Abstract
Initial research supports the use of propranolol to prevent posttraumatic stress disorder (PTSD); research has not examined pharmacological prevention for children. Twenty-nine injury patients (ages 10–18 years old) at risk for PTSD were randomized to a double-blind 10-day trial of propranolol or placebo initiated within 12 hours post admission. Six-week PTSD symptoms and heart rate were assessed. Although intent-to-treat analyses revealed no group differences, findings supported a significant interaction between gender and treatment in medication-adherent participants, ΔR2 = .21. Whereas girls receiving propranolol reported more PTSD symptoms relative to girls receiving placebo, ΔR2 = .44, boys receiving propranolol showed a nonsignificant trend toward fewer PTSD symptoms than boys receiving placebo, ΔR2 = .32. Findings inform gender differences regarding pharmacological PTSD prevention in youth.
Keywords: PTSD, pediatric trauma, propranolol
The Efficacy of Early Propranolol Administration at Reducing PTSD Symptoms in Pediatric Injury Patients: A Pilot Study To date, no randomized controlled trials have examined the efficacy of pharmacologic agents administered shortly posttrauma in the prevention of posttraumatic stress disorder (PTSD) in youth. One promising candidate, propranolol, is a centrally-acting beta-blocker that has been found to block the memory-enhancing effects of emotional arousal (Cahill et al. 1994). Pitman and colleagues (2002) tested propranolol as a secondary intervention for the prevention of PTSD in adults. Following a 10-day medication regimen, 30% (6 out of 20) of placebo recipients as compared with 10% (1 out of 10) of propranolol recipients met PTSD criteria one month post-trauma. Three months post-trauma, none of the propanolol group versus nearly half of the placebo group exhibited physiological reactivity during subsequent traumatic imagery, suggesting that early treatment with the β-blocker may be especially effective at decreasing physiological reactivity. The present study is a randomized, placebo-controlled examination of the efficacy of an acute (within 12 hours post-trauma), brief (10 days plus taper) propranolol regimen as a preventive intervention for PTSD in pediatric injury patients.
Method
Participants
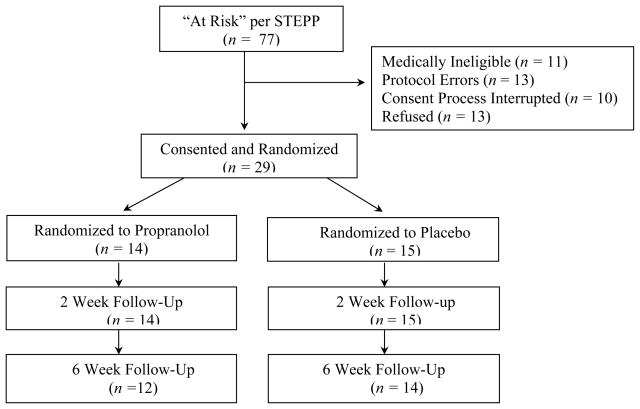
Participants included 29 pediatric trauma emergency department patients (see Table 1). Eligibility criteria included a Glasgow Coma Scale score of 14 or greater and an “at-risk” child score on a screen for risk of PTSD. Medication-specific exclusion criteria included hypersensitivity to beta-blockers, bradycardia; cardiogenic or hypovolemic shock, diabetes, preexisting heart condition, or treatment for asthma (Lacy et al., 2002). Children were also excluded if their injuries or medical treatment procedures contraindicated propranolol. Figure 1 summarizes recruitment and retention.
Table 1.
Participant Demographics.
| Total Sample N |
Propranolol n |
Placebo n |
|
|---|---|---|---|
| Age M (SD) | 14.77 (2.06) | 15.24 (1.32) | 14.32 (2.54) |
| Sex (female/male) | 14/15 | 6/8 | 8/7 |
| Race (African-American/White) | 2/27 | 0/14 | 2/13 |
| Type of trauma | |||
| Motor vehicle accident | 16 | 7 | 9 |
| Bicycle accident | 3 | 1 | 2 |
| Pedestrian vs MVA | 3 | 1 | 2 |
| Fall | 2 | 2 | 2 |
| Miscellaneousa | 5 | 3 | 2 |
| Death of family member | 4 | 4 | 0 |
| Chronic psychiatric diagnosis | 9 | 4 | 5 |
| Prior trauma | 8 | 4 | 4 |
Note. MVA = Motor Vehicle Accident.
Miscellaneous injuries include accidental gunshot injury, horse riding accident, accidental electrocution, power lawn mower accident, and sudden injury sustained while walking.
Figure 1.
Participant screen, consent, randomization, and retention (CONSORT flowchart). STEPP = Screening Tool for Early Predictors of PTSD
Measures
Heart rate and blood pressure were recorded with an automated blood pressure cuff (Model SD-700A, Industrial & Biomedical Sensors Corporations, Waltham, MA) every 60 seconds during the 5-minute trauma narrative and every 5 minutes for 20 minutes postnarrative. Standard deviation scores were calculated for heart rate using age and gender norms based on healthy children (Wuhl, Witte, Soergel, Mehls, & Schaefer, 2002).
The Screening Tool for Early Predictors of PTSD (Winston et al., 2003) is comprised of parent responses to four questions, child responses to four questions, and four questions available in medical charts. Eight items code for child PTSD risk, with 4 or more positive responses indicating risk. Initial sensitivity in predicting PTSD in pediatric injury patients was .88 (Winston et al., 2003).
Parents completed a medication log. Six participants were judged to show poor adherence (> 1 missed dose or > 4 incidents of poor time adherence). Adequate adherence was equally prevalent in propranolol (n = 9) and placebo (n = 11) groups.
Posttraumatic stress disorder symptom severity and diagnosis were determined via the Clinician Administered PTSD Scale for Children and Adolescents (Nader et al., 1996). The CAPS-CA has demonstrated adequate psychometric properties (Nader et al., 1996; Carrion et al., 2002; Newman & Ribbe, 1996). Each symptom is scored for frequency and intensity. Symptoms were coded present if they had a minimum score of 1 for frequency and 2 for intensity; consistent with criteria according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 1994), PTSD diagnosis required one re-experiencing symptom, three avoidance symptoms, and two arousal symptoms. Subsyndromal PTSD required a minimum score of 1 for frequency and 2 for intensity for at least one symptom in each diagnostic category and impairment in one or more areas of functioning (Marshall et al. 2001). Interviewers, blind to treatment group status, evidenced excellent interrater agreement (.98).
Procedure
Procedures were approved by the Institutional Review Boards of Kent State University and Akron Children’s Hospital. A trauma nurse administered the Screening Tool for Early Predictors of PTSD (Winston et al., 2003) to all admitted patients. If the patient screened positive for PTSD risk, a medical doctor determined medical eligibility and completed informed consent procedures with the family. Interested participants were randomized by the hospital pharmacy in a double-blind design.
Treatment was initiated within 12 hours of admission. Children ingested an oral solution of propranolol (HCL 20 mg/5 ml) or liquid placebo twice daily for 10 days (5-day taper; based on Pitman et al., 2002). Pediatric therapeutic dose was calculated as determined by Famularo et al. (1988) to be 2.5 mg/kg/d with a maximum dose of 40 mg bid (Green, 2001). Medication logs were collected at two-weeks.
In-home follow-up assessment occurred 6-weeks post-trauma. Children were seated in a comfortable chair and a portable, self-inflating blood pressure cuff was placed on their non-dominant arm. Following a 15-minute rest period, baseline cardiovascular measures were recorded. To orient children to the target trauma and approximate the Pitman (2002, 1987) script-driven psychophysiologic assessment, the trauma interview began with the youth’s 5-minute trauma narrative followed by the CAPS-CA (Nader et al., 1996) interview.
Data Analysis
All variables showed acceptable skewness and kurtosis and no outliers were found. Pearson Chi-Square analyses were conducted to examine treatment group differences in diagnostic status. Hierarchical linear regression analyses were conducted to examine the effects of treatment condition on subsequent PTSD symptoms after controlling for theoretically and empirically identified covariates.
Results
Six weeks posttrauma, 1 out of 26 participants (4%) met full criteria for PTSD secondary to the index trauma and an additional 11 (42%) met criteria for partial PTSD. The CAPS-CA total scores for the index trauma ranged from 0 to 71 at 6-weeks (M = 24.4, SD = 19.0), with the number of clinically significant symptoms endorsed ranging from 0 – 12 (M = 4, SD = 3). Eight participants reported prior trauma experiences including motor vehicle accidents, burglary, assault, sexual abuse/assault, and parent partner violence (prior trauma CAPS-CA M = 4.1, SD = 8.9, range 0 – 27). Participant race, parent income, and parent educational attainment were not related to CAPS-CA scores at follow-up. Girls reported significantly greater CAPS-CA scores than boys, F (1, 25) = 8.11, p < .01. Analyses revealed no association between weight and PTSD symptoms.
Intent-to-Treat Analyses
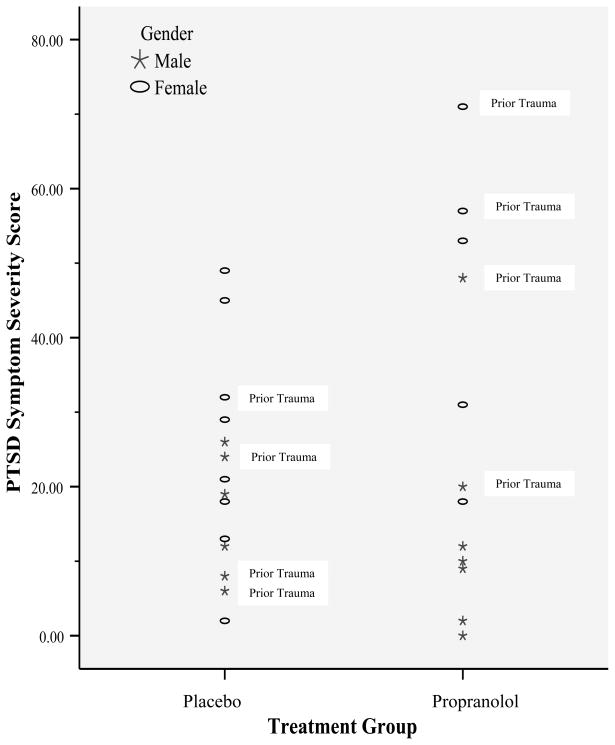
Figure 2 summarizes CAPS-CA total scores by treatment group. As only one participant met full criteria for PTSD, full and subthreshold groups were combined for Pearson Chi-Square analyses. Findings supported no significant treatment group differences in diagnostic status, χ2 < 1. Given observed gender effects, we also conducted identical regressions with the addition of a Gender × Treatment interaction term. Gender and treatment condition did not interact to predict PTSD symptoms.
Figure 2.
PTSD Symptom Severity in Propranolol versus Placebo Group
To examine heart rate responses to the trauma narrative, we conducted linear regression analyses on heart rate data collected during the trauma narrative and over the course of 20 minutes following the trauma narrative. Gender and age were not included in the regression model as they were accounted for by the standardization of cardiovascular measures. There were no treatment group differences in heart rate measured during or following the trauma narrative.
Adherent Participants
Given the pilot nature of the study, we repeated analyses including only adherent participants (n = 20). Pearson Chi-Square analyses were not possible due to cell size restraints. As shown in Table 2, hierarchical linear regression analyses revealed no treatment group differences in PTSD symptoms. A significant interaction effect was found for gender and treatment. Follow-up regression analyses revealed a nonsignificant, but suggestive, pattern of decreased PTSD symptoms reported by boys in the propranolol group relative to the placebo group, R2 = .32, p = .09. In contrast, girls in the propranolol group reported more PTSD symptoms than girls in the placebo group, R2 = .44, p = .05.
Table 2.
Hierarchical Linear Regression Analyses Predicting 6-week PTSD in Adherent Youth
| Step | Variables | R2 | ΔR2 | B | SE B | β |
|---|---|---|---|---|---|---|
| Step 1 | .31 | .31* | ||||
| Gender | 18.13 | 6.73 | .57* | |||
| Age a | −4.17 | 8.25 | −.10 | |||
| Step 2 | .33 | .03 | ||||
| Gender | 18.75 | 6.73 | .59* | |||
| Age | −0.52 | 9.56 | −.01 | |||
| Prior trauma PTSD severity | 0.50 | 0.64 | .19 | |||
| Step 3 | .40 | .06 | ||||
| Gender | 22.23 | 7.18 | .70* | |||
| Age | −2.81 | 9.58 | −.07 | |||
| Prior trauma PTSD severity | 0.63 | 0.64 | .24 | |||
| Treatment condition b | 8.87 | 7.13 | .28 | |||
| Significance of the regression model, F (4, 15) = 2.46, p = .09. | ||||||
| Step 4 | .61** | .21* | ||||
| Gender | 7.00 | 8.10 | .22 | |||
| Age | −2.18 | 7.96 | −.05 | |||
| Prior trauma PTSD severity | .35 | .54 | .13 | |||
| Treatment condition | −7.12 | 8.26 | −.22 | |||
| Treatment × Gender | 31.82 | 11.46 | .72* | |||
Note. PTSD = Posttraumatic stress disorder
Consistent with prior psychophysiological research examining age/gender differences (Gunnar et al., 2009), age was dichotomized within gender such that girls over 12 years of age and boys over 14 years of age were coded as “1.”
Treatment was coded such that propranolol was “1” and placebo was “0”.
p < .05.
p < .01.
Again, heart rate measured during and following the trauma narrative was unrelated to treatment condition in linear regression analyses controlling for prior trauma exposure.
Discussion
The present study was a pilot investigation of propranolol as a secondary intervention to prevent PTSD in pediatric injury patients. As the first study of this kind in children, we restricted eligibility to a small sample of high-risk children using established dosage recommendations for pediatric populations. Consistent with the Pitman et al (2003) investigation, the present investigation was powered to detect medium-to-large treatment effects. Although analyses failed to reveal statistically significant main effects for treatment, evidence was found for an interaction of treatment group and gender in medication-adherent youth. More specifically, whereas adherent boys reported lower (but nonsignificant; R2 = .32) PTSD symptoms in the propranolol condition relative to the placebo condition, a significant increase in PTSD symptoms was reported in girls in the propranolol condition relative to girls in the placebo condition (R2 = .44). Although PTSD symptoms secondary to a prior trauma did not significantly predict index PTSD symptoms, inspection of Figure 2 suggests that youth in the propranolol group who reported lower PTSD symptoms tended to be male and to report no prior trauma experiences relative to propranolol group youth who reported higher PTSD symptoms. Taken together, these findings introduce questions about the potential influence of factors such as gender and prior trauma history on the utility of propranolol as a preventative intervention in youth.
Important differences between the present investigation and prior research, such as the use of a measure to screen for risk of PTSD and dosing based on prior research with children rather than adults, may account for our inability to replicate prior findings. The present investigation is further limited by a small sample size (N = 26 at follow-up). Although the present findings must be interpreted with caution, these findings provide evidence for possible gender differences (for more background on biological influences on gender differences in PTSD see Olff et al., 2007) in the efficacy of propranolol in pediatric injury patients and support the need to further evaluate the effects of propranolol in pediatric populations.
Acknowledgments
This study was completed in collaboration between Kent State University and Akron Children’s Hospital and served as a doctoral dissertation (NRN), supported by a grant from the Ohio Board of Regents (DLD). Manuscript preparation was supported, in part, by NIMH grants T32 078788 (NRN), K01 MH087240 (NRN), R34 MH71201 (DLD), and R34 MH 73014 (DLD).
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. β-adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Famularo R, Kinscherff R, Fenton T. Propranolol treatment for childhood posttraumatic stress disorder, acute type. A pilot study. American Journal of Diseased Children. 1988;142:1244–1247. doi: 10.1001/archpedi.1988.02150110122036. [DOI] [PubMed] [Google Scholar]
- Green WH. Child and adolescent clinical psychopharmacology. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Lacy CF, Armstrong LL, Goldman MP, Lance LL. Drug information handbook: Pocket. Hudson, OH: Lexi-Comp Inc; 2002. [Google Scholar]
- Marshall R, Olfson M, Hellman F, Blanco C, Guarding M, Struening E. Comorbidity, impairment, and suicidality in subthreshold PTSD. American Journal of Psychiatry. 2001;158:1467–1473. doi: 10.1176/appi.ajp.158.9.1467. [DOI] [PubMed] [Google Scholar]
- Nader K, Kriegler JA, Blake DD, Pynoos RS, Newman E, Weathers FW. Clinician Administered PTSD Scale, Child and Adolescent version. White River Junction, VT: National Center for PTSD; 1996. [Google Scholar]
- Newman E, Ribbe D. Psychometric review of the Clinician Administered PTSD Scale for Children. In: Stamm BH, editor. Measurement of stress, trauma, and adaptation. Lutherville, MD: Sidran; 1996. pp. 106–114. [Google Scholar]
- Olff M, Langeland W, Draijer N, Gersons BPR. Gender differences in posttraumatic stress disorder. Psychological Bulletin. 2007;133:183–204. doi: 10.1037/0033-2909.133.2.183. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, et al. Pilot study of secondary prevention for posttraumatic stress disorder with propranolol. Biological Psychiatry. 2002;51:189–192. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- Winston FA, Kassam-Adams N, Garcia-Espana F, Ittenbach R, Cnaan A. Screening for risk of persistent posttraumatic stress in injured children and their parents. Journal of the American Medical Association. 2003;290:643–649. doi: 10.1001/jama.290.5.643. [DOI] [PubMed] [Google Scholar]
- Wuhl E, Witte K, Soergel M, Mehls O, Schaefer F. Distribution of 24-h ambulatory blood pressure in children: Normalized reference values and role of body dimensions. Journal of Hypertension. 2002;20:1995–2007. doi: 10.1097/00004872-200210000-00019. [DOI] [PubMed] [Google Scholar]