Abstract
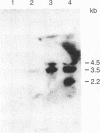
We describe an interleukin, termed interleukin 5, that is the recombinant product previously referred to as T-cell-replacing factor (TRF), B-cell growth factor II (BCGF II), or killer-helper factor (KHF). TRF has been defined as a T-cell-derived lymphokine that acts on activated B cells as a B-cell differentiation factor. We have previously demonstrated that TRF is identical to BCGF II and induces expression of receptors for interleukin 2 (IL-2) on activated B cells. We also have reported that KHF can induce not only expression of IL-2 receptors on peanut agglutinin-binding (PNA+) thymocytes but also generation of cytotoxic T lymphocytes (CTL) in PNA+ thymocytes in the presence of IL-2. We show here that culture supernatants of T-cell hybridomas that produce TRF as well as TRF purified by high-pressure liquid chromatography (HPLC-TRF) have KHF activity and generate CTL in PNA+ thymocytes in the presence of stimulator cells and IL-2. Moreover, translation products (recombinant TRF) of Xenopus oocytes injected with cDNA encoding for murine TRF (BCGF II) also exert KHF activity. A rat monoclonal anti-TRF antibody TB13 can block generation of CTL by HPLC-TRF or recombinant TRF. These results indicate that TRF acts not only on B cells as BCGF II but also on PNA+ thymocytes as KHF. In view of the diverse activities and targets of TRF, we propose that TRF refers to a different interleukin, interleukin 5.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Erard F., Corthesy P., Smith K. A., Fiers W., Conzelmann A., Nabholz M. Characterization of soluble factors that induce the cytolytic activity and the expression of T cell growth factor receptors of a T cell hybrid. J Exp Med. 1984 Aug 1;160(2):584–599. doi: 10.1084/jem.160.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk W., Männel D. N., Katzer B., Kaltmann B., Krammer P. H., Diamantstein T., Dröge W. Induction of IL 2 receptor expression and cytotoxicity of thymocytes by stimulation with TCF1. J Immunol. 1985 Aug;135(2):1160–1164. [PubMed] [Google Scholar]
- Finke J. H., Orosz C. G., Battisto J. R. Splenic T-killer cells can be generated by allogeneic thymic cells in conjunction with assisting factor. Nature. 1977 May 26;267(5609):353–354. doi: 10.1038/267353a0. [DOI] [PubMed] [Google Scholar]
- Finke J. H., Scott J., Gillis S., Hilfiker M. L. Generation of alloreactive cytotoxic T lymphocytes: evidence for a differentiation factor distinct from IL 2. J Immunol. 1983 Feb;130(2):763–767. [PubMed] [Google Scholar]
- Hamaoka T., Takatsu K., Okuno K., Tsuchida T. Functional characterization of the killer-helper factor responsible for the induction of cytotoxic T lymphocytes from thymocytes, and evidence for the nature of this factor as distinct from T cell-replacing factor (TRF) in regard to B cell triggering. J Immunol. 1981 Feb;126(2):659–665. [PubMed] [Google Scholar]
- Harada N., Kikuchi Y., Tominaga A., Takaki S., Takatsu K. BCGFII activity on activated B cells of a purified murine T cell-replacing factor (TRF) from a T cell hybridoma (B151K12). J Immunol. 1985 Jun;134(6):3944–3951. [PubMed] [Google Scholar]
- Hardt C., Diamantstein T., Wagner H. Signal requirements for the in vitro differentiation of cytotoxic T lymphocytes (CTL): distinct soluble mediators promote preactivation of CTL-precursors, clonal growth and differentiation into cytotoxic effector cells. Eur J Immunol. 1985 May;15(5):472–478. doi: 10.1002/eji.1830150511. [DOI] [PubMed] [Google Scholar]
- Howard M., Nakanishi K., Paul W. E. B cell growth and differentiation factors. Immunol Rev. 1984 Apr;78:185–210. doi: 10.1111/j.1600-065x.1984.tb00482.x. [DOI] [PubMed] [Google Scholar]
- Kanagawa O. Three different signals are required for the induction of cytolytic T lymphocytes from resting precursors. J Immunol. 1983 Aug;131(2):606–610. [PubMed] [Google Scholar]
- Kikuchi Y., Kato R., Sano Y., Takahashi H., Kanatani T., Takatsu K. Generation of cytotoxic T lymphocytes from thymocyte precursors to trinitrophenyl-modified self antigens. II. Establishment of a T cell hybrid clone constitutively producing killer-helper factor(s) (KHF) and functional analysis of released KHF. J Immunol. 1986 May 15;136(10):3553–3560. [PubMed] [Google Scholar]
- Kinashi T., Harada N., Severinson E., Tanabe T., Sideras P., Konishi M., Azuma C., Tominaga A., Bergstedt-Lindqvist S., Takahashi M. Cloning of complementary DNA encoding T-cell replacing factor and identity with B-cell growth factor II. Nature. 1986 Nov 6;324(6092):70–73. doi: 10.1038/324070a0. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. Factors affecting B-cell growth and differentiation. Annu Rev Immunol. 1985;3:133–157. doi: 10.1146/annurev.iy.03.040185.001025. [DOI] [PubMed] [Google Scholar]
- O'Garra A., Warren D. J., Holman M., Popham A. M., Sanderson C. J., Klaus G. G. Interleukin 4 (B-cell growth factor II/eosinophil differentiation factor) is a mitogen and differentiation factor for preactivated murine B lymphocytes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5228–5232. doi: 10.1073/pnas.83.14.5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M., Klimpel G. R., Kuppers R. C., Henney C. S. The differentiation of cytotoxic T cells in vitro. I. Amplifying factor(s) in the primary response is Lyt 1 + cell dependent. J Immunol. 1979 Jun;122(6):2527–2533. [PubMed] [Google Scholar]
- Okuno K., Kikuchi Y., Tsuchida T., Hamaoka T., Igarashi T., Kato R., Takatsu K. The role of I-region associated antigen (Ia)-bearing accessory cells in the generation of cytotoxic T cells in a subpopulation of thymocytes. Jpn J Cancer Res. 1986 Jul;77(7):711–721. [PubMed] [Google Scholar]
- Raulet D. H., Bevan M. J. A differentiation factor required for the expression of cytotoxic T-cell function. Nature. 1982 Apr 22;296(5859):754–757. doi: 10.1038/296754a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanderson C. J., O'Garra A., Warren D. J., Klaus G. G. Eosinophil differentiation factor also has B-cell growth factor activity: proposed name interleukin 4. Proc Natl Acad Sci U S A. 1986 Jan;83(2):437–440. doi: 10.1073/pnas.83.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimpl A., Wecker E. Replacement of T-cell function by a T-cell product. Nat New Biol. 1972 May 3;237(70):15–17. doi: 10.1038/newbio237015a0. [DOI] [PubMed] [Google Scholar]
- Shimuzu A., Kondo S., Takeda S., Yodoi J., Ishida N., Sabe H., Osawa H., Diamantstein T., Nikaido T., Honjo T. Nucleotide sequence of mouse IL-2 receptor cDNA and its comparison with the human IL-2 receptor sequence. Nucleic Acids Res. 1985 Mar 11;13(5):1505–1516. doi: 10.1093/nar/13.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L., Howard M., Kappler J., Marrack P., Watson J., Booth R., Wetzel G. D., Dutton R. W. Evidence for two distinct classes of murine B cell growth factors with activities in different functional assays. J Exp Med. 1983 Sep 1;158(3):822–835. doi: 10.1084/jem.158.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L. Role of BCGFII in the differentiation to antibody secretion normal and tumor B cells. J Immunol. 1985 Jun;134(6):3934–3943. [PubMed] [Google Scholar]
- Takatsu K., Harada N., Hara Y., Takahama Y., Yamada G., Dobashi K., Hamaoka T. Purification and physicochemical characterization of murine T cell replacing factor (TRF). J Immunol. 1985 Jan;134(1):382–389. [PubMed] [Google Scholar]
- Takatsu K., Ishizaka T., Ishizaka K. Biologic significance of disulfide bonds in human IgE molecules. J Immunol. 1975 Jun;114(6):1838–1845. [PubMed] [Google Scholar]
- Takatsu K., Kikuchi Y., Kanatani T., Okuno K., Hamaoka T., Tominaga A., Sano Y. Generation of cytotoxic T lymphocytes from thymocyte precursors to trinitrophenyl-modified self antigens. I. Requirement of both killer-helper factor(s) and interleukin 2 for CTL generation from a subpopulation of thymocytes. J Immunol. 1986 Feb 15;136(4):1161–1170. [PubMed] [Google Scholar]
- Takatsu K., Tanaka K., Tominaga A., Kumahara Y., Hamaoka T. Antigen-induced T cell-replacing factor (TRF). III. Establishment of T cell hybrid clone continuously producing TRF and functional analysis of released TRF. J Immunol. 1980 Dec;125(6):2646–2653. [PubMed] [Google Scholar]
- Taniguchi T., Matsui H., Fujita T., Takaoka C., Kashima N., Yoshimoto R., Hamuro J. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983 Mar 24;302(5906):305–310. doi: 10.1038/302305a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]