Abstract
A microanalysis of task events in a common go/no-go task was completed to examine how task events impact individual reaction times. Predictors of long reaction times were analyzed in order to better understand increased intra-individual variability (IIV) among children with ADHD compared to normal controls. 65 children with ADHD and 65 normal controls matched on gender, ethnicity, age completed a go/no-go task. Children across both groups were slower before and after omission errors than all other trials. They were also slower on the trial before successfully inhibiting their response to no-go trials. Children with ADHD exhibited a pronounced slowing on trials prior to omission errors and trials prior to successful inhibitions compared to the normal control group. Pre-error slowing in children with ADHD may represent the beginning stages of attentional disengagement that subsequently results in the absence of responding (i.e., errors of omission or successful inhibition). While these event-related increases in RT explain some of the increased IIV observed in children with ADHD, the removal of these trials did not remove the pronounced between-group differences in IIV suggesting that additional unmeasured processes are contributing to IIV in children with ADHD.
Keywords: response variability, intra-individual, post-error slowing, pre-error slowing, attention, disengagement
Attention-Deficit/Hyperactivity Disorder (ADHD) is defined behaviorally by symptoms of inattention, hyperactivity, and impulsivity (American Psychiatric Association, 2000). On cognitive and neuropsychological tests, patients diagnosed with ADHD demonstrate deficits in response inhibition, attention, and working memory (Hervey, Epstein, & Curry, 2004; Willcutt et al., 2005). In addition, patients with ADHD frequently display increased intra-individual variability (IIV) on neuropsychological tests compared to normal controls. For years, this IIV was perceived as noise. Recently however, considerable research attention has been directed to understanding IIV among children with ADHD. It has now been documented that IIV is more highly correlated with ADHD diagnostic status than most other more traditional indicators of ADHD-related deficits (e.g., Epstein et al., 2003). Also, increased IIV among patients with ADHD has been documented across a wide range of cognitive tasks (Andreou et al., 2007; Hervey et al., 2006; Klein et al., 2006; Leth-Steensen, Elbaz, & Douglas, 2000). Recent investigations into IIV have used advanced analytic strategies to model IIV response patterns to better understand the nature of IIV differences in patients with ADHD. Increased IIV appears to be the result of long reaction times (RTs) that occur frequently and periodically throughout the RT stream (Hervey et al., 2006; Leth-Steensen et al., 2000). These longer RTs may occur in predictable frequencies (i.e., approximately every 20 seconds or .05 Hz) in the RT stream (Castellanos et al., 2005).
In order to better understand IIV and examine conditions under which long RTs emerge, a micro-analysis of IIV appears warranted. In particular, an analysis of the potential predictors of long RTs would provide useful information about the origins of IIV. Task variables such as presentation rate and time on task can and have been examined as predictors of IIV and long RTs (Hervey et al., 2006; Klein et al., 2006; Leth-Steensen et al., 2000). Others have researched the effects of stimulus and response characteristics but only cursorily, in the context of examining response re-engagement, error monitoring, and negative priming deficits in children with ADHD (Schachar et al., 2004; Schachar et al., 1995; J. A. Sergeant & van der Meere, 1988).
Go/no-go tasks are measures of response inhibition that have been used to document IIV deficits (Hervey et al., 2006; Klein et al., 2006). On a go/no-go task, there are two stimulus categories and two response categories. Stimulus categories include “go” and “no-go” stimuli. Response categories include correct and incorrect responses to these stimuli, resulting in four possible trial events: 1) response to a go stimulus (i.e., correct go); 2) non-response to a go stimulus (i.e., omission error); 3) non-response to a no-go stimulus (i.e., successful inhibition); and 4) response to a no-go stimulus (i.e., commission error).
As stated above, some of these events have been examined as predictors of RTs in the context of documenting response re-engagement, error monitoring, and negative priming deficits in children with ADHD. Longer RTs on subsequent trials after errors have been proposed as indicators of deficient error monitoring (Rabbitt, 1966, 1968). Investigators have examined RT slowing post errors across ADHD and normal control groups to examine these processes (Krusch et al., 1996; Schachar et al., 2004; Sergeant & van der Meere, 1988). Multiple studies have found that after making an error, both normal controls and patients with ADHD demonstrate post-error slowing on the following trial but that children with ADHD do not slow their responses after commission errors as frequently or to the degree that normal controls do (Krusch et al., 1996; Schachar et al., 2004; Sergeant & van der Meere, 1988).
Hence, previous research seems to indicate that commission errors may influence RTs throughout the task and possibly intra-individual variability. Other trial events, such as omission errors, successful inhibition, or the effects of responding to a go trial successfully have not been examined. The effect of successful go trials is especially intriguing given the finding that children without ADHD showed increased susceptibility to making inhibition errors as the number of preceding go trials increased, whereas children with ADHD made high rates of commission errors irrespective of the context of preceding go trials (Durston et al., 2003). Another feature of trial events that has not been examined are the trials preceding error trials. Longer RTs during pre-error trials have been repeatedly documented among normal samples and have been hypothesized to reflect attentional disengagement (Manly et al., 1999; Cheyne et al., 2009).
The goal of the study was to conduct a microanalysis of task events in a common go/no-go task for which IIV is evident (Hervey et al., 2006) and examine whether task events appear to impact individual RTs. In addition to examining the impact of task events on RT, we explore differential effects across ADHD and normal control groups. We hypothesize that RTs preceding and following task events will be different than RTs unrelated to task events. Namely, we expect children to slow down after errors. Consistent with previous research, we predict that the slowing of post-error responding among children with ADHD will be less pronounced than among normal controls (Krusch et al., 1996; Schachar et al., 2004; Sergeant & van der Meere, 1988).
Methods
Participants
This project analyzes archival data originally collected as part of the 24-month follow-up evaluation of the multisite Multimodal Treatment Study of children with ADHD (MTA; MTA Cooperative Group, 2004). During baseline assessment for the MTA study, 579 children aged 7.0–9.9 years of age received a diagnosis of ADHD, Combined Type (American Psychiatric Association, 2000). This diagnosis was determined using the Diagnostic Interview Schedule for Children, Parent Report (DISC-P 4.0, Shaffer et al., 2000), supplemented with up to two symptoms identified by children’s teachers on the SNAP-IV (DSM-IV ADHD/ODD Scale; Swanson, 1992) for cases falling just below the DISC diagnostic threshold. Once a diagnosis was confirmed, children were randomly assigned to one of four treatment groups: Medication Only, Psychosocial Treatment Only, Combined Treatment, or Community Control. The treatment phase lasted for 14 months.
At the time of the 14-month assessment, families were no longer required to adhere to their randomized treatment and were allowed to seek any form of treatment for their child. At the 24-month assessment (10 months after the end of treatment), all children were thoroughly assessed again using a comprehensive battery of measures. Four of the six MTA sites administered the go/no-go task to their research participants at the 24-month assessment (n = 387). Participants were not administered this task prior to this assessment visit. The retention rate of children who had completed DISC-P data at 24 months was 92% (n = 356). Of these children, 319 had complete go/no-go task data. 151 of the children who had go/no-go task data at 24 months met diagnostic criteria for ADHD according to the DISC-P. In order to examine ADHD-related deficits in the absence of medication, only children with ADHD who were not taking medication on the day of the CPT assessment were utilized for this study (final n = 65; age range = 9.1–12.3 years of age). Parents were requested to medicate their children on the day of testing if the child was medicated regularly and to refrain from medication if the child was typically unmedicated.
A nonclinical control group was derived from the Local Normative Comparison Group (LNCG) acquired at the time of the 24-month MTA assessment. LNCG children were living in the same communities and attending the same schools as the MTA children. These children (n = 194 across the four MTA sites) were identified from school registries to match the MTA sample in terms of grade, sex, and ethnicity and then randomly chosen from among those families who volunteered to participate. Those children in the LNCG group who met criteria for any subtype of ADHD, as assessed using the DISC-Parent Report (n=20), were excluded to avoid confounding the two groups.
A matching procedure was utilized to select one LNCG child for each ADHD child (n = 65; age range = 8.5–12.7 years of age). LNCG participants were matched on four variables prioritized as follows: sex, ethnicity, age and site. The ADHD sample and matched nonclinical control group originally were created, analyzed, and described in an earlier study (Hervey et al., 2006). See Table 1 for demographic and clinical characteristics of the two groups. Note that most of the ADHD sample no longer continued to meet diagnostic criteria for ADHD, Combined Type but instead met criteria for ADHD, Predominantly Inattentive Type (40%) or ADHD, Predominantly Hyperactive-Impulsive Type (18%) at the 24-month visit. Such changes in ADHD diagnostic status are likely related to developmental changes in ADHD symptoms (Hurtig et al., 2007).
Table 1.
Demographic Characteristics for ADHD and Control Group
| ADHD (n = 65) | Control (n = 65) | Group Comparison p-value* | |
|---|---|---|---|
| Mean (SD) age in years | 10.7 (.84) | 10.6 (.93) | .52 |
| Number male | 50 | 50 | 1.0 |
| Number of each ethnicity (percentage) | |||
| Caucasian | 37 | 41 | .49 |
| African American | 20 | 14 | .25 |
| Hispanic (non-Black) | 2 | 5 | .21 |
| Asian | 1 | 1 | 1.0 |
| Mixed | 5 | 4 | .66 |
| Number of each ADHD subtype | |||
| Inattentive | 26 | 0 | <.0001 |
| Hyperactive/Impulsive | 12 | 0 | .0003 |
| Combined | 27 | 0 | <.0001 |
| Number with specified comorbid psychological disorder | |||
| Oppositional Defiant Disorder | 14 | 1 | .0006 |
| Conduct Disorder | 6 | 0 | .01 |
| Any Anxiety Disorder | 27 | 9 | .0005 |
| Any Elimination Disorder | 12 | 3 | .02 |
| Tic Disorder | 8 | 0 | .005 |
| Any Mood Disorder | 0 | 0 | 1.0 |
Note: Any Anxiety Disorders includes: Simple Phobia, Social Phobia, Agoraphobia, Panic Disorder, Overanxious Disorder, Generalized Anxiety Disorder, Obsessive-Compulsive Disorder, & Separation Anxiety Disorder; Any Mood Disorder includes Major Depression, Dysthymia, Mania, & Hypomania; Any Elimination Disorder includes enuresis (primary or secondary) and encopresis;
statistical testing was conducted using t-tests for comparing means (i.e., age) and chi-square tests for comparing proportions.
Measures
Conners continuous performance test
(CPT; Conners, 1994). The Conners Continuous Performance Test is a go/no-go task. This task was completed on an IBM-compatible desktop computer in a quiet setting with minimal distractions. Three hundred sixty (360) total letters appeared on the computer screen, one at a time, each for approximately 250 milliseconds. The 360 trials were presented in the standard format of 18 sets of 20 trials each. The sets differed only in the interstimulus interval (ISI) between letter presentations, which lasted 1, 2, or 4 seconds. ISIs were block randomized so that all three ISI conditions would occur every three sets. Transition from one set to the next was unannounced and occurred without delay. For purposes of examining time on task, the task can be divided into 6 blocks, each of which contains all 3 ISI conditions.
Participants were taking the CPT for the first time. They were instructed to press the spacebar when any letter except the letter “X” appeared on the screen. The percentage of trials when letters other than “X” appeared was 90% across all ISI blocks. Reaction time was measured from the point at which any letter other than “X” appeared on the screen until the spacebar was depressed. This is considered a go trial. Only successful go trials, or trials when the participant correctly pressed the spacebar when presented with a target stimulus, were included for data analysis. No-go trials occurred when an “X” was presented. Two types of errors were recorded. Errors of omission occurred when the participant failed to respond to a target stimulus (i.e., any non-“X” letter). Errors of commission occurred when the participant responded to a non-target stimulus (i.e., “X”). The total Conners CPT task took approximately 14 minutes for each participant to complete.
Procedure
The MTA children and their parents participated in informed consent during the baseline visit of the MTA study. The LNCG participants and their parents were consented during the 24-month MTA follow-up, which served as the baseline visit for the LNCG group. Children in both groups were administered the CPT as part of a more comprehensive assessment lasting approximately 5 hours. The CPT was administered to the children as the second measure of a fixed assessment battery. Trained interviewers administered the DISC-P to parents and the results of this administration were scored using computerized algorithms.
All participants and their families assented/consented to be part of this study. All data included in this manuscript was obtained in compliance with the Helsinki Declaration.
Statistical Analyses
A set of generalized linear mixed models were conducted to evaluate how well specific performance events predicted reaction time and whether these performance effects interacted with ADHD status. The events of interest were defined as 1) response to a go stimulus (i.e., correct go); 2) non-response to a go stimulus (i.e., omission error); 3) non-response to a no-go stimulus (i.e., successful inhibition); and 4) response to a no-go stimulus (i.e., commission error). Subject was treated as a random effects factor and the dependency among the 360 trials per subject was accommodated through generalized estimating equation (GEE) capabilities in SAS statistical software (PROC MIXED). An auto-regressive within-subject covariance matrix was selected due to the time series nature of the data. Reaction times less than 100 ms were excluded from analysis because of probable anticipation by the participants during these trials (Luce, 1986; Ulrich & Miller, 1994).
The statistical models were organized by each of the performance events, referred to as temporal performance indicators (TPIs). The models assessed the predictive effects of each TPI on RT. Three of the four TPIs were discrete events: omission errors, commission errors, and successful inhibitions. Separate models were run for each of four the trials preceding and following each of these TPIs compared to all other trials in order to assess any potential TPI effects. For example, models examining the effect of commission errors compared RTs on each of the four trials that occurred immediately before and immediately after a commission error to RTs on all other trials. If a commission or omission error interrupted the 4-trial string before or after a TPI, analyses excluded those trials and any relevant preceding or succeeding trials that would contaminate the targeted TPI effects. For example, when examining commission errors as a TPI, if an omission error occurred on the 2nd trial following a commission error, the 2nd trial following the targeted commission error would be excluded from the analysis as well as the following 3rd and 4th trials following this commission error. The number of excluded trials ranged from 44 – 387 trials across all of the analyses. Given that 36,952 total trials were used in the analyses, the maximum percent of trials excluded trials was 1% of all trials. All models included a group variable (MTA vs. LNCG) so that main effects of group and group x TPI interaction effects could be examined.
For successful go trials, the strategy was to examine the possible progressive interference effects introduced by increasing numbers of successive successful go trials. Hence, the successful go trial variable was created by using a continuous counter that represented the number of consecutive successful go trials. The counter reset when an omission error occurred or a no-go stimulus appeared. This continuous variable was used to predict RT using generalized linear mixed modeling in the same way the dichotomous TPI variables were used. Again, group (MTA vs. LNCG) was included in the statistical models.
The reported p-values are adjusted for multiple testing using the False Discovery Rate method (Benjamin & Hochberg, 1995). Effect sizes for all 1df tests were computed using Cohen’s d (Cohen, 1988).
Results
Note that no differences were found between the ADHD and LNCG groups for the overall errors of commission (F(1,128)=0.87, p>.05), but a statistically significant difference was found for errors of omission (F(1,128)=7.43, p<.01). Children with ADHD had higher rates of errors of omission than children in the LNCG group (Hervey et al., 2006). Also, children with ADHD were significantly slower (F(1,127)=9.93, p<.01) and more variable in responding (F(1,127)=23.47, p<.001) than children in the LNCG group (Hervey et al., 2006).
Effects of Temporal Performance Indicators
Consistent with the between-group differences in mean reaction time reported in Hervey et al. (2006), children with ADHD demonstrated slower RTs than children in the LNCG group across all analyses (all ps<.05; effect size range: .30–1.10).
Commission errors
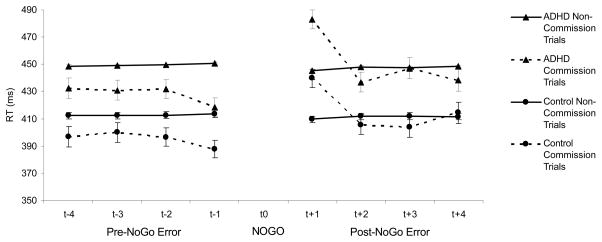
There was a significant main TPI effect for the first (F(1,128) = 38.27, p<.0075; Cohen’s d = .73), second (F(1,128) = 11.99, p=.0013; Cohen’s d = .41), third (F(1,128) = 8.84, p=.0060; Cohen’s d = .36), and fourth (F(1,128) = 8.75, p=.0069; Cohen’s d = .35) trials before a commission error. On these trials preceding a commission error, children had faster RTs compared to all other trials. Also, on the trial after a commission error, children had slower RTs compared to all other trials (F(1,128) = 51.35, p=.0013; Cohen’s d =.83). No main effects of TPI were observed for the second, third, and fourth trials after a commission error. Also, none of the interaction effects between TPI and group status were statistically significant. Post-hoc analyses revealed that the main effect of TPI were present for the LNCG group for the first, second, third, and fourth trials before a commission error and the first trial after a commission error (all ps<.05). Also, TPI main effects were significant for the ADHD group on the first, second, and third trials before a commission error and the first trial following a commission error (all ps<.05). The ADHD group did not show a main effect of TPI on the fourth trial before a commission error (p=.07). See Figure 1.
Figure 1.
Reaction times for children with ADHD and normal controls on the four trials preceding (t−1, t−2, t−3, t−4) and the four trials following (t+1, t+2, t+3, t+4) a commission error on a no-go trial (t0; NO GO) in comparison to all other responses.
Omission errors
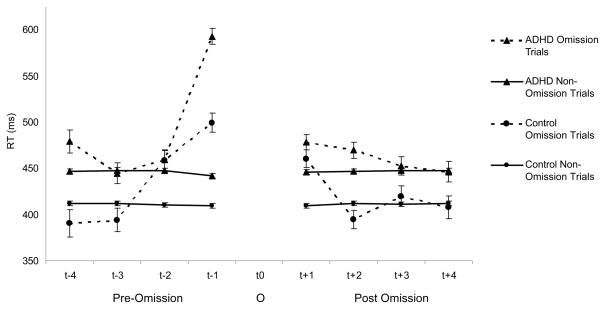
Main effects of TPI for omission errors, all indicating slower RTs for these trials compared to all other trials, were observed on the first (F(1,126) = 371.74, p=.0007; Cohen’s d = 2.19) and second trials (F(1,126) = 14.27, p=.0005; Cohen’s d = .49) before omission errors. RTs were also slower on the first trial after an omission error (F(1,126) = 44.88, p=.0004; Cohen’s d = 80). RTs that occurred three and four trials before an omission error or two, three, and four trials after an omission error did not show a main effect of TPI. There were also several statistically significant interaction effects. Specifically, there were TPI x Group interactions on the first (F(1,125)=20.79, p=.0006), second (F(1,124)=5.66, p=.03), and fourth (F(1,115)=7.75, p=.01) trial before omission errors. Also, on the second trial after an omission error, there was a significant TPI x Group interaction (F(1,124)=9.19, p=.0004).
Post-hoc analyses revealed that the LNCG group demonstrated TPI-related slowing on the first and second trials prior to an omission error as well as the trial following an omission error (all ps<.05). The LNCG group also demonstrated TPI-related RT speeding on the second trial after an omission error (p=.03). The LNCG group did not show a TPI main effect on the fourth trial prior to an omission error (p=.08) as was indicated in the overall analysis of this trial. The ADHD group, on the other hand, demonstrated TPI-related slowing on the first and fourth trials prior to and the first and second trials following an omission error (all ps<.05). Children with ADHD did not exhibit slowed RT on the second trial prior to an omission error (p=.23). See Figure 2.
Figure 2.
Reaction times for children with ADHD and normal controls on the four trials preceding (t−1, t−2, t−3, t−4) and the four trials following (t+1, t+2, t+3, t+4) an omission error on a go trial (t0; O) in comparison to all other responses..
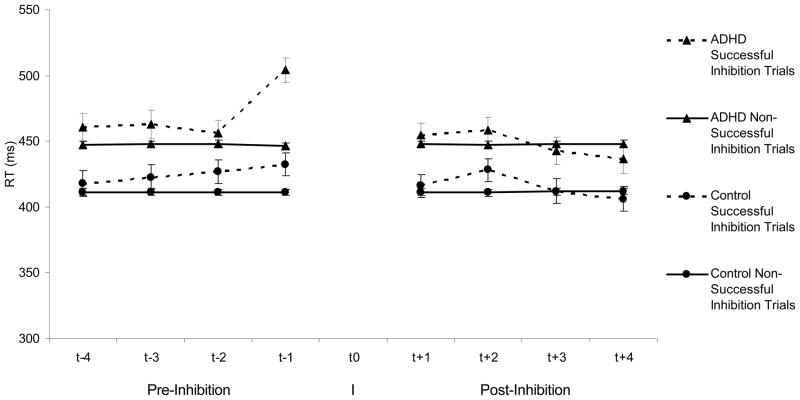
Successful inhibitions
Examining trials before and after successful inhibitions, the only trial event that showed a statistically significant TPI effect was the first trial before a successful inhibition (F(1,129) = 33.31, p=.0003; Cohen’s d = .74). The first trial before a successful inhibition also displayed a significant TPI x Group interaction (F(1,128)=8.03, p=.01). While children in the LNCG group as well as children in the ADHD group had slower RTs on these first trial before a successful inhibition (both ps<.05), children in the ADHD group exhibited a pattern of greater slowing than children in the LNCG group. See Figure 3.
Figure 3.
Reaction times for children with ADHD and normal controls on the four trials preceding (t−1, t−2, t−3, t−4) and the four trials following (t+1, t+2, t+3, t+4) a successful inhibition to a no-go trial (t0; I) in comparison to all other responses.
Successful go trials
Children had faster RTs as the number of successive successful go trials increased (F(1, 36950)=82.67, p<.0002; Cohen’s d = .13). Also, there was a significant TPI x Group interaction (F(1, 37000)=8.92, p=.006). Though an increasing number of successful go trials predisposed children in the ADHD and LNCG groups towards faster RTs (both ps<.05), this effect was more pronounced for children with ADHD compared to the LNCG group.
Effects of TPI on RT Variability
In order to determine whether the trial events that exhibited group x TPI interaction effects were the causal factor in the observed between-group differences in overall response time variability, a new set of analyses were conducted comparing the standard deviation in RT between-groups after removing the first, second, and fourth trials before and the second trial after an omission error and the trial immediately before successful inhibitions. With these trials removed, children with ADHD continued to have a higher RT standard deviation (Mean=240.02, SD=98.17) compared to children in the LNCG group (Mean=151.20, SD=98.61, t(127)=3.72, p=.0003, unadjusted; Cohen’s d = .70). For the sake of comparison, the between-group effect size (Cohen’s d) with these trials included was .78.
Discussion
An examination of the effects of stimulus and response events during the administration of a go/no-go task revealed that task events do predict RTs. Most of the effects of task events on RTs were present across groups. For example, children evidenced post-error slowing after both omission and commission errors. This post-error slowing persisted for only one trial after errors were made. No effect was observed on subsequent trials. We also found a distinct pattern of pre-event slowing that occurred before an omission error and before a successful inhibition trial. The pre-event slowing was present for only one trial prior to a successful inhibition and for the first two trials prior to an omission error. Finally, there was an opposite pattern of pre-event speeding of RTs prior to commission errors that spanned all 4 trials prior to a commission error. In terms of differential patterns of RT across the ADHD and LNCG groups, we found that pre-event RT slowing before omission errors and successful inhibitions differed in magnitude across the ADHD and LNCG groups. Children with ADHD exhibited a more pronounced slowing pattern prior to either of these events than did the normal controls. Also, two trials after an omission error, children with ADHD continued to have extended RTs whereas RTs for children in the LNCG group returned to normal.
Post-error slowing is a well-documented neuropsychological phenomenon. Rabbitt (1966, 1968) initially reported on post-error slowing and attributed it to error monitoring. Rabbitt (1968) described error monitoring as an executive control process whereby immediately after making an error, participants slow their RTs on subsequent trials in order to self-correct for making an error, thereby ensuring that another error is not made. Post-error slowing after a commission error may also represent a reactive internal self-analysis of performance which temporarily interferes with the subsequent trial’s task performance (Smallwood et al., 2003; Smallwood et al., 2004). The present study results seem to suggest that both children with ADHD and normal controls exhibit post-error slowing.
Somewhat unique to the present findings is that post-error slowing was observed after both commission errors and omission errors. The majority of research examining post-error slowing has focused only on commission errors. It is quite interesting that a similar post-error slowing effect is observed across both error types given the active nature of commission errors and the passive nature of omission errors. An error monitoring explanation for post-error slowing makes sense for post-commission error slowing but less sense for post-omission error slowing. Recall that an omission error results when a participant fails to emit a response to a go stimulus. There are two possible explanations for why omission errors may occur. First, they may represent an attentional lapse (Corkum & Siegel, 1993). Second, because the go/no-go task involves a choice discrimination (i.e., is the stimulus a go or no-go stimulus), the lack of a response may be due to a processing delay in making the discrimination. Since most go/no-go tasks, including the one used in this study, are set up to provide a simple discrimination (i.e. “X” vs. not an “X”), this second explanation seems less likely. So if omission errors are the result of a lapse in attention, why would participants slow their response on the subsequent trial? An error monitoring explanation (i.e., slowing to ensure that another error is avoided) makes little sense after an omission error since one would predict an engaged and possibly faster response on the next trial after realizing that one made an error by failing to respond on the previous trial. An alternative explanation is that post-omission error slowing may be a manifestation and continuation of the same process that initially caused the error (Gehring et al., 1993). It may be that the observed post-omission error slowing is part of a response monitoring process (Gehring et al., 1993). Indeed, both omission errors and long RTs have been interpreted as being indicative of attentional lapses (Corkum & Siegel, 1993; Leth-Steensen et al., 2000). Hence, the omission error may be the initial manifestation of an attentional lapse followed by a long RT signifying continued inattention.
This is further supported by this study’s finding that prior to an omission error there is pre-error slowing for at least 2 trials prior to the omission error. Hence, it appears that the lack of response monitoring and lack of task engagement begins prior to the actual error. Cheyne et al (2009) have posited an attentional model whereby attentional lapses or “tuning out” begins with transient disengagement of attention and moves to automatic responding without actively attending and then mind wandering. These attentional states are sequential. Using a similar go/no-go task, Cheyne et al. suggested that pre-error long RTs indicate the first stage of attentional disengagement while actual omission errors represent mind wandering or total attentional disengagement. This study presents a similar pattern of results to those of Cheyne et al. with RT slowing pre- and post-omission errors. Unique to the present study is the finding that children with ADHD appear to demonstrate a more pronounced pattern of pre-error slowing than normal controls. This pattern may suggest that children with ADHD may have a greater disengagement response than normal controls. Given that children with ADHD also demonstrated more errors of omission on this task, it may be that children with ADHD are unable to stop the attentional disengagement process during early stages (i.e., before it results in an error). Further, the finding that children with ADHD had longer RTs on the second trial following an omission error may suggest that the attentional disengagement process extends beyond the omission error. Figure 2 clearly displays that on the trial after an omission error, there is post-error slowing across both groups. However, on the second trial following an omission error, the normal controls demonstrate RTs consistent with non-event trials whereas children with ADHD continue to evidence long RTs. It seems that children with ADHD take longer to return to a normal response speed than normal controls. This may be due to an inability to end the attentional disengagement process. Alternatively, it may indicate an attenuated, delayed, or lack of a response to making an error.
Children with ADHD also exhibited a pronounced pattern of pre-event slowing prior to successful inhibitions. One possible explanation for slowing prior to a successful inhibition is as follows. RTs are autocorrelated and this relationship is stronger for those trials nearer to one another. Given that long RTs may indicate the beginnings of task disengagement, a long RT on one trial is likely to lead to a long RT or even a non-response on the following trial. A predilection towards a long RT or a non-response is helpful when trying to withhold a response (i.e., successful inhibition). Hence, we see that long RT trials often precede successful inhibition trials. This relationship is stronger for children with ADHD since a long RT response bias is likely to facilitate successful inhibition for this group of children with documented difficulties with response inhibition (Oosterlaan, Logan, & Sergeant, 1998).
Notably, successful response inhibition did not predict RTs on subsequent trials. Rieger and Gauggel (1999) examined slowing effects following inhibition trials using a stop signal paradigm with a non-clinical sample of undergraduates. Their findings revealed slowing effects following successful inhibition. Also, Schachar et al. (1995) used a change task, an adaptation of the stop-signal paradigm, to study response inhibition and re-engagement of response after inhibition in children with ADHD. Their results indicated deficits in inhibitory control and re-engagement following successful inhibition with significantly longer RTs on measures of both processes. However, this result was not replicated in a later study using a stop-signal task (Schachar et al., 2004). The inconsistency in effects across these previous studies as well as the current study may suggest that this effect is variable and dependent on task conditions.
Another finding of note is the pre-error speeding of RTs prior to commission errors. It appears that children with ADHD and normal controls both predispose themselves towards making errors of commission by adopting a fast RT response style. Most studies do not find that faster RTs correlate with errors across the entire task (e.g., Sergeant & vanderMeere, 1988). It may be that this relationship between speed and accuracy is only true for RTs immediately preceding inhibition trials.
A final finding of note is the effect of the number of successive successful go trials on RTs. As the number of successive successful go trials increased, all children exhibited faster RTs however this effect was much more pronounced among children with ADHD. An international group (Russell et al., 2006) has recently proposed that patients with ADHD have astrocytes that supply insufficient ATP to neurons. This impairs restoration of ionic gradients across neuronal membranes and ultimately impairs neuronal firing which leads to variable performance or IIV. The astrocyte dysfunction hypothesis (Russell et al., 2006) would seem to suggest that RTs should get longer and according to the number of trials in a row that patients are required to emit the same response. Our result indicating faster RTs in the ADHD group as successful go trials increase does not appear to support this prediction.
The intention of this micro-analytic examination of RTs was to discover predictors of long RT in order to better understand increased IIV among children with ADHD compared to normals. The few event variables that differentially predicted RT were the first, second, and fourth trials prior to an omission error, the second trial after an omission, the first trial prior to a successful inhibition, and the number of successive successful go trials. For most of these variables, children with ADHD had longer RTs on these trials than did normal controls which, is consistent with a pattern of increased variability (Hervey et al., 2006) and long RTs (Klein et al., 2006) among children with ADHD. While these event-related increases in RT explain some of the increased IIV observed in children with ADHD, they do not seem to account for all of the increased IIV among children in ADHD. This was evidenced by a between-group analysis comparing standard deviations across groups that excluded these datapoints and still displayed significantly more variability in RT among the children with ADHD compared to the normal controls.
It is likely that we will need to research other factors that may better predict the RT variability other than these task-related events. IIV may be predictable by some unmeasured variable such as constantly fluctuating attentional states (Gilden & Hancock, 2007; Smallwood et al., 2008). For example, Castellanos et al. (2005) found that long RTs followed an oscillating pattern with longer RTs appearing every 20 secs. The fast Fourier transform technique used to discover this pattern could not be used in the present study due to the within-task ISI manipulation.
There are several limitations in the present study that need to be considered when interpreting the findings. First, the matched sample that was used in this study was not matched for psychiatric comorbidity. It is possible that part of the performance differences between groups may be a result of differences in comorbidities and not solely ADHD. Our sample selection process for the present study also excluded the majority of the MTA sample because they did not meet diagnostic criteria for ADHD at 24 months and/or were currently taking medications for ADHD. Excluding children on medication was necessary given the documented effects of medication on IIV (Epstein et al., 2006; Spencer et al., in press). Epstein et al. (2006) has shown with this same sample that children who were not taking medication were more likely to be diagnosed with ADHD suggesting that our sample of unmedicated children was not limited to mild cases. Finally, all of the subjects with ADHD met criteria for ADHD, Combined Type at study entry. Though study subjects met criteria across all three subtypes at the time to testing, this sample comprises a unique group of ADHD patients and results may not be generalizable to all ADHD subtypes or patients with different developmental diagnostic trajectories (e.g., child diagnosed with Inattentive Type when 8 years old and later diagnosed with Combined Type at 10 years old).
This study’s micro-analytic examination of RTs during a go/no-go task revealed differential patterns of behavior between children with ADHD and normal controls. Such patterns would not have been discovered without assessing for relations across all task parameters (e.g., omission errors) and across a larger number of trials than had been assessed in previous studies. This examination led to some interesting and unexpected results that increase our understanding of moment by moment responding during such tasks. Future research must examine these error patterns across other tasks with different task parameters to assess the extent to which these results generalize to other tasks. Further, future research should likely include neurological outcomes (e.g., ERP) to better understand the neurological underpinnings of attentional fluctuations.
Acknowledgments
This study was supported by UO1 MH50461 (Drs. Hinshaw and Elliot), UO1 MH50477 (Drs. Conners, Wells, and March), UO1 MH50440 (Drs. Swanson, Cantwell, and Wigal), UO1 MH50453 (Drs. Abikoff and Hechtman), UO1 MH50454 (Drs. Greenhill and Newcorn), and UO1 MH50467 (Drs. Pelham and Hoza), from the National Institute of Mental Health, Bethesda, MD.
The National Institute of Mental Health collaborators are Peter S. Jensen, M.D. (Office of the Director), L. Eugene Arnold, M.D., M.Ed. (Department of Psychiatry, Ohio State University), Ms. Joanne B. Severe, M.S. (Biostatistics and Data Management Unit, Division of Services and Intervention Research), Benedetto Vitiello, M.D. (Child & Adolescent Treatment and Preventive Interventions Research Branch), Kimberly Hoagwood, Ph.D. (Office of the Director). Principal investigators and co investigators from the 6 sites are as follows: University of California, Berkeley/San Francisco: Stephen P. Hinshaw, Ph.D. (Department of Psychology, University of California, Berkeley), Glen R. Elliott, M.D., Ph.D. (Department of Psychiatry, University of California, San Francisco); Duke University: C. Keith Conners, Ph.D., Karen C. Wells, Ph.D., John March, M.D., M.P.H. (Department of Psychiatry & Behavioral Sciences); University of California, Irvine/Los Angeles: James Swanson, Ph.D. (Department of Pediatrics and Cognitive Science, University of California, Irvine), Dennis P. Cantwell, M.D., deceased (Department of Psychiatry, Neuropsychiatric Institute, University of California, Los Angeles), Timothy Wigal, Ph.D. (Department of Pediatrics, University of California, Irvine); Long Island Jewish Medical Center/Montreal Children’s Hospital: Howard B. Abikoff, Ph.D. (Department of Psychiatry, New York University School of Medicine), Lily Hechtman, M.D. (Department of Psychiatry, McGill University); New York State Psychiatric Institute/Columbia University/Mount Sinai Medical Center: Laurence L. Greenhill, M.D. (Department of Psychiatry, Columbia University), Jeffrey H. Newcorn, M.D. (Department of Psychiatry, Mount Sinai School of Medicine); University of Pittsburgh: William E. Pelham, Ph.D. (Department of Psychology, State University of New York at Buffalo), Betsy Hoza, Ph.D. (Department of Psychological Sciences, Purdue University). Helena C. Kraemer, Ph.D. (Stanford University, Department of Psychiatry & Behavioral Science) is statistical and design consultant. Robert D. Gibbons, Ph.D. (Center for Health Statistics, University of Illinois at Chicago) is statistical consultant for the follow-up. The Office of Special Education Programs/US Department of Education principal collaborator is Ellen Schiller, Ph.D.
The opinions and assertions contained in this report are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of Health and Human Services, the National Institutes of Health, or the National Institute of Mental Health.
References
- Andreou P, Neale BM, Chen W, Christiansen H, Gabriels I, Heise A, et al. Reaction time performance in ADHD: improvement under fast-incentive condition and familial effects. Psychological Medicine. 2007:1–13. doi: 10.1017/S0033291707000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (IV-Text Revision) Wahington, DC: Author; 2000. [Google Scholar]
- Benjamini Yoav, Hochberg Yosef. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Scheres A, Di Martino A, Hyde C, Walters JR, et al. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biological Psychiatry. 2005;57:1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyne JA, Solman GJF, Carriere JSA, Smilek D. Anatomy of an error: A bidirectional state model of task engagement/disengagement and attention-related errors. Cognition. 2009;111:98–113. doi: 10.1016/j.cognition.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. New York: Academic Press; 1988. [Google Scholar]
- Conners CK. The Conners Continuous Performance Test. Toronto, Canada: Multi- Health Systems, Inc; 1994. [Google Scholar]
- Corkum PV, Siegel LS. Is the Continuous Performance Task a valuable research tool for use with children with Attention-Deficit-Hyperactivity Disorder? Journa of Child Psychology and Psychiatry. 1993;34(7):1217–1239. doi: 10.1111/j.1469-7610.1993.tb01784.x. [DOI] [PubMed] [Google Scholar]
- Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, et al. Differential patterns of striatal activation in young children with and without ADHD. Biological Psychiatry. 2003;53:871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Conners CK, Hervey AS, Tonev ST, Arnold LE, Abikoff HB, et al. Assessing medication effects in the MTA study using neuropsychological outcomes. Journal of Child Psychology and Psychiatry. 2006;47:446–456. doi: 10.1111/j.1469-7610.2005.01469.x. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Erkanli A, Conners CK, Klaric J, Costello JE, Angold A. Relations Between Continuous Performance Test Performance Measures and ADHD Behaviors. Journal of Abnormal Child Psychology. 2003;31:543–554. doi: 10.1023/a:1025405216339. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gilden DL, Hancock H. Response variability in attention deficit disorders. Psychological Science. 2007;18:796–802. doi: 10.1111/j.1467-9280.2007.01982.x. [DOI] [PubMed] [Google Scholar]
- Hervey AS, Epstein JN, Curry JF. The neuropsychology of adults with Attention Deficit Hyperactivity Disorder: A meta-analytic review. Neuropsychology. 2004;18:485–503. doi: 10.1037/0894-4105.18.3.485. [DOI] [PubMed] [Google Scholar]
- Hervey AS, Epstein JN, Curry JF, Tonev S, Arnold LE, Conners CK, et al. Reaction time distribution analysis of neuropsychological performance in an ADHD sample. Child Neuropsychology. 2006;12:125–140. doi: 10.1080/09297040500499081. [DOI] [PubMed] [Google Scholar]
- Hurtig T, Ebeling H, Taanila A, Miettunen J, Smalley SL, McGough JJ, et al. ADHD symptoms and subtypes: Relationship between childhood and adolescent symptoms. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:1605–1613. doi: 10.1097/chi.0b013e318157517a. [DOI] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, Peper M, Klein C, et al. Intra-subject variability in attention-deficit hyperactivity disorder. Biological Psychiatry. 2006;60:1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Krusch DA, Klorman R, Brumaghim JT, Fitzpatrick PA, Borgstedt AD, Strauss J. Methylphenidate slows reactions of children with attention deficit disorder during and after an error. Journal of Abnormal Child Psychology. 1996;24:633–650. doi: 10.1007/BF01670104. [DOI] [PubMed] [Google Scholar]
- Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychologica. 2000;104(2):167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Luce RD. Response times: Their role in inferring elementary mental organization. New York: Oxford University Press; 1986. [Google Scholar]
- Manly T, Robertson IH, Galloway M, Hawkins K. The absent mind: Further investigations of sustained attention to response. Neuropsychologia. 1999;37:661–670. doi: 10.1016/s0028-3932(98)00127-4. [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group. National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: 24-Month outcomes of treatment strategies for Attention-Deficit/Hyperactivity Disorder. Pediatrics. 2004;113:754–761. doi: 10.1542/peds.113.4.754. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Logan GD, Sergeant JA. Response inhibition in AD/HD, CD, comorbid AD/HD + CD, anxious, and control children: a meta-analysis of studies with the stop task. Journal of Child Psychology & Psychiatry & Allied Disciplines. 1998;39:411–425. [PubMed] [Google Scholar]
- Rabbitt PMA. Errors and error correction in choice reaction tasks. Journal of Experimental Psychology. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Rabbitt PMA. Repetition effects and signal classification strategies in serial choice- response tasks. Quarterly Journal of Experimental Psychology A. 1968;20:232–240. doi: 10.1080/14640746808400157. [DOI] [PubMed] [Google Scholar]
- Rieger M, Gauggel S. Inhibitory after-effects in the stop signal paradigm. British Journal of Psychology. 1999;90:509–518. [Google Scholar]
- Russell VA, Oades RD, Tannock R, Killeen PR, Auerbach JG, Johansen EB, et al. Response variability in Attention-Deficit/Hyperactivity Disorder: A neuronal and glial energetics hypothesis. Behavioral and Brain Functions. 2006;2 doi: 10.1186/1744-9081-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachar RJ, Chen S, Logan GD, Ornstein TJ, Crosbie J, Ickowicz A, et al. Evidence for an error monitoring deficit in attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology. 2004;32:285–293. doi: 10.1023/b:jacp.0000026142.11217.f2. [DOI] [PubMed] [Google Scholar]
- Schachar R, Tannock R, Marriott M, Logan G. Deficient inhibitory control in attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology. 1995;23(4):411–437. doi: 10.1007/BF01447206. [DOI] [PubMed] [Google Scholar]
- Sergeant JA, vanderMeere J. What happens after a hyperactive child commits an error? Psychiatry Research. 1988;24:157–164. doi: 10.1016/0165-1781(88)90058-3. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Smallwood JM, Baracaia SF, Lowe M, Obonsawin M. Task unrelated thorugh whilst encoding information. Consciousness and Cognition. 2003;12:452–484. doi: 10.1016/s1053-8100(03)00018-7. [DOI] [PubMed] [Google Scholar]
- Smallwood JM, Davies JB, Heim D, Finnigan F, Sudberry M, O’Connor R, et al. Subjective experience and the attentional lapse: Task engagement and disengagement during sustained attention. Consciousness and Cognition. 2004;13:657–690. doi: 10.1016/j.concog.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Smallwood JM, McSpadden M, Luus B, Schooler JW. Segmenting the stream of consciousness: The psychological correlates of temporal structures in the time series data of a continuous performance test. Brain and Cognition. 2008;66:50–56. doi: 10.1016/j.bandc.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Spencer SV, Hawk LW, Richards JB, Shiels K, Pelham WE, Waxmonsky JG. Stimulant treatment reduces lapses in attention among children with ADHD: The effects of methylphenidate on intra-individual response time distributions. Journal of Abnormal Child Psychology. doi: 10.1007/s10802-009-9316-2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM. School Based Assessments and Interventions for ADD Students. Irvine, CA: K.C.; 1992. [Google Scholar]
- Ulrich R, Miller J. Effects of truncation on reaction time analysis. Journal of Experimental Psychology: General. 1994;123:34–80. doi: 10.1037//0096-3445.123.1.34. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of Attention-Deficit/Hyperactivity Disorder: A meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]