post-test variance [73]. An RCI criterion of ≥ 1.96 (95% confidence interval) was utilized [74]. An RCI was calculated for each participant and categorized as ‘Recovered’, ‘Improved’, ‘Unchanged’ or ‘Deteriorated’ based on previously published methods [75]. The number of participants in each category was summed and the mean RCI across participants was calculated.
Linear mixed models as described above were used to determine differences in ROI activation between pre- and post-CRC assessments.
Effect sizes for linear mixed models were calculated by dividing the estimated slope (change) from Time 1 to Time 2 by the standard deviation of the observed outcome at Time 1. Therefore, these effect sizes can be interpreted as magnitudes of change in relation to the observed outcome distribution at baseline.
Secondary, exploratory analyses
ROIs that were significantly different from baseline were correlated with significantly improved cognitive test scores and RCI category to explore the relationship between improved cognitive performance and increased brain activation. Cancer diagnosis (1 = brain tumour, 2 = ALL), age at diagnosis, time since treatment completion and gender were entered as independent variables in multiple regressions with ROI slope and cognitive test slope to explore the effect of these variables on intervention outcome. Increasing time since treatment completion and female gender have been associated with increasing cognitive deficit in ALL and brain tumour [76–78].
Results
CRC compliance
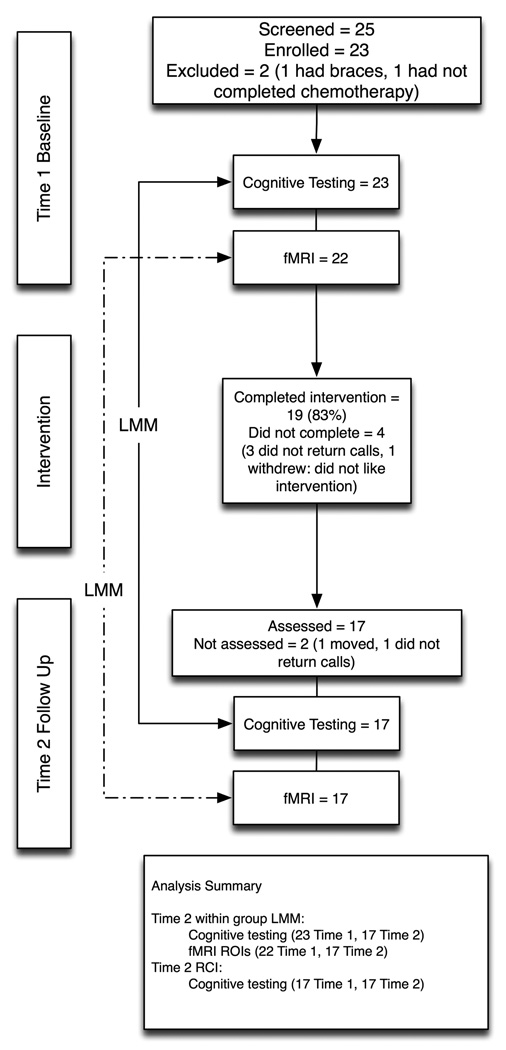
As shown in Figure 1, 23 children participated in the CRC. One child was excluded for MRI contraindications and one had not yet completed chemotherapy. Nineteen children completed the entire curriculum (83%) and 17 of these successfully completed both the pre- and post-CRC assessment. Although the CRC was designed to be an 8-week program, most children required longer (mean = 14, SD = 7.5 weeks) to complete the curriculum. Reasons for falling behind schedule included forgetting, illness, vacation and too much schoolwork, the latter being the most common reason. There were no differences between those who completed or did not complete the CRC in terms of age, gender, minority status, maternal education level, cancer type, age at diagnosis, time since anti-cancer treatment completion or baseline cognitive test scores.
Figure 1.
Summary of sample sizes and analyses. Of those who participated in the intervention, three were lost to follow up and one withdrew from the study (did not like the intervention). One of the children who did not complete follow-up assessments was lost to follow-up and the other moved out of state.
Changes in cognitive function
Children with cancer showed significantly increased Processing Speed Index (p = 0.002), Sort Test (p = 0.001), List Memory (p = 0.03) and Picture Memory (p = 0.02) scores following the CRC (Table III).
Table III.
Cognitive testing, CRC and demographic data. All cognitive test data are shown as T-scores.
| LMM | |||||
|---|---|---|---|---|---|
| Time 1 Mean (SD) (n = 23) | Time 2 Mean (SD) (n = 17) | slope | p | d | |
| Processing Speed Index | 44.1 (9.9) | 55.6 (9.9) | 9.87 | 0.002 | 1.0 |
| Working Memory Index | 49.1 (9.7) | 51.5 (10.3) | 2.82 | 0.10 | 0.29 |
| Sort Test | 37.6 (11.3) | 48.7 (7.4) | 10.56 | 0.001 | 1.1 |
| Cancellation | 44.9 (12.1) | 50.1 (13.2) | 3.65 | 0.12 | 0.30 |
| List Memory | 43.6 (11.1) | 50.9 (13.5) | 6.55 | 0.03 | 0.59 |
| Picture Memory | 38.4 (10.2) | 48.4 (8.1) | 6.24 | 0.02 | 0.61 |
| MVPT3 | 44.8 (22.7) | 63.8 (11.3) | 7.58 | 0.09 | 0.33 |
| Full Scale IQ | 50.8 (10.3) | ||||
| Age at participation (years) | 12.6 (4.1) | ||||
| Age at diagnosis (years) | 6.5 (3.7), 2.3–14 | ||||
| Last anti-cancer TX (months) | 37.3 (30.1), 6–126 | ||||
| Males | 61% | ||||
| Minority status | 52% | ||||
| Maternal education (years) | 17.7 (2.0) | ||||
| CRC duration (weeks) | 14 (7.5) | ||||
‘average’ range for T-score = 44–56. MVPT3 = Motor-free Visual Perception Test; Last TX = months since all anti-cancer treatments were completed; CRC = Cognitive Rehabilitation Curriculum; SD = standard deviation, p = significance value (alpha), d = effect size, LMM = Linear Mixed Model.
Changes in brain activation
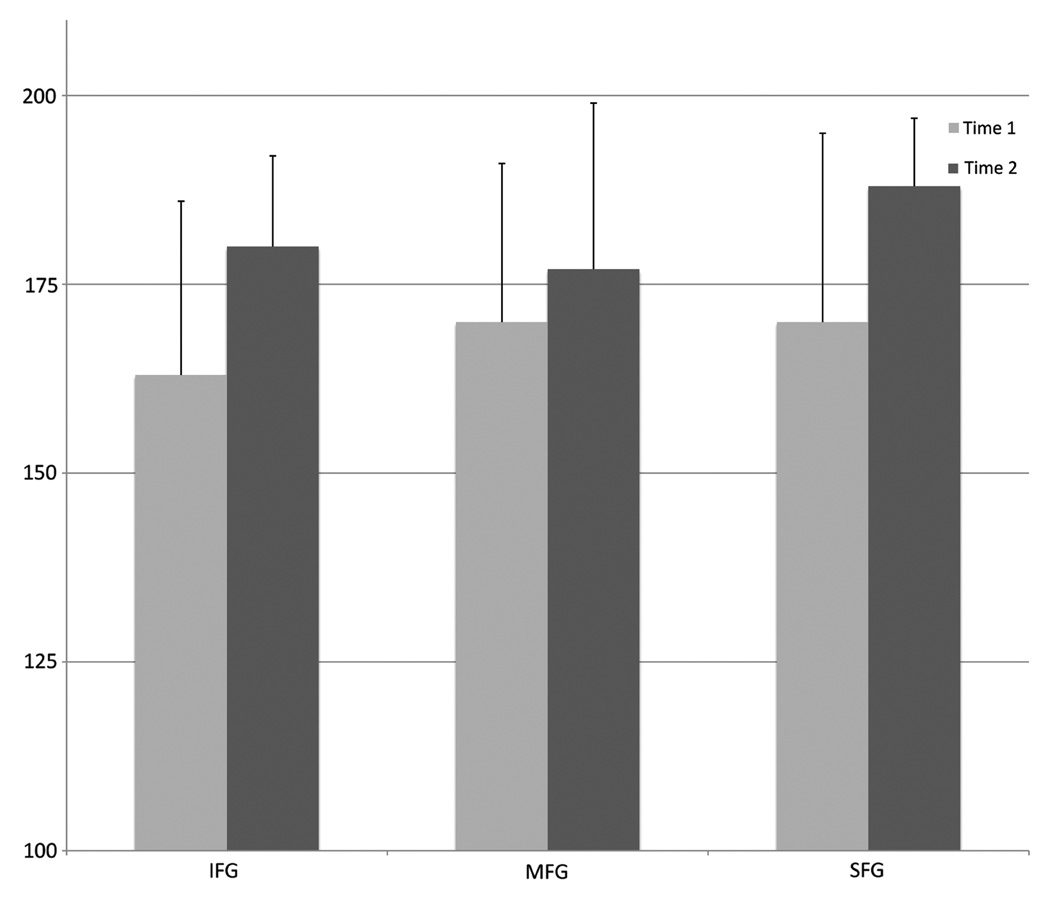
Linear modelling demonstrated significantly increased inferior (p = 0.02), middle (p = 0.04) and superior frontal gyrus activation (p = 0.01) and decreased reaction time (p = 0.009) following the CRC compared to baseline (Table IV, Figure 2). Due to significantly poor quality (signal and/or motion artifacts in > 20% of images), fMRI data for one child (Time 1) were excluded from this analysis.
Table IV.
Function MRI (fMRI) region of interest (ROI) activation and behavioural performance data for the card sort task.
| LMM | |||||
|---|---|---|---|---|---|
| Time 1 Mean (SD) (n = 22) | Time 2 Mean (SD) (n = 17) | slope | p | d | |
| Inferior middle frontal gyrus | 163.45 (23.3) | 179.74 (11.6) | 10.33 | 0.02 | 0.44 |
| Middle frontal gyrus | 169.74 (21.2) | 176.95 (21.5) | 6.88 | 0.04 | 0.32 |
| Superior frontal gyrus | 170.18 (24.9) | 187.57 (8.8) | 13.83 | 0.01 | 0.56 |
| Percentage accuracy | 0.61 (0.11) | 0.66 (0.04) | 0.007 | 0.83 | 0.06 |
| Reaction time (msec) | 1482 (212) | 1429 (225) | −165.6 | 0.009 | 0.78 |
msec = milliseconds, SD = standard deviation, p = significance value (alpha), d = effect size.
Figure 2.
Participants demonstrated significantly increased percentage activation, on average, in the inferior (IFG), middle (MFG) and superior (SFG) frontal gyri following the cognitive rehabilitation curriculum. Error bars indicate standard deviation.
Reliable change index (RCI) analysis
RCI analyses indicated that Processing Speed Index and Sort Test scores were ‘recovered’, on average (mean RCI = 1.96, 2.03, respectively). Sixty percent of participants demonstrated recovered or improved Processing Speed Index scores and 62% showed recovered or improved Sort Test scores. Memory scores were classified as ‘unchanged’ (Table V).
Table V.
Reliable change index (RCI) data for the cognitive rehabilitation curriculum (CRC).
| Test | Mean RCI | Cut score | Mean post-test score | Recovered | Improved | Unchanged | Deteriorated |
|---|---|---|---|---|---|---|---|
| PSI | 1.96* | 48 | 55 | 50% | 10% | 40% | 0% |
| Sort Test | 2.42* | 40 | 50 | 62% | 0% | 38% | 0% |
| List Memory | 1.19 | 47 | 53 | 15% | 0% | 85% | 0% |
| Picture Memory | 0.678 | 45 | 48 | 14% | 0% | 86% | 0% |
PSI = Processing Speed Index. Data are for paediatric cancer survivors with both pre- and post-CRC only (n = 17).
Significant at 95% confidence interval.
Exploratory analyses
There were no significant correlations between ROI activation and cognitive outcome following the CRC or between ROI and cognitive slopes, cancer type, time since treatment completion or gender.
Discussion
This study demonstrated that a computerized curriculum of executive function skills practice completed at home can be feasible for children and adolescents with a history of cancer. Overall compliance level was 83%, similar to the compliance level reported for a previous cognitive rehabilitation study involving paediatric cancer survivors that utilized in-person sessions (84%) [24]. Participants required longer than expected to complete the program. Health issues contributed to the need for extended program time in many children, although schoolwork burden was the most common factor. These findings suggest that home-based cognitive skills practice can successfully be implemented in young survivors of paediatric cancer, but optimal duration parameters require further study.
The executive function program also was effective for improving processing speed and cognitive flexibility as well as visual and verbal declarative memory. Improvements in test scores following the intervention occurred even after controlling for age, intervention duration and cancer type. Given the young age of the participants, some improvement over time might be expected naturally, irrespective of the intervention, due to developmental increases in cognitive ability, particularly in executive functions [53, 79, 80]. However, previous studies have indicated that children with a history of cancer tend to actually show declines in cognitive function over time [77, 78, 81]. The findings of significant improvements in cognitive function are therefore contrary to what typically occur in these children.
An RCI analysis was also employed to control for practice effects. Results of this analysis showed that processing speed and cognitive flexibility scores also were clinically significant, classified across participants as ‘recovered’. This classification indicates that, on average, performance on these tests was not only improved but also within the normative distribution following the CRC. Furthermore, these results suggest that children with ALL or brain tumour showing certain executive impairments may be successfully trained to improve their performance. The CRC improved both trained and untrained cognitive performance, suggesting some skills transfer.
The CRC was associated with significant increases in dorsolateral prefrontal cortex activation. This finding suggests that the intervention impacted neurobiologic status in participants, possibly increasing neural resources. The prefrontal region is known to subserve several executive skills in children including cognitive flexibility [82] and processing speed [83]. Other groups have also shown significantly increased prefrontal activation following other one-armed executive skills training [48, 84]. Theories regarding experience-dependent neuroplasticity mechanisms suggest that cognitive training should result in neurobiologic changes that support recovery of function [85, 86]. The results presented here appear to be consistent with this theory given that participants showed significantly improved test scores—on both trained and untrained tests—as well as significantly increased prefrontal activation.
An increase in activation following cognitive training putatively stems from recruitment of additional neural resources and/or heighted regional response [87], but has previously been associated only with sensorimotor training [86]. The current findings of increased rather than decreased prefrontal activation related to higher level, executive function tasks may reflect the use of adaptive training. Adaptive training is designed to continuously increase learning and challenge neural systems resulting in long-term neuroplastic changes [85]. Adaptive skills training may therefore be associated with higher cognitive load in certain brain regions and an associated increase, rather than decrease, in activation of these networks.
However, working memory and visual attention were not improved by the training program, despite their reliance on prefrontal-executive networks. These skills have previously been shown to be improved in association with changes in prefrontal function within other populations [47, 88]. The reason these skills were not improved by the CRC is unclear, although it may reflect the choice of cognitive tests. For example, the Working Memory Index involves auditory stimuli, whereas the CRC used only visual stimuli. A visual working memory test such as Spatial Span may have elucidated change in this area. It is also possible that the CRC exercises in these domains were not effective or that this sample of children required more and/or longer sessions related to these domains.
The relationship between brain activation and test score change could not be quantified, possibly because the association between cognitive testing and neuroimaging data can be difficult to elucidate. Many factors mitigate this relationship, particularly the inherently complex nature of cognitive function. Changes in neurobiologic status related to the CRC may have involved structural and/or microstructural changes, for example, that are not detected by fMRI. Additionally, this analysis may have lacked enough statistical power due to the relatively small sample size. Further research utilizing multimodal imaging in larger samples is required to explore these possibilities.
The current results are very preliminary and have several limitations. The lack of a no-treatment or alternate treatment comparison group increases the probability that the findings were due to practice effects. Recent studies have successfully utilized a similar one-arm design for executive function training in healthy adults [84, 89]. Similar to these studies, significant changes in neuroimaging metrics were demonstrated here. FMRI tasks have been shown to have strong test–re-test reliability for complex tasks that have minimal behavioural practice effects [90]. The present fMRI task was based on the Wisconsin Card Sorting Task, which has been shown to have minimal to no test–re-test differences in activation [91]. Alternate forms of this task were utilized at baseline and post-test to further minimize practice effects. Unlike the previous one-arm trial studies, the current analyses were complimented with RCI analysis, which accounts for practice effects [73]. Additionally, advanced statistical methods and robust covariates were employed to reduce the variability in baseline scores that tends to increase the influence of regression towards the mean. Nevertheless, a randomized trial is necessary to demonstrate the true potential of the intervention in paediatric cancers. Additionally, the effects of demographic and medical variables on intervention efficacy could not be addressed due to lack of statistical power. Only children who met a certain definition of executive function deficit were included and therefore other definitions of deficit may have resulted in a different outcome. Furthermore, the stability of the findings over time are unknown. Longitudinal followup is required to address this limitation.
Acknowledgements
This work was supported by the Neuro-Cognitive Rehabilitation Research Network (R24 HD050836) and the National Cancer Institute (K07 CA134639).
Footnotes
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Buizer AI, de Sonneville LM, Veerman AJ. Effects of chemotherapy on neurocognitive function in children with acute lymphoblastic leukemia: A critical review of the literature. Pediatric Blood & Cancer. 2009;52:447–454. doi: 10.1002/pbc.21869. [DOI] [PubMed] [Google Scholar]
- 2.Palmer SL. Neurodevelopmental impact on children treated for medulloblastoma: A review and proposed conceptual model. Developmental Disabilities Research Reviews. 2008;14:203–210. doi: 10.1002/ddrr.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kadan-Lottick NS, Brouwers P, Breiger D, Kaleita T, Dziura J, Northrup V, Chen L, Nicoletti M, Bostrom B, Stork L, et al. Comparison of neurocognitive functioning in children previously randomly assigned to intrathecal methotrexate compared with triple intrathecal therapy for the treatment of childhood acute lymphoblastic leukemia. Journal of Clinical Oncology. 2009 doi: 10.1200/JCO.2009.23.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu D, Leung LHT, Kwong DLW, Chan GCF, Khong P-L. Mapping radiation dose distribution on the fractional anisotropy map: Applications in the assessment of treatment-induced white matter injury. NeuroImage. 2006;31:109–115. doi: 10.1016/j.neuroimage.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Dellani PR, Eder S, Gawehn J, Vucurevic G, Fellgiebel A, Muller MJ, Schmidberger H, Stoeter P, Gutjahr P. Late structural alterations of cerebral white matter in long-term survivors of childhood leukemia. Journal of Magnetic Resonance Imaging. 2008;27 doi: 10.1002/jmri.21364. 1250-1205. [DOI] [PubMed] [Google Scholar]
- 6.Waldrop SM, Davis PC, Padgett CA, Shapiro MB, Morris R. Treatment of brain tumors in children is associated with abnormal MR spectroscopic ratios in brain tissue remote from the tumor site. AJNR. American Journal of Neuroradiology. 1998;19:963–970. [PMC free article] [PubMed] [Google Scholar]
- 7.Khong PL, Kwong DL, Chan GC, Sham JS, Chan FL, Ooi GC. Diffusion-tensor imaging for the detection and quantification of treatment-induced white matter injury in children with medulloblastoma: A pilot study. AJNR American Journal of Neuroradiology. 2003;24:734–740. [PMC free article] [PubMed] [Google Scholar]
- 8.Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. Journal of Biology. 2006;5:22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seigers R, Schagen SB, Coppens CM, van der Most PJ, van Dam FS, Koolhaas JM, Buwalda B. Methotrexate decreases hippocampal cell proliferation and induces memory deficits in rats. Behavourial Brain Research. 2009;201:279–284. doi: 10.1016/j.bbr.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 10.Ficek K, Blamek S, Sygula D, Miszczyk L, Sonta-Jakimczyk D, Tarnawski R. Evaluation of the late effects of CNS prophylactic treatment in childhood acute lymphoblastic leukemia (ALL) using magnetic resonance spectroscopy. Acta Neurochirurgica Supplement. 2010;106:195–197. doi: 10.1007/978-3-211-98811-4_36. [DOI] [PubMed] [Google Scholar]
- 11.Wei CW, Guo G, Mikulis DJ. Tumor effects on cerebral white matter as characterized by diffusion tensor tractography. The Canadian journal of neurological sciences. Le Journal Canadien des Sciences Neurologiques. 2007;34:62–68. doi: 10.1017/s0317167100005801. [DOI] [PubMed] [Google Scholar]
- 12.Aukema EJ, Caan MW, Oudhuis N, Majoie CB, Vos FM, Reneman L, Last BF, Grootenhuis MA, Schouten-van Meeteren AY. White matter fractional anisotropy correlates with speed of processing and motor speed in young childhood cancer survivors. International Journal of Radiation Oncology Biology Physics. 2009;74:837–843. doi: 10.1016/j.ijrobp.2008.08.060. [DOI] [PubMed] [Google Scholar]
- 13.Fuss M, Wenz F, Scholdei R, Essig M, Debus J, Knopp MV, Wannenmacher M. Radiation-induced regional cerebral blood volume (rCBV) changes in normal brain and low-grade astrocytomas: Quantification and time and dose-dependent occurrence. International Journal of Radiation Oncology Biology Physics. 2000;48:53–58. doi: 10.1016/s0360-3016(00)00590-3. [DOI] [PubMed] [Google Scholar]
- 14.Mulhern RK, White HA, Glass JO, Kun LE, Leigh L, Thompson SJ, Reddick WE. Attentional functioning and white matter integrity among survivors of malignant brain tumors of childhood. Journal of the International Neuropsychological Society : JINS. 2004;10:180–189. doi: 10.1017/S135561770410204X. [DOI] [PubMed] [Google Scholar]
- 15.Shan ZY, Liu JZ, Glass JO, Gajjar A, Li CS, Reddick WE. Quantitative morphologic evaluation of white matter in survivors of childhood medulloblastoma. Magnetic Resonance Imaging. 2006;24:1015–1022. doi: 10.1016/j.mri.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Paakko E, Harila-Saari A, Vanionpaa L, Himanen S, Pyhtinen J, Lanning M. White matter changes on MRI during treatment in children with acute lymphoblastic leukemia: Correlation with neuropsychological findings. Medical & Pediatric Oncology. 2000;35:456–461. doi: 10.1002/1096-911x(20001101)35:5<456::aid-mpo3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 17.Paakko E, Lehtinen S, Harila-Saari A, Ahonen A, Jauhiainen J, Torniainen P, Pyhtinen J, Lanning M. Perfusion MRI and SPECT of brain after treatment for childhood acute lymphoblastic leukemia. Medical & Pediatric Oncology. 2003;40:88–92. doi: 10.1002/mpo.10210. [DOI] [PubMed] [Google Scholar]
- 18.Schonberg T, Pianka P, Hendler T, Pasternak O, Assaf Y. Characterization of displaced white matter by brain tumors using combined DTI and fMRI. NeuroImage. 2006;30:1100–1111. doi: 10.1016/j.neuroimage.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Zou P, Mulhern RK, Butler RW, Li CS, Langston JW, Ogg RJ. BOLD responses to visual stimulation in survivors of childhood cancer. Neuroimage. 2005;24:61–69. doi: 10.1016/j.neuroimage.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 20.Morris J. Cognitive rehabilitation: Where we are and what is on the horizon. Physical Medicine and Rehabilitation Clinics of North America. 2007;18:27–42. doi: 10.1016/j.pmr.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Laatsch L, Harrington D, Hotz G, Marcantuono J, Mozzoni MP, Walsh V, Hersey KP. An evidence-based review of cognitive and behavioral rehabilitation treatment studies in children with acquired brain injury. Journal of Head Trauma Rehabilitation. 2007;22:248–256. doi: 10.1097/01.HTR.0000281841.92720.0a. [DOI] [PubMed] [Google Scholar]
- 22.Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlstrom K, Gillberg CG, Forssberg H, Westerberg H. Computerized training of working memory in children with ADHD–a randomized, controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Keller TA, Just MA. Altering cortical connectivity: Remediation-induced changes in the white matter of poor readers. Neuron. 2009;64:624–631. doi: 10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butler RW, Copeland DR, Fairclough DL, Mulhern RK, Katz ER, Kazak AE, Noll RB, Patel SK, Sahler OJ. A multicenter, randomized clinical trial of a cognitive remediation program for childhood survivors of a pediatric malignancy. Journal of Consulting & Clinical Psychology. 2008;76:367–378. doi: 10.1037/0022-006X.76.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell LK, Scaduto M, Van Slyke D, Niarhos F, Whitlock JA, Compas BE. Executive function, coping, and behavior in survivors of childhood acute lymphocytic leukemia. J Pediatr Psychol. 2009;34:317–327. doi: 10.1093/jpepsy/jsn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carey ME, Haut MW, Reminger SL, Hutter JJ, Theilmann R, Kaemingk KL. Reduced frontal white matter volume in long-term childhood leukemia survivors: A voxel-based morphometry study. AJNR American Journal of Neuroradiology. 2008;29:792–797. doi: 10.3174/ajnr.A0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powell KB, Voeller KK. Prefrontal executive function syndromes in children. Journal of Child Neurology. 2004;19:785–797. doi: 10.1177/08830738040190100801. [DOI] [PubMed] [Google Scholar]
- 28.Rende B. Cognitive flexibility: Theory, assessment, and treatment. Seminars in Speech & Language. 2000;21:121–132. doi: 10.1055/s-2000-7560. quiz 133. [DOI] [PubMed] [Google Scholar]
- 29.Repovs G, Baddeley A. The multi-component model of working memory: Explorations in experimental cognitive psychology. Neuroscience. 2006;139:5–21. doi: 10.1016/j.neuroscience.2005.12.061. [DOI] [PubMed] [Google Scholar]
- 30.Geurts HM, Corbett B, Solomon M. The paradox of cognitive flexibility in autism. Trends in Cognitive Sciences. 2009;13:74–82. doi: 10.1016/j.tics.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levin HS, Hanten G. Executive functions after traumatic brain injury in children. Pediatric Neurology. 2005;33:79–93. doi: 10.1016/j.pediatrneurol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Nelson CJ, Nandy N, Roth AJ. Chemotherapy and cognitive deficits: Mechanisms, findings, and potential interventions. Palliative & Supportive Care. 2007;5:273–280. doi: 10.1017/s1478951507000442. [DOI] [PubMed] [Google Scholar]
- 33.Hong D, Scaletta Kent J, Kesler S. Cognitive profile of Turner syndrome. Developmental Disabilities Research Reviews. 2009;15:270–278. doi: 10.1002/ddrr.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woods SP, Weinborn M, Posada C, O’Grady J. Preliminary evidence for impaired rapid verb generation in schizophrenia. Brain Language. 2007;102:46–51. doi: 10.1016/j.bandl.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thorell LB, Lindqvist S, Bergman Nutley S, Bohlin G, Klingberg T. Training and transfer effects of executive functions in preschool children. Developmental Sciences. 2009;12:106–113. doi: 10.1111/j.1467-7687.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- 36.Bergquist T, Gehl C, Mandrekar J, Lepore S, Hanna S, Osten A, Beaulieu W. The effect of internet-based cognitive rehabilitation in persons with memory impairments after severe traumatic brain injury. Brain Injury. 2009;23:790–799. doi: 10.1080/02699050903196688. [DOI] [PubMed] [Google Scholar]
- 37.Gontkovsky ST, McDonald NB, Clark PG, Ruwe WD. Current directions in computer-assisted cognitive rehabilitation. NeuroRehabilitation. 2002;17:195–199. [PubMed] [Google Scholar]
- 38.Meeske KA, Siegel SE, Globe DR, Mack WJ, Bernstein L. Prevalence and correlates of fatigue in long-term survivors of childhood leukemia. Journal of Clinical Oncology. 2005;23:5501–5510. doi: 10.1200/JCO.2005.03.210. [DOI] [PubMed] [Google Scholar]
- 39.Pelletier G, Verhoef MJ, Khatri N, Hagen N. Quality of life in brain tumor patients: The relative contributions of depression, fatigue, emotional distress, and existential issues. Journal of Neurooncology. 2002;57:41–49. doi: 10.1023/a:1015728825642. [DOI] [PubMed] [Google Scholar]
- 40.Butler RW, Copeland DR. Attentional processes and their remediation in children treated for cancer: A literature review and the development of a therapeutic approach. Journal of the International Neuropsychological Society. 2002;8:115–124. [PubMed] [Google Scholar]
- 41.Butler RW, Haser JK. Neurocognitive effects of treatment for childhood cancer. Mental Retardation & Developmental Disabilities Research Reviews. 2006;12:184–191. doi: 10.1002/mrdd.20110. [DOI] [PubMed] [Google Scholar]
- 42.Laatsch LK, Thulborn KR, Krisky CM, Shobat DM, Sweeney JA. Investigating the neurobiological basis of cognitive rehabilitation therapy with fMRI. Brain Injury. 2004;18:957–974. doi: 10.1080/02699050410001672369. [DOI] [PubMed] [Google Scholar]
- 43.Laatsch L. The use of functional MRI in traumatic brain injury diagnosis and treatment. Physical Medicine & Rehabilitation Clinics of North America. 2007;18:69–85. vi. doi: 10.1016/j.pmr.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Miotto EC, Savage CR, Evans JJ, Wilson BA, Martins MG, Iaki S, Amaro E., Jr Bilateral activation of the prefrontal cortex after strategic semantic cognitive training. Human Brain Mapping. 2006;27:288–295. doi: 10.1002/hbm.20184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laatsch L, Krisky C. Changes in fMRI activation following rehabilitation of reading and visual processing deficits in subjects with traumatic brain injury. Brain Injury. 2006;20:1367–1375. doi: 10.1080/02699050600983743. [DOI] [PubMed] [Google Scholar]
- 46.Westerberg H, Klingberg T. Changes in cortical activity after training of working memory–a single-subject analysis. Physiology & Behaviour. 2007;92:186–192. doi: 10.1016/j.physbeh.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 47.Tomasi D, Ernst T, Caparelli EC, Chang L. Practice-induced changes of brain function during visual attention: A parametric fMRI study at 4 Tesla. Neuroimage. 2004;23:1414–1421. doi: 10.1016/j.neuroimage.2004.07.065. [DOI] [PubMed] [Google Scholar]
- 48.Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nature Neuroscience. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- 49.Wechsler D. Wechsler Intelligence Scale for Children. 4th ed. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 50.Kavale K, Holdnack J, Mostert M. Responsiveness to intervention and the identification of specific learning disability: A critique and alternative proposal. Learning Disability Quarterly. 2005;28:2–16. [Google Scholar]
- 51.Steinbach JP, Blaicher HP, Herrlinger U, Wick W, NÑgele T, Meyermann R, Tatagiba M, Bamberg M, Dichgans J, Karnath HO, et al. Surviving glioblastoma for more than 5 years: The patient’s perspective. Neurology. 2006;66:239–242. doi: 10.1212/01.wnl.0000194221.89948.a0. [DOI] [PubMed] [Google Scholar]
- 52.Glover GH, Lai S. Self-navigated spiral fMRI: Interleaved versus single-shot. Magnetic Resonance in Medicine. 1998;39:361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- 53.Bujoreanu IS, Willis WG. Developmental and neuropsychological perspectives on the Wisconsin Card Sorting Test in children. Developmental Neuropsychology. 2008;33:584–600. doi: 10.1080/87565640802254364. [DOI] [PubMed] [Google Scholar]
- 54.Lie CH, Specht K, Marshall JC, Fink GR. Using fMRI to decompose the neural processes underlying the Wisconsin Card Sorting Test. Neuroimage. 2006;30:1038–1049. doi: 10.1016/j.neuroimage.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 55.Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin Card Sorting revisited: Distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. Journal of Neuroscience. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hardy J, Scanlon M. [accessed 08/21/2008];The science behind lumosity. 2009 Available online at: http://www.lumosity.com/documents/the_science_behind_lumosity.pdf.
- 57.Bangirana P, Giordani B, John CC, Page C, Opoka RO, Boivin MJ. Immediate neuropsychological and behavioral benefits of computerized cognitive rehabilitation in Ugandan pediatric cerebral malaria survivors. Journal of Developmental & Behavioural Pediatrics. 2009;30:310–318. doi: 10.1097/DBP.0b013e3181b0f01b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shalev L, Tsal Y, Mevorach C. Computerized progressive attentional training (CPAT) program: Effective direct intervention for children with ADHD. Child Neuropsychology. 2007;13:382–388. doi: 10.1080/09297040600770787. [DOI] [PubMed] [Google Scholar]
- 59.Klingberg T, Forssberg H, Westerberg H. Training of working memory in children with ADHD. Journal of Clinical & Experimental Neuropsychology. 2002;24:781–791. doi: 10.1076/jcen.24.6.781.8395. [DOI] [PubMed] [Google Scholar]
- 60.McDermott JM, Perez-Edgar K, Fox NA. Variations of the flanker paradigm: Assessing selective attention in young children. Behaviour Research Methods. 2007;39:62–70. doi: 10.3758/bf03192844. [DOI] [PubMed] [Google Scholar]
- 61.Hyafil A, Summerfield C, Koechlin E. Two mechanisms for task switching in the prefrontal cortex. Journal of Neuroscience. 2009;29:5135–5142. doi: 10.1523/JNEUROSCI.2828-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Friston KJ. Commentary and opinion: II. Statistical parametric mapping: Ontology and current issues. Journal of Cerebral Blood Flow & Metabolism. 1995;15:361–370. doi: 10.1038/jcbfm.1995.45. [DOI] [PubMed] [Google Scholar]
- 63.Mazaika P. [accessed 05/12/2008];ArtRepair. Available online at: http://cibsr.stanford.edu.
- 64.Mazaika P, Whitfield-Gabrielli S, Reiss AL. Aritfact repair for fMRI data from high motion clinical subjects. Poster presented at Organization for Human Brain Mapping Meeting; 2007; Chicago, IL. [Google Scholar]
- 65.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 66.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 67.Whitfield-Gabrieli S. [accessed 05/16/2008];REX Toolbox. Available online at: http://web.mit.edu/swg/software.htm.
- 68.Diggle PJ, Liang KY, Zeger SL. The analysis of longitudinal data. Oxford: Oxford University Press; 1994. [Google Scholar]
- 69.Laird MN, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 70.Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 71.Little RJA, Rubin DB. Statistical analysis with missing data. New York: Wiley; 2002. [Google Scholar]
- 72.Kesler SR, Hopkins RO, Blatter DD, Edge-Booth H, Bigler ED. Verbal memory deficits associated with fornix atrophy in carbon monoxide poisoning. Journal of the International Neuropsychological Society. 2001;7:640–646. doi: 10.1017/s1355617701005112. [DOI] [PubMed] [Google Scholar]
- 73.Maassen GH. The standard error in the Jacobson and Truax Reliable Change Index: The classical approach to the assessment of reliable change. Journal of the International Neuropsychological Society. 2004;10:888–893. doi: 10.1017/s1355617704106097. [DOI] [PubMed] [Google Scholar]
- 74.Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting & Clinical Psychology. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 75.Bauer S, Lambert MJ, Nielsen SL. Clinical significance methods: A comparison of statistical techniques. Journal of Personality Assessment. 2004;82:60–70. doi: 10.1207/s15327752jpa8201_11. [DOI] [PubMed] [Google Scholar]
- 76.Mulhern RK, Butler RW. Neurocognitive sequelae of childhood cancers and their treatment. Pediatric Rehabilitation. 2004;7:1–14. doi: 10.1080/13638490310001655528. discussion 15–16. [DOI] [PubMed] [Google Scholar]
- 77.Mabbott DJ, Spiegler BJ, Greenberg ML, Rutka JT, Hyder DJ, Bouffet E. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. Journal of Clinical Oncology. 2005;23:2256–2263. doi: 10.1200/JCO.2005.01.158. [DOI] [PubMed] [Google Scholar]
- 78.Harila MJ, Winqvist S, Lanning M, Bloigu R, Harila-Saari AH. Progressive neurocognitive impairment in young adult survivors of childhood acute lymphoblastic leukemia. Pediatric Blood & Cancer. 2009;53:156–161. doi: 10.1002/pbc.21992. [DOI] [PubMed] [Google Scholar]
- 79.Fry AF, Hale S. Processing speed, eorking memory, and fluid intelligence: Evidence for a developmental cascade. Psychological Science. 1996;7:237–241. [Google Scholar]
- 80.Klingberg T. Development of a superior frontal-intraparietal network for visuo-spatial working memory. Neuropsychologia. 2006;44:2171–2177. doi: 10.1016/j.neuropsychologia.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 81.Palmer SL, Gajjar A, Reddick WE, Glass JO, Kun LE, Wu S, Xiong X, Mulhern RK. Predicting intellectual outcome among children treated with 35–40 Gy craniospinal irradiation for medulloblastoma. Neuropsychology. 2003;17:548–555. doi: 10.1037/0894-4105.17.4.548. [DOI] [PubMed] [Google Scholar]
- 82.Crone EA, Donohue SE, Honomichl R, Wendelken C, Bunge SA. Brain regions mediating flexible rule use during development. Journal of Neuroscience. 2006;26:11239–11247. doi: 10.1523/JNEUROSCI.2165-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Turken A, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JD. Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. Neuroimage. 2008;42:1032–1044. doi: 10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dux PE, Tombu MN, Harrison S, Rogers BP, Tong F, Marois R. Training improves multitasking performance by increasing the speed of information processing in human prefrontal cortex. Neuron. 2009;63:127–138. doi: 10.1016/j.neuron.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moucha R, Kilgard MP. Cortical plasticity and rehabilitation. Progress in Brain Research. 2006;157:111–122. doi: 10.1016/s0079-6123(06)57007-4. [DOI] [PubMed] [Google Scholar]
- 86.Kelly C, Foxe JJ, Garavan H. Patterns of normal human brain plasticity after practice and their implications for neurorehabilitation. Archives of Physical Medicine & Rehabilitation. 2006;87:20–29. doi: 10.1016/j.apmr.2006.08.333. [DOI] [PubMed] [Google Scholar]
- 87.Poldrack RA. Imaging brain plasticity: Conceptual and methodological issues – a theoretical review. Neuroimage. 2000;12:1–13. doi: 10.1006/nimg.2000.0596. [DOI] [PubMed] [Google Scholar]
- 88.Klingberg T. Training and plasticity of working memory. Trends in Cognitive Science. 2010;14:317–324. doi: 10.1016/j.tics.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 89.McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, Klingberg T. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323:800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- 90.Aron AR, Gluck MA, Poldrack RA. Long-term test-retest reliability of functional MRI in a classification learning task. Neuroimage. 2006;9:1000–1006. doi: 10.1016/j.neuroimage.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nyhus E, Barcelo F. The Wisconsin Card Sorting Test and the cognitive assessment of prefrontal executive functions: A critical update. Brain Cognition. 2009;71:437–451. doi: 10.1016/j.bandc.2009.03.005. [DOI] [PubMed] [Google Scholar]