Abstract
Presenilin-1 (PS1) is the catalytic core of the aspartyl protease γ-secretase. Previous genetic studies using germ-line deletion of PS1 and conditional knock-out mice demonstrated that PS1 plays an essential role in neuronal differentiation during neural development, but it remained unclear whether PS1 plays a similar role in neurogenesis in the adult brain. Here we show that neural progenitor cells infected with lentiviral vectors-expressing short interfering RNA (siRNA) for the exclusive knockdown of PS1 in the neurogenic microenvironments, exhibit a dramatic enhancement of cell differentiation. Infected cells differentiated into neurons, astrocytes and oligodendrocytes, suggesting that multipotentiality of neural progenitor cells is not affected by reduced levels of PS1. Neurosphere cultures treated with γ-secretase inhibitors exhibit a similar phenotype of enhanced cell differentiation, suggesting that PS1 function in neural progenitor cells is γ-secretase-dependent. Neurospheres infected with lentiviral vectors expressing siRNA for the targeting of PS1 differentiated even in the presence of the proliferation factors epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF), suggesting that PS1 dominates EFG and bFGF signaling pathways. Reduction of PS1 expression in neural progenitor cells was accompanied by a decrease in EGF receptor and β-catenin expression level, suggesting that they are downstream essential transducers of PS1 signaling in adult neural progenitor cells. These findings suggest a physiological role for PS1 in adult neurogenesis, and a potential target for the manipulation of neural progenitor cell differentiation.
Introduction
Neural progenitor cells (NPCs) in the adult brain reside in discrete microenvironments, the subventricular zone (SVZ) and the subgranular layer (SGL) of the dentate gyrus (DG) (Kriegstein and Alvarez-Buylla, 2009; Aimone et al., 2010). These niches support NPC self-renewal, proliferation and differentiation into neurons and glia throughout life (Suh et al., 2009). However, the molecular basis for the transition of NPCs from proliferation to differentiation has remained largely elusive. Presenilin-1 (PS1) is the catalytic core of the aspartyl protease γ-secretase (De Strooper et al., 1998, 1999; Wolfe et al., 1999; Herreman et al., 2000; Li et al., 2000; Zhang et al., 2000) that catalyzes numerous molecules implicated in neurogenesis (for review, see Wines-Samuelson and Shen, 2005; Lazarov and Marr, 2010). In humans, mutations in PS1 cause familial Alzheimer's disease (FAD) (Selkoe, 2001). Mice with targeted disruption in the PS1 locus showed impairments in neurogenesis. Beginning at embryonic day 14.5 the ventricular zone is substantially thinner, indicating a drastic reduction in the number of NPCs (Shen et al., 1997). In PS conditional double knock-out mice there is a severe depletion of NPCs characterized by their disrupted interkinetic nuclear migration (Kim and Shen, 2008). Reduced numbers of NPCs are thought to be due to premature differentiation, rather than a decrease in proliferation or cell death (Handler et al., 2000; Yang et al., 2000; Kim and Shen, 2008).
PS1 knock-out mice die in late embryogenesis, hampering the examination of the role of PS1 in regulation of adult neurogenesis. Information from transgenic mice harboring FAD-linked PS1 variants is controversial and inconclusive (Feng et al., 2001; Wang et al., 2004; Wen et al., 2004; Chevallier et al., 2005; Choi et al., 2008). In fact, this controversy is not surprising, as FAD-linked PS1 transgenic mice generated so far offer little advantage when it comes to processes that take place in restricted areas of the postnatal brain with a unique population of NPCs, as transgenes are expressed in a ubiquitously, nonspecific manner, in large neuronal populations in the forebrain, making the relevance of these studies to adult neurogenesis highly questionable. Equally troubling is the neuropathology expressed in these mice, such as high levels of β-amyloid 42 (Aβ42), that may alter neurogenesis indirectly.
To establish a paradigm that examines PS1 function in the neurogenic niches of the adult brain exclusively we developed a lentiviral vector system that expresses small interfering RNAs (siRNAs) for the targeting of PS1, and a green fluorescent protein (GFP) marker for the tracking of targeted cells. Here we show that knocking down PS1 expression in NPCs in the adult brain decreases their proliferation and enhances their differentiation. NPCs expressing reduced levels of PS1 differentiate faster into neurons and glia than NPCs expressing endogenous levels of PS1. PS1 function in NPCs dominates the critical proliferation signals epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF). Finally, PS1 regulates NPC differentiation in a γ-secretase-dependent manner and may exert its effect through EGF receptor (EGFR) and β-catenin signaling pathways. This study suggests a physiological role for PS1 in regulation of NPC differentiation in the adult brain.
Materials and Methods
Development of lentiviral vectors expressing siRNA for the targeting of PS1.
The development of a lentiviral vector system to knockdown PS1 expression was designed to expresses siRNA sequences for targeting PS1, and to coexpress a GFP marker to track targeted cells. For this purpose, third generation (self-inactivating) lentiviral vectors were designed to express small-hairpin RNAs (shRNA) from the 3′ remnant U3 sequence as previously described (Brummelkamp et al., 2002; Tiscornia et al., 2006a). Two siRNA sequences targeting murine PS1 were chosen. One is based on a previously published sequence (5′AAGGCCCACTTCGTATGCTGG 3′) (1.1) (Xie et al., 2004) and one by algorithm (S-fold, http://sfold.wadsworth.org) (5′GGACCAACTTGCATTCCAT3′; 4.11) (Fig. 1A). As controls we generated vectors expressing irrelevant siRNAs: IR-8.1 (5′CTTCATTGTCGGCATGGGT 3′) and IR-9.1 (5′GTATAATACACCGCGCTAC3′). As an additional control, a lentiviral vector expressing GFP alone was used. For validation of shRNA constructs, N2a cells were transduced with the purified shRNA vector preparation followed by anti-PS1 immunoblot. Purification of viral stocks was done as previously described (Tiscornia et al., 2006b). Briefly, HEK-293T cells were transfected with the lentiviral vectors and packaging plasmids and lentiviral vectors concentrated/purified by centrifugation of cell culture supernatants.
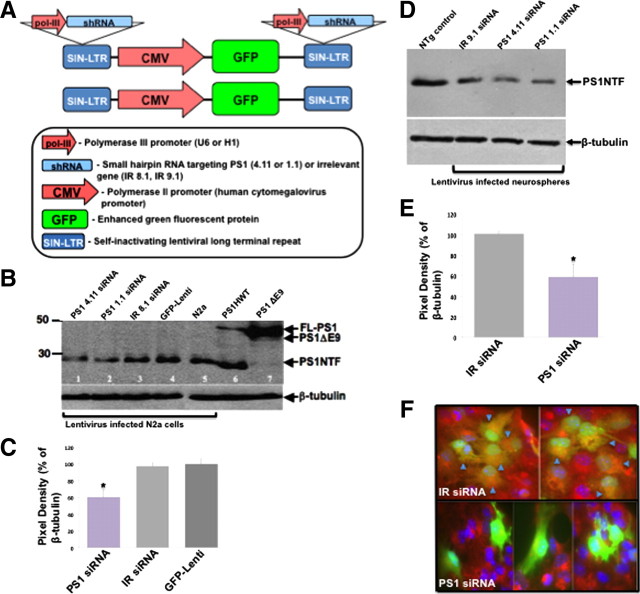
Figure 1.
Reduced PS1 expression in adult neural progenitor cells following transduction with lentiviral vectors expressing siRNA for the targeting of PS1. A, Designs for lentiviral vectors used in this study. shRNA for the silencing of PS1 (PS1 1.1, PS1 4.11) or an irrelevant control shRNA (IR 8.1, IR 9.1) were inserted in the duplicated LTR regions. As an additional control GFP-Lenti was used. B, Western blot analysis shows reduced expression of PS1 in N2a cells infected with lentiviral vectors expressing PS1 1.1, PS1 4.11 shRNA for the targeting of PS1 (lanes 1,2) compared with PS1 expression in N2a cells infected with lentiviral vectors expressing irrelevant control shRNA (IR 8.1, lane 3) or GFP-Lenti (lane 4) or noninfected N2a cells (lane 5). Lanes 6 and 7 serve as expression controls: brain extracts of transgenic mice harboring PS1HWT (lane 6), PS1ΔE9 (lane 7). C, Densitometric analysis shows the average reduction in PS1 expression in N2a cells infected with lentiviral vectors expressing PS1 1.1, PS1 4.11 shRNA for the targeting of PS1 (n = 3). D, Western blot analysis showing reduced expression of PS1 in neural progenitor cell cultures (neurospheres) following transduction with lentiviral vectors expressing PS1 1.1, PS1 4.11 shRNA for the targeting of PS1 compared with neurospheres transduced with lentiviral vectors expressing an irrelevant control shRNA (IR 9.1) or noninfected neurospheres. E, Densitometric analysis shows the average reduction in PS1 expression in neurospheres infected with lentiviral vectors expressing PS1 1.1, PS1 4.11 shRNA for the targeting of PS1 (n = 3). F, Immunostaining of neurospheres infected with lentiviral vectors using anti-PS1 antibodies shows reduced expression of PS1 in GFP+ neurospheres infected with lentiviral vectors expressing PS1 1.1 or PS1 4.11 shRNA, compared with PS1 expression in GFP+ neurospheres infected with lentiviral vectors expressing IR 8.1 or IR 9.1 (arrowheads point to GFP+PS1+ double-labeled cells; red, PS1; green, GFP; blue, DAPI).
Mouse lines.
Adult male C57BL/6 mice used in these studies were purchased from the Jackson Laboratories (JAX Mice). Mice were used at 6–8 months of age for stereotaxic injections. C57BL/6 mice 2–4 months old were used for all in vitro neurosphere cultures.
Stereotaxic injection of lentiviral vectors expressing siRNA for PS1 targeting.
Lentiviral vectors coexpressing either (1) GFP and siRNA for PS1 targeting (1.1 or 4.11) or (2) GFP and an irrelevant siRNA (IR 8.1 or 9.1) or (3) GFP alone, were stereotaxically injected (n = 80) unilaterally into the SGL of C57BL/6 mice (1 μl/site; 0.25 μl/min) using the following DG coordinates: (anteroposterior, −3.0 mm; mediolateral, +2.0 mm; dorsoventral, −2.5). Mice were anesthetized using a mixture of ketamine and xylazine. The mice then had their heads shaved and wiped with 70% ethanol (EtOH). Animals were placed into the stereotaxic frame and a one-inch incision was made in the midline to reveal the bregma. The scalp was then cleared of tissue and wiped with 30% hydrogen peroxide to dehydrate the scalp. A small hole was drilled at the coordinate site as measured from the bregma according the mouse atlas of Paxinos and Franklin (2004). Animals then received unilateral injections of 1 μl of lentivirus directly in the SGL. The virus was delivered using a 5 μl Hamilton syringe connected to a hydraulic system to inject the solution at a rate of 0.20 μl/min. The injection needle was then left in place for another minute to ensure distribution of the lentivirus. The needle was slowly removed and the wounds were closed with sterile EZ-clips from Stoelting. The mice were placed in cages individually and survived for 6 weeks after surgery.
BrdU injections.
Animals were given intraperitoneal injections of 100 mg/kg 5′-bromo-2′deoxyuridine (BrdU) (Sigma) every 12 h for 3 d. Animals were perfused 12 h after the last BrdU injection to study stem cell proliferation and differentiation. All animals were anesthetized with a mixture of ketamine and xylazine and transcardially perfused with 200 ml of cold 1× PBS followed by 100 ml of 4% paraformaldehyde (PFA). The brains were then dissected and placed into 4% PFA for 24 h at 4°C. They were then transferred into a solution of 30% sucrose and kept at 4°C.
Neurosphere culture.
SVZ derived neural stem cells were isolated as previously described (Demars et al., 2010). Briefly, SVZ of 2- to 4-month-old C57BL/6 mice were dissected and chopped. The tissue was incubated in a mixture of activated papain (Sigma), EDTA (Sigma), and l-cysteine (Sigma), and 0.1% DNase for 40 min at 37°C. Tissue was then disassociated and centrifuged at 1000 × g for 5 min. Cells were then plated in complete medium [(water, DMEM-F-12; Invitrogen), glucose (Sigma), NaHCO3 (Sigma), HEPES (Sigma), l-glutamine (Invitrogen), penicillin/streptomycin (Invitrogen), putrescine (Sigma), apo-transferrin (Sigma), insulin (Roche), selenium (Sigma), progesterone (Sigma), BSA (Sigma), heparin (Sigma), EGF (20 ng/ml; Peprotech), bFGF (10 ng/ml; Peprotech)] and incubated in 37°C, 5% CO2 for 10 d. Cells were passaged two to three times to clear debris before using in experiments.
L-685,458, DAPT γ-secretase inhibitors.
Neurosphere cultures were treated with the γ-secretase inhibitor N-[N-(3,5-difluorophenacetyl)-l-alanyl]-(S)-phenylglycine tert-butyl ester (DAPT) (0.5 μm, Calbiochem) or with the transition state analog inhibitor L-685,458 [(5S)-(tert-butoxycarbonylamino)-6-phenyl-(4R)-hydroxy-(2R)-benzylhexanoyl)-l-leucy-l-phenylalaninamide] (10 μm, Sigma) for 48 h.
Antibodies.
The following primary antibodies were used in this study: Rat anti-BrdU polyclonal (1:500), GFP marker mouse anti-GFP monoclonal (1:200; Santa Cruz Biotechnology), chicken anti-TUJ-1 polyclonal (1:100 Millipore Bioscience Research Reagents), mouse anti-TUJ-1 monoclonal (1:5000; Promega) early neuronal marker goat anti-doublecortin polyclonal (DCX) (1:400; Santa Cruz Biotechnology), rabbit anti-PS1NTF polyclonal (1:5000, a generous gift from Dr. Gopal Thinakaran, University of Chicago, Chicago, IL), glial marker rabbit anti-glial fibrillary acidic protein polyclonal (GFAP) (1:400 Dako), glial marker GFAP (1:500 Millipore), mouse anti-NeuN monoclonal (1:400; Millipore Bioscience Research Reagents), mouse anti-actin monoclonal (1:5000; Millipore Bioscience Research Reagents), neural stem cell marker mouse anti-nestin monoclonal (1:200; Millipore Bioscience Research Reagents). The following secondary antibodies were used from Jackson ImmunoResearch Laboratories: Cy3-conjugated donkey anti-rat (1:500), biotin-conjugated donkey anti-mouse (1:250), Cy2-conjugated streptavidin (1:250), Cy5-conjugated donkey anti-chicken (1:250), Cy5-conjugated donkey anti-mouse (1:250), Cy3-conjugated donkey anti-mouse (1:500), Cy5-conjugated donkey anti-goat (1:250), Cy3-conjugated donkey anti-rabbit (1:500), Cy5-conjugated donkey anti-rabbit (1:250). Alexa 488 goat anti-mouse IgM (1:400; Invitrogen), peroxidase-conjugated rabbit anti-mouse (1:5000; Pierce Biotechnology), protein A-peroxidase (1:1000; Sigma), and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) nucleic acid stain (1:50,000; Invitrogen) were also used.
BrdU proliferation assay.
Neural progenitor cells were plated at a density of 1.5 × 104 cells/well into a round-bottom 96-well plate in complete medium. Cells are incubated for 24 h at 37°C, 5% CO2. A 5 μm BrdU solution was added to each well and incubate at 37°C, 5% CO2 for 48 h. Plates were spun down at 1000 × g for 10 min to pellet the cells. The cells were fixed with 70% EtOH and 0.1N NaOH for 30 min at room temperature. Cells were incubated in mAb BrdU (1:300, Novocastra) primary antibody for 1 h. After washing, cells were incubated in secondary antibody rabbit anti-mouse HRP (1:5000, Pierce) for 30 min. The cells were then washed and incubated with tetramethylbenzidine (TMB) substrate solution (Invitrogen) for 15 min in the dark. To stop the reaction, a solution of 2.5N sulfuric acid was added to the cells with TMB. The plates were read using a spectrophotometric microtiter plate reader set at a dual wavelength of 450–595. Each experimental group includes 4 replicates (n = 10).
Clonogenic assay.
Lentiviral infected or γ-secretase inhibitor-treated neurospheres were singly disassociated and plated at a density of 2500 cells/ ml onto a 2 cm grid plate. Neurospheres were counted after 7 d using an upright microscope. n = 5 for each experimental group.
Neurosphere differentiation assay.
For differentiation assays, neurospheres or single cells were plated onto glass coverslips coated with Matrigel (BD Biosciences) in complete media without EGF and bFGF and with the addition of 5% fetal bovine serum. Cells were allowed to differentiate for various time points at 37°C, 5% CO2. The cells were then fixed with 4% PFA for 30 min and immunostained. n = 5 for each experimental group.
Immunohistochemistry.
Brains were sectioned sagittally at 50 μm using a microtome (Leica) and placed into cryoprotectant (glycerol, ethylene glycol, PBS). Every sixth section was used for immunohistochemistry. To expose the BrdU antigen, sections were pretreated with deionized formamide and SSC for 2 h at 65° in a shaking water bath. Sections were then rinsed with SSC and incubated in a 2N HCl solution for 30 min at 37°C. Sections were then neutralized with 0.1 m borate buffer for 10 min. Sections were blocked for 1–3 h in TBS, normal donkey serum, and Triton X-100 (TBS++) before being incubated in primary antibodies in TBS and Triton X-100 (TBS+) for 72 h at 4°C. Sections were blocked again in TBS++ before being incubated in secondary antibodies for 2 h at room temperature. Sections were then mounted onto glass coverslips using polyvinyl acetate-1,4-diazabicyclo-[2.2.2]octane (PVA-DABCO). Immunohistochemistry was followed by stereological analysis.
Cell culture immunostaining.
Differentiated cells were fixed using 4% PFA for 30 min at room temperature. Cells were washed three times in TBS and blocked in TBS++ for 1 h. Cells were then incubated in primary antibodies for 2 h in TBS+. Following a second block in TBS++ for 30 min, cells were incubated in secondary antibodies in TBS+ for 1 h at room temperature. Cells were incubated in a DAPI solution for 5 min. Cells were then washed and mounted using PVA-DABCO.
Stereological analysis.
Quantification of immunostained sections were done using Stereo Investigator (MicroBrightField Bioscience). Immunostained sections were counted using the following parameters: the counting frame was set to 115 μm × 115 μm, and sampling grid was set at 122 μm × 122 μm. The section thickness was averaged at 35 μm. The entire dentate gyrus was counted due to the scarcity of BrdU+ cells.
Western blot analysis.
Protein extraction of tissue was prepared using a 1× TNE buffer [50 mm Tris, 150 mm NaCl, 5 mm EDTA, 0.5% SDS, 100 mm PMSF, and Protease Inhibitor Cocktail (Sigma)]. Protein extraction of in vitro cells was prepared using a 1× immunoprecipitation buffer (150 mm NaCl, 50 mm Tris-Cl, 0.5% Triton X-100, 0.5% sodium deoxycholate, 5 mm EDTA, 0.25% SDS, 100 mm PMSF, and Protease Inhibitor Cocktail), or 50 mm HEPES and 1% SDS lysis buffer. Protein was extracted by homogenization of tissue in the appropriate lysis buffer. Protein concentration was determined using the BCA (bicinchoninic acid) method (Pierce). Equal amounts of protein were taken for immunoblotting.
Real time reverse transcriptase PCR.
Total RNA was extracted using TRI Reagent (Sigma). Two micrograms of total RNA was treated with DNase for 1 h (RNase free, Ambion) and then reverse transcribed into cDNA using a mix of oligodT and random primers (High-Capacity cDNA Reverse Transcription Kit, Applied Biosystems). Primer sets were CycloA-5′ GGC CGA TGA CGA GCC C, CycloA-3′ TGT CTT TGG AAC TTT GTC TGC AA at, Egfr 5′ ATCCTCTGCAGGCTCAGAAA, Egfr 3′ GGCGTTGGAGGAAAAGAAAG, Bmi1–5′ AGCAGCAATGACTGTGATGCACTTGAG, Bmi1–3′ GCTCTCCAGCATTCGTCAGTCCATCCC, Catnb-5′ CAGCTTGAGTAGCCATTGTCC, Catnb-3′ GAGCCGTCAGTGCAGGAG, Tlx1–5′ TCACGTTCCTCTGCTGTCTG, Tlx1–3′ CAAGGCGCTCAAAATGACC. DCX-5′ TTCAGGACCACAAGCAATGA, DCX-3′ GGAAACCGGAGTTGTCAAAA, β-III tubulin-5′ ATGCTTGGGACTACGTTTGG, β-III tubulin-3′ TGAGGCCTCCTCTCACAAGT, GFAP-5′ CACGAAGCTAACGACTATCGC, GFAP-3′ CTCTAGGGACTCGTTCGTGC, PDGF-5′ CTGCACCAAGTCAGGTCCC, PDGF-3′ CTTCTCTGGGTGTTGGCTCA, Nestin-5′ GGTCACTGT CGCCGCTACTC, Nestin-3′ CGGACGTGGAGCACTAGAGAA. Quantitative real-time (RT)-PCR was performed with SYBRGreen PCR Master Mix (Applied Biosystems) using an ABI Prism 7700 sequence detection system. The results were analyzed using the comparative ΔΔCt method (normalized to the amplification cycle counts of the cyclophilin reference gene). Unpaired t test and ANOVA used for statistical analysis.
Results
Targeting PS1 in NPCs and in neurogenic niches exclusively
To examine the role of PS1 in regulation of NPCs and their progeny in the adult brain we developed lentiviral vectors expressing siRNA for the targeting of PS1 (Fig. 1A). Viral vectors coexpress GFP for the identification of infected cells and their progeny. To rule out off-target effects of the siRNA, we developed 2 shRNA for the targeting of PS1 (designated 1.1 and 4.11 PS1 siRNA) and 2 control shRNA expressing irrelevant sequences (IR 8.1, 9.1 siRNA). As an additional control we infected neurospheres in vitro and in vivo with lentiviral vectors expressing GFP only (GFP-Lenti, Fig. 1A). To validate that infection of cells with lentiviral vectors expressing siRNA for the targeting of PS1 results in a reduction of PS1 expression, we first infected murine neuroblastoma N2a cells with the five lentiviral vector preparations. As expected, infection of N2a cells with both lentiviral vectors expressing siRNA for the targeting of PS1 induced a reduction in PS1 expression (Fig. 1B,C). Likewise, infection of neurosphere culture revealed a reduction in PS1 expression in cultures infected with lentiviral vectors expressing PS1 1.1 and PS1 4.11 siRNA, but not in cultures infected with lentiviral vectors expressing IR 8.1, IR 9.1 siRNA compared with noninfected neurospheres (Fig. 1D,E). To confirm a reduction in PS1 expression in neurospheres cultures infected with lentiviral vectors expressing PS1 1.1 and PS1 4.11 siRNA, neurospheres were immunostained with anti-PS1 antibodies 5 d after lentiviral infection. PS1 immunoreactivity was significantly reduced in GFP+ neurospheres infected with lentiviral vectors expressing PS1 1.1 and PS1 4.11 siRNA, compared with GFP+ neurospheres expressing IR 8.1, IR 9.1 siRNA (Fig. 1F). We then injected lentiviral vectors into the SGL and the SVZ of adult mice stereotaxically (Fig. 2). Examination of PS1 expression in protein extracts of SGL (n = 16; Fig. 2A,B) and SVZ (data not shown) of mice infected with lentiviral vectors expressing PS1 1.1 and PS1 4.11 siRNA revealed a decrease in PS1 expression compared with SGL and SVZ of mice infected with lentiviral vectors expressing IR 8.1, IR 9.1 siRNA or GFP-Lenti (Fig. 2A,B). To verify specificity of infection in the neurogenic microenvironments, brain sections of mice infected with lentiviral vectors were subject to immunohistochemical analysis (Fig. 2C). Six weeks after stereotaxic injection of lentiviral vectors, GFP+ cells were confined to the SGL with some GFP+ cells in the granule layer of the dentate gyrus. No GFP+ cells could be detected in any other area of the hippocampus or in any other brain area (Fig. 2Ca–Cd; supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Likewise, injection of lentiviral vectors into the SVZ resulted in massive GFP+ expression of progenitor cells (Fig. 2Ce). These results suggest that lentiviral vectors expressing siRNA for PS1 targeting, successfully downregulate PS1 expression in the neurogenic microenvironments and in neurosphere cultures derived from these niches.
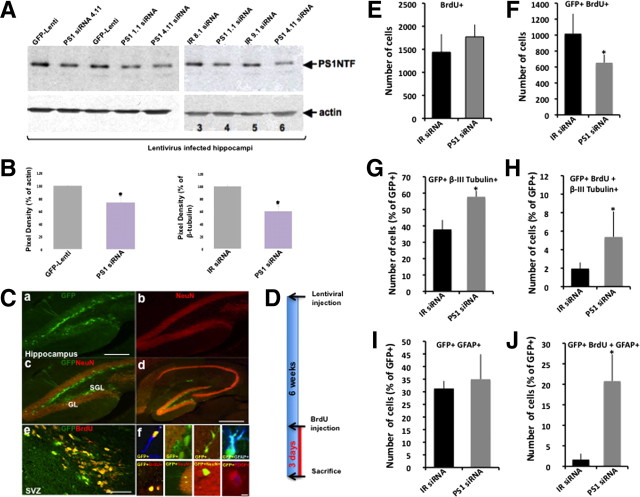
Figure 2.
Downregulation of PS1 expression in NPCs in the SGL of adult mice enhances their differentiation into neurons and glia. A, Reduced PS1 expression in protein extracts of mouse hippocampi infected with lentiviral vectors expressing PS1 1.1, PS1 4.11 shRNA for the targeting of PS1, compared with hippocampi infected with lentiviral vectors expressing control IR 8.1, IR 9.1 shRNA or GFP-Lenti. B, Densitometric analysis shows the average reduction in PS1 expression in hippocampi infected with lentiviral vectors expressing PS1 1.1, PS1 4.11 shRNA for the targeting of PS1 compared with hippocampi infected with GFP-Lenti or IR shRNA (n = 3). C, Confocal images of brain sections of 6-month-old C57BL/6 mice 6 weeks after stereotaxic injection of lentiviral vectors expressing GFP and PS1 siRNA into the SGL (Ca–Cd) or into the SVZ (Ce). Ca, A mass of GFP+ cells in the SGL of the dentate gyrus. GFP+ cells are present mainly in the SGL. Some GFP+ cells can be detected in the granule layer (GL). Cb, NeuN+ immunolabeled neurons in the DG. c, d, Merged images of NeuN+ immunolabeling and GFP+ staining in the DG (Cc) and in whole hippocampus (Cd) reveals that GFP+ cells in the SGL are NeuN negative and in turn NeuN+ cells in other hippocampal areas are GFP negative. Ce, Confocal image of GFP+ cells (green) immunolabeled with antibodies raised against BrdU (red), as detected in the SVZ of mice injected with lentiviral vectors 6 weeks after injection. Note that the vast majority of cells are GFP+BrdU+ double labeled. Scale bar, 150 μm (Ca–Cc), 250 μm (Cd) and 75 μm (Ce). Cf, GFP+ neural progenitor cells express a variety of progenitor and lineage-specific markers detected by confocal analysis. Top from left: GFP+DCX+ cell; GFP+ cells at the SGL are NeuN (red) negative; GFP+BrdU+ cells at the SGL extend processes toward the granular layer of the DG; GFP+GFAP+; bottom from left: GFP+BrdU+ cell in the granule layer; GFP+NeuN+ cell (NeuN red) in the granular layer of the dentate gyrus; GFP+ cells that migrated to the granule cell layer of the DG and extend processes toward the molecular cell layer of the dentate gyrus; PDGFR+BrdU+GFP+ cell. Scale bar, 50 μm. D, A diagram showing the experimental procedures of in vivo experiments. E, F, Analysis of cell proliferation in the DG of adult mice injected with lentiviral vectors shows that the number of total proliferating (BrdU+) cells in the SGL is comparable in the brains of animals injected with lentiviral vectors expressing PS1 1.1, PS1 4.11 IR 8.1, IR 9.1 shRNA (E). However, the number of GFP+BrdU+ is significantly lower in the brains of mice injected with lentiviral vectors expressing PS1 1.1, PS1 4.11, compared with number in the brains of mice injected with lentiviral vectors expressing IR 8.1, IR 9.1 or GFP-Lenti control (F), suggesting that downregulation of PS1 in target NPCs decreased their proliferation. G, An increased number of neurons (GFP+β-III tubulin+) in the brains of mice injected with lentiviral vectors expressing PS1 1.1, PS1 4.11, compared with number in the brains of mice injected with lentiviral vectors expressing IR 8.1, IR 9.1 or GFP-Lenti control. H, An increase in the number of new neurons in the brains of mice injected with lentiviral vectors expressing PS1 1.1, PS1 4.11, compared with number in the brains of mice injected with lentiviral vectors expressing IR 8.1, IR 9.1 or GFP-Lenti control. t test *p < 0.05. I, A slight increase in the number of GFP+GFAP+ cells in the brains of mice injected with lentiviral vectors expressing PS1 1.1, PS1 4.11. J, A significant increase in the number of newly formed astrocytes (GFP+BrdU+GFAP+) in the brains of mice injected with lentiviral vectors expressing PS1 1.1, PS1 4.11, compared with number in the brains of mice injected with lentiviral vectors expressing IR 8.1, IR 9.1 or GFP-Lenti control.
Targeting PS1 in the SGL of the dentate gyrus reduces the number of rapidly proliferating NPCs and increases the number of differentiating cells
To examine the effect of reduced PS1 expression on neurogenesis, lentiviral vectors expressing PS1 1.1, PS1 4.11, IR 8.1, IR 9.1 siRNA or GFP-Lenti were injected into the SGL. Six weeks later mice were injected with BrdU and killed 3 d after injection (Fig. 2D). Unbiased stereology of brain sections revealed that the total number of BrdU+ cells was comparable in mice injected with lentiviral vectors expressing IR siRNA or GFP-Lenti and in mice injected with vectors expressing siRNA for PS1 targeting (Fig. 2E), suggesting that infection with lentiviral vectors did not have a secondary effect on proliferation of cells in the SGL. However, there was a significant reduction in the number of GFP+BrdU+ cells in the SGL of mice injected with lentiviral vectors expressing PS1 1.1, PS1 4.11 (Fig. 2F), suggesting that downregulation of PS1 expression decreased NPC proliferation in the SGL of these mice. To examine whether reduction in rate of proliferation in cells expressing siRNA for PS1 targeting is accompanied by alterations in extent of differentiation, brain sections were coimmunolabeled with neuronal and glial lineage markers (Fig. 2Cf). The number of infected neurons (GFP+β-tubulin+) and astrocytes (GFP+GFAP+) was quantified by stereological analysis. The results show a significant increase in the number of neurons (GFP+β-tubulin+) in the SGL of mice injected with lentiviral vectors expressing siRNA for PS1 targeting (Fig. 2G). In addition we observed an increase in the number of neuroblasts (GFP+BrdU+β-tubulin+) in the SGL of mice injected with lentiviral vectors expressing siRNA for PS1 targeting (Fig. 2H), suggesting that reduced expression of PS1 enhances neuronal differentiation in the SGL. Examination of the number of infected astrocytes revealed a slight but insignificant increase in the number of astrocytes (GFP+GFAP+, Fig. 2I), but a dramatic increase in the number of newly formed astrocytes (GFP+BrdU+GFAP+; Fig. 2J) in the SGL of mice injected with lentiviral vectors expressing siRNA for PS1 targeting. Together, these results suggest that downregulation of PS1 in NPCs in the SGL of adult mice enhances neuronal differentiation and increases the number of astrocytes, and this increase is accompanied by a decrease in the number of rapidly proliferating NPCs.
Downregulation of PS1 expression enhances NPC differentiation into neurons and glia
To determine whether downregulation of PS1 expression in NPCs enhances their differentiation, neurosphere cultures were infected with lentiviral vectors expressing PS1 1.1 or PS1 4.11 siRNA, or with lentiviral vectors expressing IR 8.1, IR 9.1 siRNA, or with GFP-Lenti (Fig. 3A). To induce cell differentiation, infected neurospheres were then cultured on Matrigel for 24 h. Neurospheres infected with lentiviral vectors expressing IR 8.1, IR 9.1 siRNA, or with GFP-Lenti exhibited round sphere-like morphology (Fig. 3B). In contrast, neurospheres infected with lentiviral vectors expressing PS1 1.1 or PS1 4.11 siRNA lost the sphere morphology; cells migrated out and differentiated into process-bearing cells (Fig. 3B). NPCs infected with lentiviral vectors expressing PS1 1.1 or PS1 4.11 siRNA differentiated faster than NPCs infected with lentiviral vectors expressing IR 8.1, IR 9.1 siRNA, or with GFP-Lenti even when cultured as single-cells (Fig. 3C). To quantify extent of cell differentiation following PS1 downregulation, neurospheres infected with lentiviral vectors expressing PS1 1.1, PS1 4.11, IR 8.1, IR 9.1 siRNA, or GFP-Lenti were singly dissociated and an equal number of single cells were cultured for each infected group. Seven days later, the number of neurospheres in cultures infected with PS1 siRNA was significantly lower compared with the number of neurospheres in cultures infected with IR siRNA (Fig. 3D). In contrast, the number of differentiated cells was significantly higher in cultures infected with PS1 siRNA compared with cultures infected with IR siRNA (Fig. 3D). It should be noted that neurospheres differentiated in cultures infected with PS1 siRNA despite the presence of the potent proliferation factors bFGF and EGF, suggesting that PS1 dominates the proliferative effect of these growth factors. To examine whether reduced PS1 levels in NPCs affect multipotentiality, differentiating NPCs infected with lentiviral vectors expressing PS1 or IR siRNA were immunostained for cell-lineage markers. GFP+ NPCs could differentiate into neurons, astrocytes and oligodendrocytes independently of PS1 expressing levels (Fig. 3E). Quantification of the number of differentiated neurons, astrocytes and oligodendrocytes revealed an increase in all three lineages in cultures infected with lentiviral vectors expressing PS1 siRNA (Fig. 3F), suggesting that reduced levels of PS1 do not alter multipotentiality.
Figure 3.
Downregulation of PS1 in neurosphere cultures enhances their differentiation without affecting multipotentiality. A, Neurosphere culture transduced with lentiviral vectors expressing PS1 1.1, PS1 4.11, IR 8.1, IR 9.1 express GFP (left and right panel), as well as the neural progenitor cell marker nestin (right panel; orange; nestin+GFP+; blue, DAPI). B, Neurospheres transduced with lentiviral vectors expressing PS1 1.1, PS1 4.11 for PS1 targeting readily differentiate, while neurospheres transduced with lentiviral vectors expressing IR 8.1 or IR 9.1, or GFP-Lenti control maintain sphere morphology (green, GFP; red, β-tubulin; blue, DAPI). C, Singly dissociated neural progenitor cells transduced with lentiviral vectors expressing PS1 1.1, PS1 4.11 for PS1 targeting exhibit enhanced differentiation compared with progenitors transduced with lentiviral vectors expressing IR 8.1 or IR 9.1 control (green, GFP; red, β-tubulin; blue, DAPI). D, Number of neurospheres (left panel) generated from neural progenitor cells infected with lentiviral vectors expressing PS1 1.1, PS1 4.11, is significantly reduced, while the number of differentiated cells is increased (right panel) compared with neural progenitor cells infected with lentiviral vectors expressing IR 8.1, IR 9.1 siRNA, or GFP-Lenti. E, Neural progenitor cells infected with lentiviral vectors expressing PS1 1.1, PS1 4.11 can give rise to neurons (GFP+β-tubulin+), astrocytes (GFP+GFAP+) and oligodendrocytes (GFP+PDGFR+), suggesting that downregulation of PS1 does not affect their multipotentiality. F, Neural progenitor cells infected with lentiviral vectors expressing PS1 1.1, PS1 4.11 give rise to increased number of differentiated cells of all three lineages, neurons, astrocytes and oligodendrocytes. t test *p < 0.05.
Regulation of NPC differentiation by PS1 is γ-secretase dependent
To determine whether enhancement of NPC differentiation following PS1 downregulation is γ-secretase-dependent, neurospheres were treated with the γ-secretase inhibitor DAPT or with the transition state analog inhibitor L-685,458 or with vehicle, and cultured on Matrigel. Twenty-four hours later, neurospheres treated with vehicle exhibited sphere morphology with some initial processes indicating cell differentiation (Fig. 4A). In contrast, and similar to neurospheres infected with PS1 siRNA, neurospheres treated with either DAPT or L-685,458 lost sphere morphology. Cells migrated out of the neurosphere, and differentiated (Fig. 4A). Similarly, singly dissociated NPCs treated with either DAPT or L-685,458 differentiated faster than NPCs treated with vehicle (Fig. 4B). Quantification of the length of cell processes revealed a dramatic increase in process length in L-685,458-treated NPCs (Fig. 4C). To examine whether inhibition of γ-secretase affects multipotentiality, NPCs were immunostained using lineage-specific markers (Fig. 4E) and the number of lineage-specific cells was counted (Fig. 4D). NPCs treated with either DAPT or L-685,458 differentiated into all three lineages, suggesting that multipotentiality is not affected by γ-secretase activity (Fig. 4D,E). We further observed a slight increase in levels of all three lineages neurons, astrocytes and oligodendrocytes in NPCs treated with DAPT or L-685,458 compared with NPCs treated with vehicle (Fig. 4D). Together, these results suggest that lack of γ-secretase activity in NPCs induces a similar phenotype to that of downregulated PS1, implying that PS1 regulates NPCs differentiation in a γ-secretase-dependent manner.
Figure 4.
PS1 effect on neural progenitor cells is γ-secretase-dependent. A, B, Neural progenitor cells treated with the γ-secretase inhibitor L-685,458, either as neurospheres (A) or as singly dissociated cells (B) differentiate faster than progenitors treated with vehicle (green, GFAP; red, β-tubulin; blue, DAPI). C, Quantification of the length of cell processes reveals significantly more processes in neural progenitor cells treated with L-685,458 compared with vehicle. D, Quantitative analysis of cell lineage reveals a slight increase in the number of new neurons and astrocytes in cells treated with L-685,458 or DAPT and a significant increase in the number of new oligodendrocytes. E, Neural progenitor cells treated with L-685,458 or DAPT differentiate into β-tubulin+ neurons, S100β+ astrocytes and PDGFR+ oligodendrocytes. t test *p < 0.05.
Downregulation of PS1 expression decreases extent of NPC proliferation and clone-formation capacity
To examine whether enhanced NPC differentiation following PS1 downregulation was accompanied by reduced rate of proliferation, extent of BrdU intake was examined in neurospheres infected with lentiviral vectors expressing PS1 1.1 or PS1 4.11 siRNA or IR 8.1, IR 9.1 siRNA, or GFP-Lenti, using a proliferation assay. We observed that the number of cells incorporating BrdU was significantly lower in NPCs infected with siRNA for PS1 targeting than in NPCs infected with an IR siRNA (Fig. 5A). To examine whether reduction in NPC proliferation is the result of an effect of PS1 on NPC clone-formation capacity we quantified the number of NPCs giving rise to neurospheres following infection with lentiviral vectors. Hence, neurosphere cultures were infected with lentiviral vectors expressing PS1 1.1 or PS1 4.11 siRNA or IR 8.1, IR 9.1 siRNA, or GFP-Lenti. Infected cells were singly dissociated. The number of primary neurospheres formed was lower in NPCs infected with lentiviral vectors expressing PS1 siRNA compared with the number formed in NPCs infected with IR siRNA (Fig. 5B). To examine whether reduced proliferation is γ-secretase-dependent we repeated the BrdU uptake experiment using neurospheres treated with γ-secretase inhibitor. A significant reduction in BrdU uptake was observed in cells treated with the γ-secretase inhibitor L-685,458 (Fig. 5C; supplemental Fig. 2B, available at www.jneurosci.org as supplemental material) or DAPT (Fig. 5D; supplemental Fig. 2A, available at www.jneurosci.org as supplemental material).
Figure 5.
Reduced PS1 expression impairs proliferation and affects clone formation capacity. A, Neurospheres infected with lentiviral vectors expressing siRNA for PS1 targeting exhibit reduced uptake of the thymidine analog BrdU compared with neurospheres infected with an irrelevant IR 8.1, 9.1 siRNA. B, A clonogenic assay shows that the number of clone forming cells in NPCs infected with lentiviral vectors expressing siRNA for PS1 targeting is significantly reduced compared with NPCs infected with an irrelevant IR 8.1, 9.1 siRNA. C, D, Neurospheres treated with L-685,458 (C) or DAPT (D) exhibit reduced uptake of BrdU compared with neurospheres treated with vehicle. t test *p < 0.05.
PS1 regulates NPC differentiation through β-catenin and EGFR signaling pathways
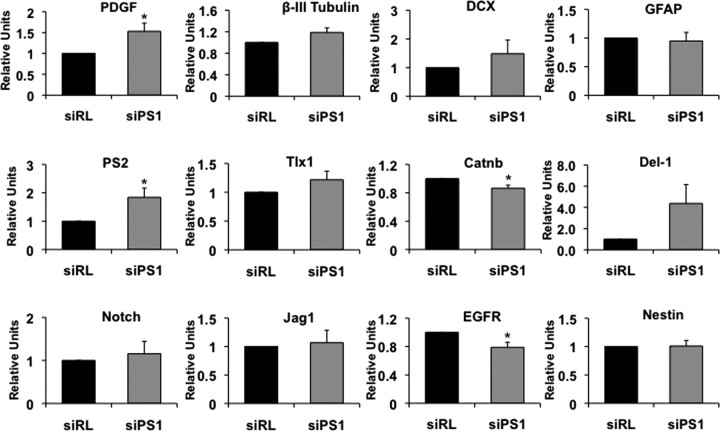
To gain further insight into the mechanism by which PS1 exerts its effect on NPC proliferation, we examined expression level of neurogenic signals that are functionally associated with PS1, namely, notch-1, notch-1 ligands Del-1 and Jagged-1, β-catenin and EGFR as well as levels of the proliferation signal Tlx, the PS1 homolog PS2, and differentiation markers: PDGF, GFAP, β-III tubulin, and DCX (Fig. 6). Thus, neurosphere cultures were transduced with lentiviral vector expressing siRNA for PS1 targeting or IR siRNA and gene expression was analyzed by quantitative real-time RT-PCR. We observed a significant decrease in expression levels of β-catenin and EGFR, suggesting that these neurogenic signaling are downstream players in PS1 regulation of NPC differentiation. Expression of Tlx, Notch-1 and Jag-1 was slightly increased, while Del-1 was significantly increased. In addition, PS2 was significantly increased. PDGF expression levels were significantly higher in cultures with reduced PS1 expression, while β-III tubulin, DCX, and GFAP were slightly increased but were not significantly different, suggesting that the effect of PS1 on the latter three proteins is not regulated on the transcriptional level.
Figure 6.
Gene expression in neurospheres after PS-1 knockdown. Neurosphere cultures were transduced with lentiviral vectors expressing PS1 shRNA for PS1 targeting or control IR shRNA and RNA was extracted for quantitative RT-PCR analysis of gene expression. Genes analyzed were EGFR, β-catenin (Catnb), presenilin-2 (PS2), PDGF, Δ-1 (Del-1), β-III tubulin (bTub), DCX, Tlx1, Notch, Jagged-1 (Jag1), nestin, and GFAP. Values are mean ± SEM of values relative to control (n = 6). Unpaired t test, *p < 0.05, and ANOVA, p < 0.05, with F > Fcrit.
Discussion
Here we show that PS1 regulates NPC differentiation in the adult brain. Downregulation of PS1 expression reduces proliferation of NPCs, and enhances their differentiation without interfering with their multipotentiality. Our study suggests that PS1 may exert its effect through EGFR and β-catenin signaling, and that PS1 dominates major proliferation pathways in NPCs, namely, EGF and bFGF signaling. PS1 effect on NPC differentiation is γ-secretase dependent.
Lack of PS1 during embryonic development was suggested to impair neurogenesis through enhanced neuronal differentiation, without an effect on cell proliferation or apoptosis (Handler et al., 2000; Yang et al., 2000). Recent studies show that in PS1/PS2 conditional double knock-out mice, in which expression of both PSs are inactivated in NPC beginning at embryonic day 11 suggests that NPCs exit cell cycle and prematurely differentiate into neurons between E13.5 and E14.5 (Kim and Shen, 2008). Other studies examining the role of PS1 in stem cell maintenance during development suggest that PS1 plays a role in the development and/or maintenance of the anterior and intermediate lobes of the pituitary gland (Nakajima et al., 2009). The present study determined that PS1 regulates neurogenesis postnatally as well. We show that downregulation of PS1 expression contracted the population of proliferating NPC in the SVZ and SGL, while enhancing the number of mature neurons and glia. This suggests that PS1 regulates a fundamental checkpoint that determines whether a cell would keep proliferating or differentiate.
Enhanced NPC differentiation observed following PS1 knockdown was similar to the effect exerted by treatment of NPCs with γ-secretase inhibitors. While the IC50 of L-685,458 and DAPT is ∼3–17 nm and 100–170 nm, respectively (Shearman et al., 2000; Weihofen and Martoglio, 2003; Morohashi et al., 2006), both inhibitors enhanced NPC differentiation in the range of 0.5–20 μm. IC50 of these inhibitors was determined using in vitro cell-free assays with purified or enriched γ-secretase membrane prep and substrate. In addition, the readout of these experiments is the inhibition of Aβ production and the accumulation of amyloid precursor protein (APP)-C′-terminal fragments. In contrast, our experiments are based on whole-cell (primary culture) assays. APP is expressed at endogenous levels. The readout is a cellular phenotype that may involve the concerted action of several signaling pathways regulated by γ-secretase in addition to APP metabolites. In addition, the time course of our experiments is days (rather than hours). In support of that, previous studies examining the effect of these inhibitors on stem cell biology used doses similar to ours (Mori et al., 2006; Borghese et al., 2010).
We further show that downregulation of PS1 in neurosphere culture reduces the expression of two critical neurogenic signaling molecules, β-catenin and EGFR. PS1 is implicated in regulation of epidermal growth factor receptor EGFR and the Wnt/β-catenin signaling, critical regulators of NSC self-renewal and proliferation (for review, see Shi et al., 2008). Substantial evidence exists for an interaction between PS1 and β-catenin and for the formation of a β-catenin/PS1 complex (Tesco et al., 1998; Tesco and Tanzi, 2000). β-Catenin is a critical downstream component of the canonical Wnt pathway, a central regulator of mammalian neural development and a regulator of NPCs in the SVZ, promoting proliferation of Mash1+ cells in the SVZ. Modulation of β-catenin signaling specifically in dividing cells in the adult mouse SVZ suggests β-catenin signaling is sufficient to increase the percentage of dividing Mash1 cells (Adachi et al., 2007). Wnt signaling plays a role in adult hippocampal neurogenesis as well, regulating proliferation of progenitor cells and neuronal differentiation (Lie et al., 2005). In the nucleus, stabilized β-catenin functions through TCF/LEF1 transcription factors and the notch-1/RBP-J complex, promoting proliferation of NPCs and suppressing their differentiation, respectively (Shimizu et al., 2008), possibly by activating the expression of target genes that are involved in the G1-S transition, such as cyclin D1 and c-Myc.
In addition, β-catenin and Notch intracellular domain (NICD) form a complex with the promoter region of the antineurogenic hes1 gene, allowing its expression. Reduced expression of β-catenin following downregulation of PS1 in NPCs may suggest that less β-catenin reaches the nucleus, leading to attenuation of proliferation-inducing genes. In addition, our results show that expression level of the notch-1 ligand Del-1 is upregulated following PS1 knockdown. Notch1 is thought to be involved in the regulation of radial glia differentiation into committed NPCs during postnatal neurogenesis in the SGL, as well as during newborn neuron maturation (Breunig et al., 2007). Interestingly we observed an increase in PS2 and notch ligand expression, suggesting unsuccessful compensatory mechanisms to resume normal levels of PS and notch signaling, respectively. Shimizu et al. (2008) have shown that GSK3β inactivation and β-catenin stabilization in NPCs potentiated the promoter activity of hes1 and hes5 genes only when Notch signaling was activated. Further, stabilized β-catenin associates with N1IC and enhances its RBP-J-dependent transcription activity to induce hes1 and hes5 gene expression. Interestingly, in hyh mutant mice reduced β-catenin correlates with early neurogenic differentiation (Chae et al., 2004). Conditional knock-out of β-catenin in the cortex and hippocampus resulted in reduced cell proliferation as a consequence of deficits in the neuroepithelium during development (Machon et al., 2003).
In summary, we show that PS1 plays a major role in adult neurogenesis, regulating NPC differentiation in a γ-secretase-dependent manner. This study reveals a new molecular target for the manipulation of NPCs in the adult brain.
Footnotes
This study was supported by National Institutes of Health Grant AG033570 and an Alzheimer's Association Young Investigator Award (O.L.).
References
- Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, Peltz G, Gong L, Kawase T, Alvarez-Buylla A, Okano H, Sawamoto K. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Deng W, Gage FH. Adult neurogenesis: integrating theories and separating functions. Trends Cogn Sci. 2010;14:325–337. doi: 10.1016/j.tics.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese L, Dolezalova D, Opitz T, Haupt S, Leinhaas A, Steinfarz B, Koch P, Edenhofer F, Hampl A, Brüstle O. Inhibition of notch signaling in human embryonic stem cell-derived neural stem cells delays G1/S phase transition and accelerates neuronal differentiation in vitro and in vivo. Stem Cells. 2010;28:955–964. doi: 10.1002/stem.408. [DOI] [PubMed] [Google Scholar]
- Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:20558–20563. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Chae TH, Kim S, Marz KE, Hanson PI, Walsh CA. The hyh mutation uncovers roles for alpha Snap in apical protein localization and control of neural cell fate. Nat Genet. 2004;36:264–270. doi: 10.1038/ng1302. [DOI] [PubMed] [Google Scholar]
- Chevallier NL, Soriano S, Kang DE, Masliah E, Hu G, Koo EH. Perturbed neurogenesis in the adult hippocampus associated with presenilin-1 A246E mutation. Am J Pathol. 2005;167:151–159. doi: 10.1016/S0002-9440(10)62962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Veeraraghavalu K, Lazarov O, Marler S, Ransohoff RM, Ramirez JM, Sisodia SS. Non-cell-autonomous effects of presenilin 1 variants on enrichment-mediated hippocampal progenitor cell proliferation and differentiation. Neuron. 2008;59:568–580. doi: 10.1016/j.neuron.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demars M, Hu YS, Gadadhar A, Lazarov O. Impaired neurogenesis is an early event in the etiology of familial Alzheimer's disease in transgenic mice. J Neurosci Res. 2010;88:2103–2117. doi: 10.1002/jnr.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain [see comments] Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- Feng R, Rampon C, Tang YP, Shrom D, Jin J, Kyin M, Sopher B, Miller MW, Ware CB, Martin GM, Kim SH, Langdon RB, Sisodia SS, Tsien JZ. Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron. 2001;32:911–926. doi: 10.1016/s0896-6273(01)00523-2. [DOI] [PubMed] [Google Scholar]
- Handler M, Yang X, Shen J. Presenilin-1 regulates neuronal differentiation during neurogenesis. Development. 2000;127:2593–2606. doi: 10.1242/dev.127.12.2593. [DOI] [PubMed] [Google Scholar]
- Herreman A, Serneels L, Annaert W, Collen D, Schoonjans L, De Strooper B. Total inactivation of gamma-secretase activity in presenilin-deficient embryonic stem cells. Nat Cell Biol. 2000;2:461–462. doi: 10.1038/35017105. [DOI] [PubMed] [Google Scholar]
- Kim WY, Shen J. Presenilins are required for maintenance of neural stem cells in the developing brain. Mol Neurodegener. 2008;3:2. doi: 10.1186/1750-1326-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Marr RA. Neurogenesis and Alzheimer's disease: At the crossroads. Exp Neurol. 2010;223:267–281. doi: 10.1016/j.expneurol.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YM, Xu M, Lai MT, Huang Q, Castro JL, DiMuzio-Mower J, Harrison T, Lellis C, Nadin A, Neduvelil JG, Register RB, Sardana MK, Shearman MS, Smith AL, Shi XP, Yin KC, Shafer JA, Gardell SJ. Photoactivated gamma-secretase inhibitors directed to the active site covalently label presenilin 1. Nature. 2000;405:689–694. doi: 10.1038/35015085. [DOI] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Désiré L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Machon O, van den Bout CJ, Backman M, Kemler R, Krauss S. Role of beta-catenin in the developing cortical and hippocampal neuroepithelium. Neuroscience. 2003;122:129–143. doi: 10.1016/s0306-4522(03)00519-0. [DOI] [PubMed] [Google Scholar]
- Mori H, Ninomiya K, Kino-oka M, Shofuda T, Islam MO, Yamasaki M, Okano H, Taya M, Kanemura Y. Effect of neurosphere size on the growth rate of human neural stem/progenitor cells. J Neurosci Res. 2006;84:1682–1691. doi: 10.1002/jnr.21082. [DOI] [PubMed] [Google Scholar]
- Morohashi Y, Kan T, Tominari Y, Fuwa H, Okamura Y, Watanabe N, Sato C, Natsugari H, Fukuyama T, Iwatsubo T, Tomita T. C-terminal fragment of presenilin is the molecular target of a dipeptidic gamma-secretase-specific inhibitor DAPT (N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester) J Biol Chem. 2006;281:14670–14676. doi: 10.1074/jbc.M513012200. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Watanabe S, Okuyama S, Shen J, Furukawa Y. Restricted growth and insulin-like growth factor-1 deficiency in mice lacking presenilin-1 in the neural crest cell lineage. Int J Dev Neurosci. 2009;27:837–843. doi: 10.1016/j.ijdevneu.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KJB. The mouse brain in stereotaxic coordinates. San Diego: Academic; 2004. [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Shearman MS, Beher D, Clarke EE, Lewis HD, Harrison T, Hunt P, Nadin A, Smith AL, Stevenson G, Castro JL. L-685,458, an aspartyl protease transition state mimic, is a potent inhibitor of amyloid beta-protein precursor gamma-secretase activity. Biochemistry. 2000;39:8698–8704. doi: 10.1021/bi0005456. [DOI] [PubMed] [Google Scholar]
- Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- Shi Y, Sun G, Zhao C, Stewart R. Neural stem cell self-renewal. Crit Rev Oncol Hematol. 2008;65:43–53. doi: 10.1016/j.critrevonc.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Kagawa T, Inoue T, Nonaka A, Takada S, Aburatani H, Taga T. Stabilized beta-catenin functions through TCF/LEF proteins and the Notch/RBP-Jkappa complex to promote proliferation and suppress differentiation of neural precursor cells. Mol Cell Biol. 2008;28:7427–7441. doi: 10.1128/MCB.01962-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol. 2009;25:253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- Tesco G, Tanzi RE. GSK3 beta forms a tetrameric complex with endogenous PS1-CTF/NTF and beta-catenin. Effects of the D257/D385A and FAD-linked mutations. Ann N Y Acad Sci. 2000;920:227–232. doi: 10.1111/j.1749-6632.2000.tb06927.x. [DOI] [PubMed] [Google Scholar]
- Tesco G, Kim TW, Diehlmann A, Beyreuther K, Tanzi RE. Abrogation of the presenilin 1/beta-catenin interaction and preservation of the heterodimeric presenilin 1 complex following caspase activation. J Biol Chem. 1998;273:33909–33914. doi: 10.1074/jbc.273.51.33909. [DOI] [PubMed] [Google Scholar]
- Tiscornia G, Singer O, Verma IM. Design and cloning of lentiviral vectors expressing small interfering RNAs. Nat Protoc. 2006a;1:234–240. doi: 10.1038/nprot.2006.36. [DOI] [PubMed] [Google Scholar]
- Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc. 2006b;1:241–245. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
- Wang R, Dineley KT, Sweatt JD, Zheng H. Presenilin 1 familial Alzheimer's disease mutation leads to defective associative learning and impaired adult neurogenesis. Neuroscience. 2004;126:305–312. doi: 10.1016/j.neuroscience.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Weihofen A, Martoglio B. Intramembrane-cleaving proteases: controlled liberation of proteins and bioactive peptides. Trends Cell Biol. 2003;13:71–78. doi: 10.1016/s0962-8924(02)00041-7. [DOI] [PubMed] [Google Scholar]
- Wen PH, Hof PR, Chen X, Gluck K, Austin G, Younkin SG, Younkin LH, DeGasperi R, Gama Sosa MA, Robakis NK, Haroutunian V, Elder GA. The presenilin-1 familial Alzheimer disease mutant P117L impairs neurogenesis in the hippocampus of adult mice. Exp Neurol. 2004;188:224–237. doi: 10.1016/j.expneurol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Wines-Samuelson M, Shen J. Presenilins in the developing, adult, and aging cerebral cortex. Neuroscientist. 2005;11:441–451. doi: 10.1177/1073858405278922. [DOI] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- Xie Z, Romano DM, Kovacs DM, Tanzi RE. Effects of RNA interference-mediated silencing of gamma-secretase complex components on cell sensitivity to caspase-3 activation. J Biol Chem. 2004;279:34130–34137. doi: 10.1074/jbc.M401094200. [DOI] [PubMed] [Google Scholar]
- Yang X, Handler M, Shen J. Role of presenilin-1 in murine neural development. Ann N Y Acad Sci. 2000;920:165–170. doi: 10.1111/j.1749-6632.2000.tb06918.x. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Nadeau P, Song W, Donoviel D, Yuan M, Bernstein A, Yankner BA. Presenilins are required for gamma-secretase cleavage of beta-APP and transmembrane cleavage of Notch-1. Nat Cell Biol. 2000;2:463–465. doi: 10.1038/35017108. [DOI] [PubMed] [Google Scholar]