Abstract
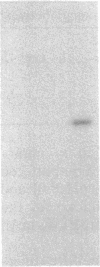
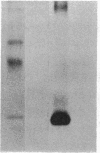
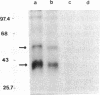
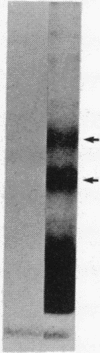
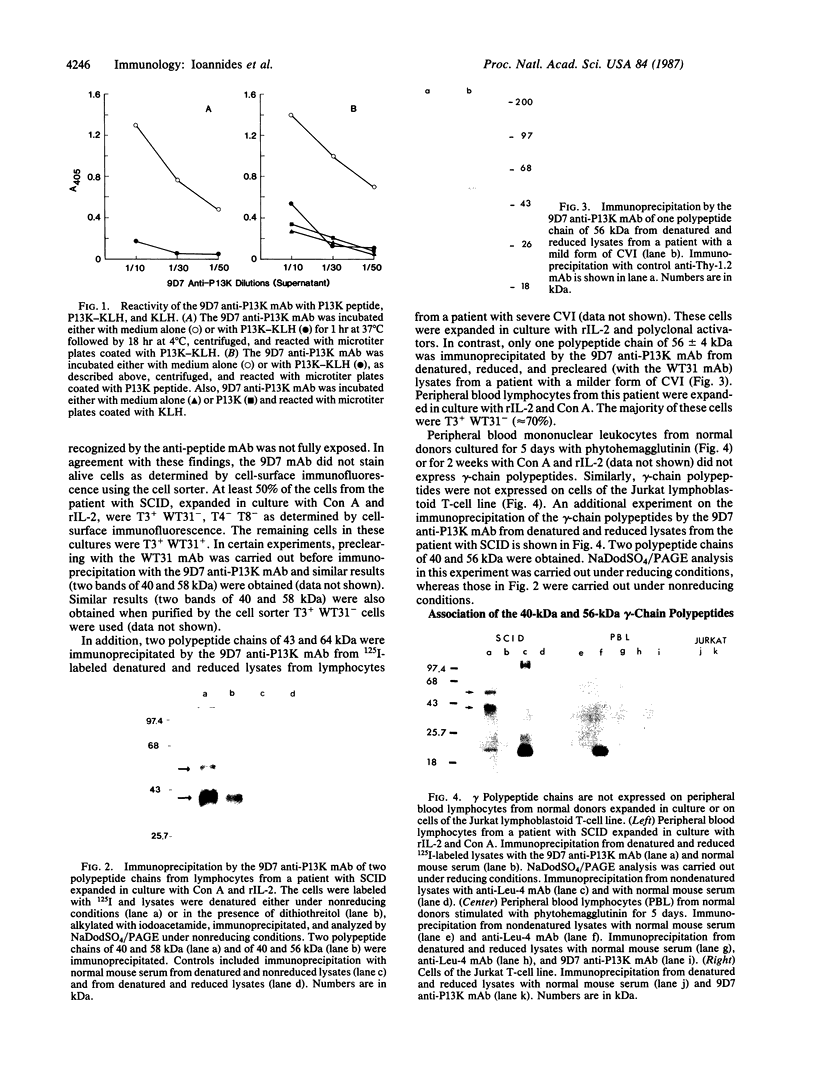
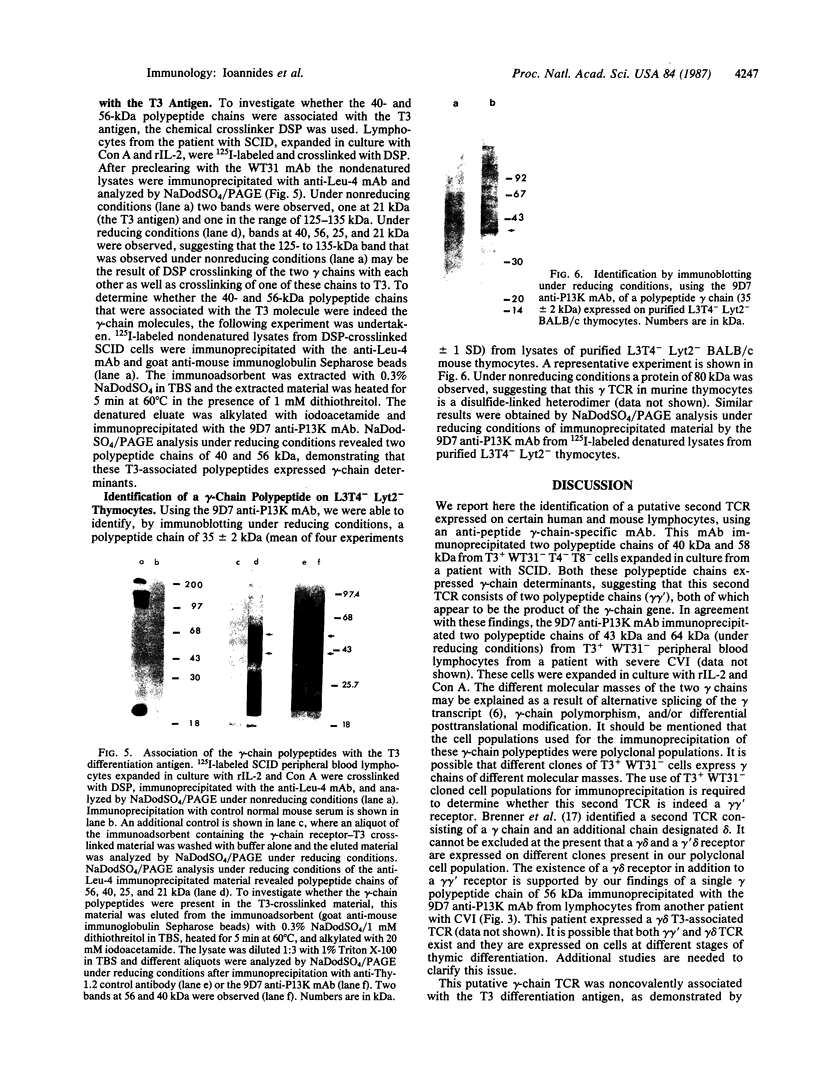
We developed a monoclonal antibody (mAb) (9D7) against a synthetic peptide (P13K) selected from the deduced amino acid sequence of the constant region of the gamma chain of the murine T-cell antigen receptor (TCR) (amino acids 118-130). Using this mAb, we identified a putative second TCR expressed on peripheral blood lymphocytes from a patient with severe combined immunodeficiency (SCID) that were propagated in culture with recombinant interleukin 2 (rIL-2) and Con A. This mAb immunoprecipitated two polypeptide chains of 40 and 58 kDa under nonreducing conditions and of 40 and 56 kDa under reducing conditions from 125I-labeled denatured lysates of T3+ WT31- lymphocytes expanded in culture from a SCID patient. These polypeptide chains were not disulfide linked and were not present on human peripheral blood lymphocytes from normal donors cultured for 5 days with phytohemagglutinin or for 2 weeks with rIL-2 and polyclonal activators or on cells of the Jurkat lymphoblastoid human T-cell line. Chemical crosslinking of 125I-labeled cells followed by immunoprecipitation with anti-Leu-4 mAb under nonreducing or reducing conditions revealed that the 40- and 56-kDa polypeptide chains were associated with the T3 differentiation antigen. These results were confirmed by sequential immunoprecipitation with anti-Leu-4 mAb followed by 9D7 anti-P13K mAb. The 9D7 anti-P13K mAb immunoprecipitated two polypeptide chains of 43 and 64 kDa from denatured lysates of lymphocytes from a patient with severe common variable immunodeficiency (CVI) that were expanded in culture with rIL-2 and Con A. Thus, this second TCR may be composed of two polypeptide chains (gamma gamma'), both of which appear to be the product of the gamma-chain gene. These experiments were done with polyclonal cell populations. Cloned T3+ WT31- cell populations are required to determine whether this TCR contains two gamma polypeptide chains. In contrast, only one polypeptide chain of 56 kDa was immunoprecipitated by the 9D7 anti-P13K mAb from peripheral blood lymphocytes from a patient with mild CVI expanded in culture with rIL-2 and polyclonal activators. Using the same 9D7 anti-P13K mAb and immunoblotting analysis, we identified a 35 kDa gamma-chain polypeptide under reducing conditions expressed on purified L3T4- Lyt2- BALB/c mouse thymocytes. This gamma-chain TCR is disulfide linked and has a molecular mass of 80 kDa under nonreducing conditions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bank I., DePinho R. A., Brenner M. B., Cassimeris J., Alt F. W., Chess L. A functional T3 molecule associated with a novel heterodimer on the surface of immature human thymocytes. Nature. 1986 Jul 10;322(6075):179–181. doi: 10.1038/322179a0. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Dialynas D. P., Strominger J. L., Smith J. A., Owen F. L., Seidman J. G., Ip S., Rosen F., Krangel M. S. Identification of a putative second T-cell receptor. Nature. 1986 Jul 10;322(6075):145–149. doi: 10.1038/322145a0. [DOI] [PubMed] [Google Scholar]
- Chang T. W., Kung P. C., Gingras S. P., Goldstein G. Does OKT3 monoclonal antibody react with an antigen-recognition structure on human T cells? Proc Natl Acad Sci U S A. 1981 Mar;78(3):1805–1808. doi: 10.1073/pnas.78.3.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig J. S., Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. 1986 Aug 28-Sep 3Nature. 322(6082):836–840. doi: 10.1038/322836a0. [DOI] [PubMed] [Google Scholar]
- Iwamoto A., Rupp F., Ohashi P. S., Walker C. L., Pircher H., Joho R., Hengartner H., Mak T. W. T cell-specific gamma genes in C57BL/10 mice. Sequence and expression of new constant and variable region genes. J Exp Med. 1986 May 1;163(5):1203–1212. doi: 10.1084/jem.163.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz D. M., Saito H., Heller M., Takagaki Y., Haas W., Eisen H. N., Tonegawa S. Limited diversity of the rearranged T-cell gamma gene. 1985 Feb 28-Mar 6Nature. 313(6005):752–755. doi: 10.1038/313752a0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lefranc M. P., Rabbitts T. H. Two tandemly organized human genes encoding the T-cell gamma constant-region sequences show multiple rearrangement in different T-cell types. Nature. 1985 Aug 1;316(6027):464–466. doi: 10.1038/316464a0. [DOI] [PubMed] [Google Scholar]
- Lew A. M., Pardoll D. M., Maloy W. L., Fowlkes B. J., Kruisbeek A., Cheng S. F., Germain R. N., Bluestone J. A., Schwartz R. H., Coligan J. E. Characterization of T cell receptor gamma chain expression in a subset of murine thymocytes. Science. 1986 Dec 12;234(4782):1401–1405. doi: 10.1126/science.3787252. [DOI] [PubMed] [Google Scholar]
- Lieberman J., Verret C. R., Kranz D. M., Hubbard S. C., Saito H., Raulet D. H., Tonegawa S., Eisen H. N. A phosphorylated, disulfide-linked membrane protein in murine cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7870–7874. doi: 10.1073/pnas.83.20.7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murre C., Waldmann R. A., Morton C. C., Bongiovanni K. F., Waldmann T. A., Shows T. B., Seidman J. G. Human gamma-chain genes are rearranged in leukaemic T cells and map to the short arm of chromosome 7. Nature. 1985 Aug 8;316(6028):549–552. doi: 10.1038/316549a0. [DOI] [PubMed] [Google Scholar]
- Niman H. L., Thompson A. M., Yu A., Markman M., Willems J. J., Herwig K. R., Habib N. A., Wood C. B., Houghten R. A., Lerner R. A. Anti-peptide antibodies detect oncogene-related proteins in urine. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7924–7928. doi: 10.1073/pnas.82.23.7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. Interaction of cross-linking agents with the insulin effector system of isolated fat cells. Covalent linkage of 125I-insulin to a plasma membrane receptor protein of 140,000 daltons. J Biol Chem. 1979 May 10;254(9):3375–3381. [PubMed] [Google Scholar]
- Platsoucas C. D., Good R. A. Inhibition of specific cell-mediated cytotoxicity by monoclonal antibodies to human T cell antigens. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4500–4504. doi: 10.1073/pnas.78.7.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platsoucas C. D. Human T cell antigens involved in cytotoxicity against allogeneic or autologous chemically modified targets. Association of the Leu 2a/T8 antigen with effector-target cell binding and of the T3/Leu 4 antigen with triggering. Eur J Immunol. 1984 Jun;14(6):566–577. doi: 10.1002/eji.1830140615. [DOI] [PubMed] [Google Scholar]
- Quertermous T., Murre C., Dialynas D., Duby A. D., Strominger J. L., Waldman T. A., Seidman J. G. Human T-cell gamma chain genes: organization, diversity, and rearrangement. Science. 1986 Jan 17;231(4735):252–255. doi: 10.1126/science.3079918. [DOI] [PubMed] [Google Scholar]
- Reilly E. B., Kranz D. M., Tonegawa S., Eisen H. N. A functional gamma gene formed from known gamma-gene segments is not necessary for antigen-specific responses of murine cytotoxic T lymphocytes. 1986 Jun 26-Jul 2Nature. 321(6073):878–880. doi: 10.1038/321878a0. [DOI] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984 Nov 1;312(5989):36–40. doi: 10.1038/312036a0. [DOI] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. 1984 Jun 28-Jul 4Nature. 309(5971):757–762. doi: 10.1038/309757a0. [DOI] [PubMed] [Google Scholar]
- Spits H., Borst J., Tax W., Capel P. J., Terhorst C., de Vries J. E. Characteristics of a monoclonal antibody (WT-31) that recognizes a common epitope on the human T cell receptor for antigen. J Immunol. 1985 Sep;135(3):1922–1928. [PubMed] [Google Scholar]
- Tax W. J., Willems H. W., Reekers P. P., Capel P. J., Koene R. A. Polymorphism in mitogenic effect of IgG1 monoclonal antibodies against T3 antigen on human T cells. Nature. 1983 Aug 4;304(5925):445–447. doi: 10.1038/304445a0. [DOI] [PubMed] [Google Scholar]
- Van Wauwe J. P., De Mey J. R., Goossens J. G. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J Immunol. 1980 Jun;124(6):2708–2713. [PubMed] [Google Scholar]
- Weiss A., Newton M., Crommie D. Expression of T3 in association with a molecule distinct from the T-cell antigen receptor heterodimer. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6998–7002. doi: 10.1073/pnas.83.18.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wussow P., Platsoucas C. D., Wiranowska-Stewart M., Stewart W. E., 2nd Human gamma interferon production by leukocytes induced with monoclonal antibodies recognizing T cells. J Immunol. 1981 Sep;127(3):1197–1200. [PubMed] [Google Scholar]