Abstract
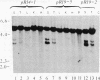
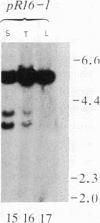
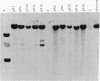
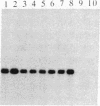
To determine whether a foreign unrearranged immunoglobulin gene can be functionally rearranged and expressed in vivo, a rabbit b9 kappa light chain gene construct containing a single germ-line kappa chain variable (V) region gene (V kappa), the five kappa chain joining (J) segments (J kappa), and the kappa chain constant (C) region germ-line gene (C kappa) was introduced into fertilized mouse eggs. Eleven transgenic mice carrying 1-30 copies of the rabbit kappa gene construct were obtained. Rearrangement of the transgene due to V kappa-J kappa recombination was observed in the spleen of all the mice lines analyzed. Only the J kappa 1 and J kappa 2 segments, which have canonical sequences for rearrangement and high-level expression, are utilized in assembly of the rabbit gene. V kappa-J kappa 1 and V kappa-J kappa 2 joining was also observed in the thymus but not in nonlymphoid tissue. Polyadenylylated rabbit kappa chain transcripts of 1.2 kilobases were found in the spleen of the transgenic mice. The level of transcription was low despite a high level of rearrangement. Three transgenic mice lines secreted kappa light chains encoded by the foreign rabbit gene. Serum rabbit kappa chains were associated with mouse mu and gamma 1 heavy chains. However, hybrid antibody molecules containing both rabbit and mouse kappa light chains were also found in the serum of these animals. These results suggest that, although the rabbit kappa chain gene construct contains the necessary sequences for gene assembly, sequences implicated in stage- and tissue-specific regulation of kappa chain gene rearrangement are either missing or not recognized by mouse lymphoid cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akimenko M. A., Heidmann O., Rougeon F. Complex allotypes of the rabbit immunoglobulin kappa light chains are encoded by structural alleles. Nucleic Acids Res. 1984 Jun 11;12(11):4691–4701. doi: 10.1093/nar/12.11.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimenko M. A., Mariamé B., Rougeon F. Evolution of the immunoglobulin kappa light chain locus in the rabbit: evidence for differential gene conversion events. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5180–5183. doi: 10.1073/pnas.83.14.5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F. W., Blackwell T. K., DePinho R. A., Reth M. G., Yancopoulos G. D. Regulation of genome rearrangement events during lymphocyte differentiation. Immunol Rev. 1986 Feb;89:5–30. doi: 10.1111/j.1600-065x.1986.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Yancopoulos G. D., Blackwell T. K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984 Jun;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Alt F. W. Site-specific recombination between immunoglobulin D and JH segments that were introduced into the genome of a murine pre-B cell line. Cell. 1984 May;37(1):105–112. doi: 10.1016/0092-8674(84)90305-2. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Moore M. W., Yancopoulos G. D., Suh H., Lutzker S., Selsing E., Alt F. W. Recombination between immunoglobulin variable region gene segments is enhanced by transcription. Nature. 1986 Dec 11;324(6097):585–589. doi: 10.1038/324585a0. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M. E., Yagle M. K., Palmiter R. D. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. L., Ritchie K. A., Hammer R. E., O'Brien R. L., Arp B., Storb U. Expression of a microinjected immunoglobulin gene in the spleen of transgenic mice. Nature. 1983 Nov 24;306(5941):332–336. doi: 10.1038/306332a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S., Tyler B. M., Adams J. M. Sets of immunoglobulin V kappa genes homologous to ten cloned V kappa sequences: implications for the number of germline V kappa genes. J Mol Appl Genet. 1981;1(2):103–116. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Weaver D., Baltimore D., Costantini F. Introduction of a mu immunoglobulin gene into the mouse germ line: specific expression in lymphoid cells and synthesis of functional antibody. Cell. 1984 Oct;38(3):647–658. doi: 10.1016/0092-8674(84)90259-9. [DOI] [PubMed] [Google Scholar]
- Heidmann O., Rougeon F. Diversity in the rabbit immunoglobulin kappa chain variable regions is amplified by nucleotide deletions and insertions at the V-J junction. Cell. 1983 Oct;34(3):767–777. doi: 10.1016/0092-8674(83)90533-0. [DOI] [PubMed] [Google Scholar]
- Heidmann O., Rougeon F. Immunoglobulin kappa light-chain diversity in rabbit is based on the 3' length heterogeneity of germ-line variable genes. Nature. 1984 Sep 6;311(5981):74–76. doi: 10.1038/311074a0. [DOI] [PubMed] [Google Scholar]
- Heidmann O., Rougeon F. Multiple sequences related to a constant-region kappa light chain gene in the rabbit genome. Cell. 1982 Mar;28(3):507–513. doi: 10.1016/0092-8674(82)90205-7. [DOI] [PubMed] [Google Scholar]
- Hinds K. R., Litman G. W. Major reorganization of immunoglobulin VH segmental elements during vertebrate evolution. Nature. 1986 Apr 10;320(6062):546–549. doi: 10.1038/320546a0. [DOI] [PubMed] [Google Scholar]
- Kronenberg M., Siu G., Hood L. E., Shastri N. The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol. 1986;4:529–591. doi: 10.1146/annurev.iy.04.040186.002525. [DOI] [PubMed] [Google Scholar]
- Kurosawa Y., von Boehmer H., Haas W., Sakano H., Trauneker A., Tonegawa S. Identification of D segments of immunoglobulin heavy-chain genes and their rearrangement in T lymphocytes. Nature. 1981 Apr 16;290(5807):565–570. doi: 10.1038/290565a0. [DOI] [PubMed] [Google Scholar]
- Lewis S., Gifford A., Baltimore D. Joining of V kappa to J kappa gene segments in a retroviral vector introduced into lymphoid cells. 1984 Mar 29-Apr 4Nature. 308(5958):425–428. doi: 10.1038/308425a0. [DOI] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Leder P. Sequences of five potential recombination sites encoded close to an immunoglobulin kappa constant region gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3450–3454. doi: 10.1073/pnas.76.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani S., Ford A. M., Wiedemann L. M., Chan L. C., Furley A. J., Greaves M. F., Molgaard H. V. Rearrangement of immunoglobulin heavy chain genes in human T leukaemic cells shows preferential utilization of the D segment (DQ52) nearest to the J region. EMBO J. 1986 Dec 20;5(13):3467–3473. doi: 10.1002/j.1460-2075.1986.tb04671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Coleclough C., Kearney J. F. Organization and expression of immunoglobulin genes in fetal liver hybridomas. Proc Natl Acad Sci U S A. 1981 Jan;78(1):247–251. doi: 10.1073/pnas.78.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primi D., Viale G., Cazenave P. A. The commitment of secretory cells to the selective expression of immunoglobulin CH genes is determined by the available concentrations of the triggering ligand. Eur J Immunol. 1986 Apr;16(4):339–344. doi: 10.1002/eji.1830160404. [DOI] [PubMed] [Google Scholar]
- Reth M. G., Ammirati P., Jackson S., Alt F. W. Regulated progression of a cultured pre-B-cell line to the B-cell stage. 1985 Sep 26-Oct 2Nature. 317(6035):353–355. doi: 10.1038/317353a0. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Dahan A., Weill J. C. A single rearrangement event generates most of the chicken immunoglobulin light chain diversity. Cell. 1985 Feb;40(2):283–291. doi: 10.1016/0092-8674(85)90142-4. [DOI] [PubMed] [Google Scholar]
- Ritchie K. A., Brinster R. L., Storb U. Allelic exclusion and control of endogenous immunoglobulin gene rearrangement in kappa transgenic mice. Nature. 1984 Dec 6;312(5994):517–520. doi: 10.1038/312517a0. [DOI] [PubMed] [Google Scholar]
- Rusconi S., Köhler G. Transmission and expression of a specific pair of rearranged immunoglobulin mu and kappa genes in a transgenic mouse line. 1985 Mar 28-Apr 3Nature. 314(6009):330–334. doi: 10.1038/314330a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Storb U., Ritchie K. A., O'Brien R., Arp B., Brinster R. Expression, allelic exclusion and somatic mutation of mouse immunoglobulin kappa genes. Immunol Rev. 1986 Feb;89:85–102. doi: 10.1111/j.1600-065x.1986.tb01474.x. [DOI] [PubMed] [Google Scholar]
- Storb U., Wilson R., Selsing E., Walfield A. Rearranged and germline immunoglobulin kappa genes: different states of DNase I sensitivity of constant kappa genes in immunocompetent and nonimmune cells. Biochemistry. 1981 Feb 17;20(4):990–996. doi: 10.1021/bi00507a053. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Weaver D., Costantini F., Imanishi-Kari T., Baltimore D. A transgenic immunoglobulin mu gene prevents rearrangement of endogenous genes. Cell. 1985 Aug;42(1):117–127. doi: 10.1016/s0092-8674(85)80107-0. [DOI] [PubMed] [Google Scholar]
- Weaver D., Reis M. H., Albanese C., Costantini F., Baltimore D., Imanishi-Kari T. Altered repertoire of endogenous immunoglobulin gene expression in transgenic mice containing a rearranged mu heavy chain gene. Cell. 1986 Apr 25;45(2):247–259. doi: 10.1016/0092-8674(86)90389-2. [DOI] [PubMed] [Google Scholar]
- Yamamura K., Kudo A., Ebihara T., Kamino K., Araki K., Kumahara Y., Watanabe T. Cell-type-specific and regulated expression of a human gamma 1 heavy-chain immunoglobulin gene in transgenic mice. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2152–2156. doi: 10.1073/pnas.83.7.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Blackwell T. K., Suh H., Hood L., Alt F. W. Introduced T cell receptor variable region gene segments recombine in pre-B cells: evidence that B and T cells use a common recombinase. Cell. 1986 Jan 31;44(2):251–259. doi: 10.1016/0092-8674(86)90759-2. [DOI] [PubMed] [Google Scholar]