Introduction
Duchenne muscular dystrophy (DMD) is an X-linked recessive (1), fatal disorder, caused by mutations in the dystrophin gene, that affects approximately 1 in 3,600 to 6,000 live male births, worldwide (2-4). Deletion mutations, which are the most common cause of DMD (5), alter the open reading frame of the dystrophin gene and usually result in a complete absence of the protein (6;7). Dystrophin protein is an important structural component of muscle fibers connecting their cytoplasmic cytoskeleton to the membrane-bound dystroglycan complex (DGC) (8-11), which in turn binds to laminin in the extracellular matrix (9-11). Lack of a functional dystrophin protein in muscle leads to destabilization of the sarcolemmal membrane, causing muscle fiber degeneration and necrosis (12;13). Over time, skeletal muscle tissue in DMD patients is replaced with fat and connective tissue. Despite extensive research to date, currently there is no cure for this disease.
Gene replacement has potential utility in the treatment of single-gene disorders, such as DMD. Dystrophin cDNA transfer using viral vectors for delivery has been extensively tested for therapeutic efficacy in the mdx mouse, a genetic and biochemical model of DMD (6;14). Recently, the first studies of gene replacement for muscular dystrophy in human patients were reported (15).
Since the dystrophin gene is very large with a 11 kb of coding sequence, transfer of the full-length dystrophin cDNA into muscles of dystrophic mice was only possible due to the development of gutted or high capacity adenoviral (HC-Ad) vectors, which retain no viral genes and thus have a large capacity for an inserted DNA expression cassette (16-18). The lack of viral genes in the HC-Ad vector leads to a lower induction of anti-vector immunity than prior generation Ad vectors and has also been shown to facilitate prolonged dystrophin protein expression in mouse models of DMD (16). However, in the case of a disease such as DMD, in which the endogenous therapeutic protein can be largely absent in the host, the immunological barrier to a successful gene transfer is not limited to the host immune reaction to vector particles, but also to the transferred therapeutic gene product (19-21).
Previous studies found that anti-dystrophin antibodies were induced by HC-Ad vector-mediated dystrophin cDNA delivery to muscles of adult mdx mice as early as two weeks post-gene transfer (21). The response of the host immune system to the transferred gene reflects the normal function of the host defense system against neo-antigens. This anti-dystrophin immunity is detected (22-25) despite the rare dystrophin-expressing ‘revertant’ fibers that are found in mdx muscle (19-21). However, very little is known about the anti-dystrophin immune response raised by dystrophin-deficient recipients to dystrophin gene transfer.
Therefore, we investigated the anti-dystrophin immune response by manipulating the immune system of adult mdx mice through a temporal removal of immune cells prior to vector-mediated murine dystrophin cDNA delivery. We utilized complementary approaches to temporarily remove the host immune system before gene transfer. In the first set of experiments, a low dose of irradiation was administered to deplete only the peripheral immune cells of adult mice and was followed by self-reconstitution of the host’s peripheral immune cells after gene transfer. In the second set of experiments, a high dose of irradiation was administered prior to gene transfer. After gene delivery, the host central and peripheral immune system was reconstituted by bone marrow (BM) transfer from a syngeneic wild-type donor; this BM should comprise cells that are fully tolerant to dystrophin protein. By employing the 2 different doses of irradiation, we explored the relative contributions of the peripheral and central components of the immune response to recombinant murine dystrophin. We determined whether the returning or new host immune cells recognized the full-length murine dystrophin protein as a self-protein. We further explored the role of regulatory T (Treg) cells (26-30) in the peripheral and central immune response to recombinant, murine dystrophin protein.
Results
Low dose irradiation delays or eliminates anti-dystrophin humoral response
We first examined the effect of a sub-lethal 600 rad dose of whole-body-irradiation of 6-week-old mdx mice intended to temporarily remove peripheral immune cells. Immune responses against HC-Ad vector-mediated murine dystrophin expression in dystrophic muscles was studied longitudinally. A control group was not irradiated prior to gene delivery. Within 24 hours post-irradiation mice in both groups each received an intramuscular injection of HC-Ad vector carrying the murine dystrophin cDNA driven by the MCK promoter (1.5-2.0 × 1010 genome copies in each tibialis anterior (TA) muscle). Treated and control mice were analyzed at 4, 8, and 12 weeks after irradiation and gene delivery.
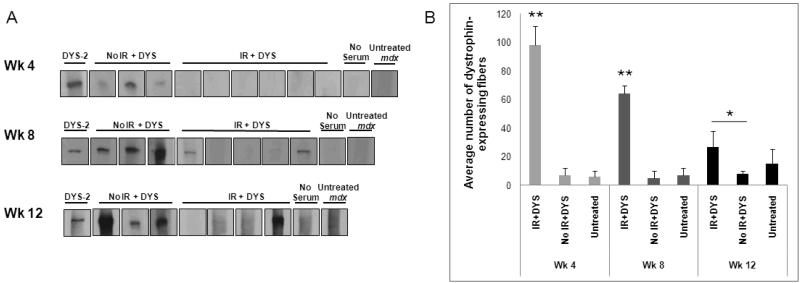
At 4 weeks post-gene transfer, mdx mice that had been irradiated prior to vector injection had no detectable anti-dystrophin humoral response (Fig. 1A, wk4), whereas control mice demonstrated an anti-dystrophin humoral response at 4 weeks post-treatment (Fig. 1A, wk4). At 8 weeks post-vector transfer the irradiated mdx mice demonstrated a variable level of anti-dystrophin humoral immunity. Three of the 5 mice showed a dystrophin-specific humoral response and two mice showed no response to dystrophin protein. The level of the response in mice that did produce anti-dystrophin antibodies was lower compared to control mice, all of which demonstrated a dystrophin-specific humoral response (Fig. 1A, wk8). A third group of mice was analyzed at 12 weeks post-vector injection. One of 4 irradiated mice produced an anti-dystrophin humoral response, while the other 3 mice did not (Fig. 1A, wk12). All control mice showed a dystrophin-specific humoral response at 12 weeks (Fig. 1A and Table 1).
Fig. 1. The anti-dystrophin humoral response and loss of muscle dystrophin expression was delayed in mdx mice treated with low dose irradiation followed by dystrophin gene transfer using a high-capacity adenoviral vector.
(A) Sera from mdx mice that were irradiated at 600 rads and received an intramuscular injection of dystrophin vector (IR + DYS), received an intramuscular injection of dystrophin vector without irradiation (no IR + DYS), or received neither irradiation nor dystrophin vector (untreated mdx) were incubated with membrane-immobilized dystrophin protein. Sera were collected for analysis at 4, 8, and 12 weeks (Wk) after treatment (n=6 for wk 4 and n=5 for wks 8 and 12). No serum indicates a negative immunoblotting control without mouse serum primary antibody. DYS-2 indicates a positive immunoblotting control using a monoclonal anti-dystrophin antibody as the primary antibody. All experimental samples and representative control samples are presented here. (B) Dystrophin-expressing fibers per TA muscle section were counted for all experimental mice shown in (A) and untreated mdx control mice (n=3). Dystrophin-expressing fibers were labeled on muscle cryosections using a monoclonal anti-dystrophin antibody and were counted per section of injected TA muscles. Muscle cross-sections from different parts of each injected TA muscle were analyzed and the mean of the counted sections from treated and control mice were calculated. Dystrophin-expressing fibers were counted to be 22%, 15%, and 7% of total muscle fibers per section at weeks 4, 8, and 12, respectively. Data is expressed as mean ± standard error (SE). *(P<0.05) and **(P<0.001) indicate significant differences from untreated mdx control.
Table 1. Summary of humoral immune response and dystrophin expression data in low- and high-dose irradiated groups.
In this table (+) indicates high level, (+/−) indicates low level, and (−) indicates an absence of response or expression. Each symbol represents an individual mouse.
| IR(600rad)+DYS | IR(900rad)+DYS+ B10 BM |
IR(900rad)+ B10 BM only |
No IR+DYS | Untreated | ||
|---|---|---|---|---|---|---|
| Dys+ Fiber |
Wk 4 | (+ + + + + ±) | (+ + + ± ±) | (− − −) | (− − −) | (− − −) |
| Wk 8 | (+ + + ± ±) | (+ + + ± ±) | (− − −) | (− − −) | (− − −) | |
| Wk 12 | (+ + ± ±) | (+ ± ± ± ±) | (− − ±) | (− − −) | (− − −) | |
| Anti-Dys Ab |
Wk 4 | (− − − − −) | (− − − − −) | (− − −) | (+ + +) | (− − −) |
| Wk 8 | (− − − ± +) | (− − − − −) | (− − −) | (+ + +) | (− − −) | |
| Wk 12 | (− − − +) | (+ + + + +) | (− − −) | (+ + +) | (− − −) | |
HC-Ad vector-mediated dystrophin expression in low-dose irradiated mdx muscles
Dystrophin expression in the vector-injected muscles of mdx mice was analyzed by immuno-staining of muscle cryo-sections. Dystrophin-expressing fibers were scattered throughout the vector-injected muscle tissue of irradiated mice at 4, 8, and 12 weeks post-treatment. In contrast, control mice did not show muscle dystrophin expression above the background level of revertant fibers observed in untreated muscle at any time-point (Fig. 1B and Table 1). The number of dystrophin-expressing muscle fibers in the irradiated, vector-treated mice decreased with time correlating temporally with the production of anti-dystrophin antibodies (Fig. 1B). The reduction in the number of dystrophin-expressing fibers over time was observed even in mice that did not produce anti-dystrophin antibody, suggesting that the humoral response was not solely responsible for the decrease in dystrophin protein expression.
Lymphocytes in draining lymph nodes and vector-injected muscle after low-dose irradiation
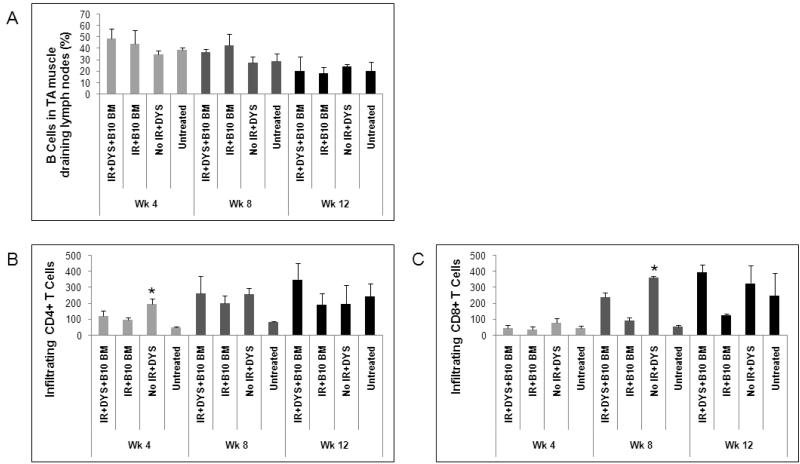
Since antibody production and B cell population are directly related to each other, B cell levels in the lymph nodes draining the vector-injected TA muscles were analyzed to ensure that the peripheral B cells in the irradiated mice had returned to levels comparable to untreated mdx mice. At all 3 time-points, the level of B cells, which were identified by CD19 expression, in draining lymph nodes of vector-treated mice was not significantly different from untreated mdx mice suggesting a return to the full complement of B cells after irradiation (Fig. 2A). The number of B cells in the irradiated, vector-treated mice, however, increased gradually from week 4 to week 12. The increase in the number of B cells coincided with the development of anti-dystrophin antibodies suggesting that the self-reconstituted B cells eventually recognized dystrophin protein as a neo-antigen and generated an anti-dystrophin immune response. At 4 weeks post-treatment, the number of B cells in the draining lymph nodes of the control mice was significantly higher than both the irradiated, vector-injected and the untreated groups (Fig. 2A), suggesting an early vector-mediated B cell expansion that correlated with the high level of anti-dystrophin humoral response observed in control mice.
Fig. 2. Infiltrating T cells, but not peripheral lymphocytes from vector-injected muscle draining lymph nodes in low-dose irradiated mdx mice are different from untreated mdx muscle.
(A) The B cell population in draining lymph nodes was analyzed by flow cytometry in low-dose irradiated and non-irradiated mdx mice at 4, 8, and 12 weeks post-treatment. Infiltrating CD4+ (B) and CD8+ (C) T cells were examined by immunohistochemistry in the treated muscles of low-dose irradiated and non-irradiated mdx mice at the three time points mentioned above. Number of mice in each group is the same as in Fig. 1. Data is expressed as mean ± standard error (SE). * (P<0.05) indicates significant difference from untreated mdx control.
We also investigated the infiltration of CD4+ and CD8+ T cells in vector-injected muscles of mdx mice to assess the cellular immune reaction following murine dystrophin expression. Since muscle infiltration of CD4+ and CD8+ T cells is a disease-associated phenotype of mdx mouse muscle and is thought to promote muscle fiber necrosis (31), infiltrating T cells are seen in dystrophic mdx muscles with and without dystrophin gene transfer. We compared the number of T cells infiltrating vector-injected muscle, with or without irradiation, to age-matched untreated muscle.
Levels of infiltrating CD4+ and CD8+ T cells in muscles of low-dose irradiated, vector-treated or control mice were not significantly different from untreated mice at week 4 post-treatment (Fig. 2B and 2C). At week 8 CD4+ and CD8+ T cells increased in the vector-injected TA muscles of both irradiated and non-irradiated hosts compared to untreated muscle, indicating the development of a T cell-mediated response following dystrophin vector expression that was not affected by irradiation (Fig. 2B and 2C). By week 12, levels of CD4+ and CD8+ T cells had increased in all groups to similar levels (Fig. 2B and 2C).
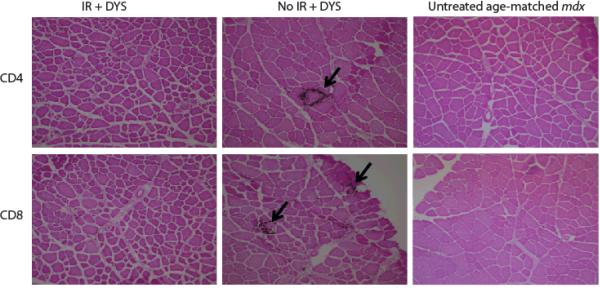
The pattern of T cell infiltration for both CD4+ and CD8+ T cells observed on muscle sections was consistently different in the vector-treated, irradiated mice compared to the control group (Fig. 3). At week 4, T cells were scattered throughout the muscle tissue in irradiated, vector-injected mdx muscles, similar to those in age-matched untreated mdx muscles. In the control group, however, in addition to scattered infiltrating cells, large numbers of cells were seen to cluster around one or multiple adjacent fibers, and groups of necrotic muscle fibers were seen as early as 4 weeks post-treatment. Therefore, it appeared that the transduced muscle fibers were targeted by a specific cell-mediated host immune response in the absence of irradiation prior to gene transfer. In the irradiated group a similar pattern was only seen in the vector-injected muscles at week 12 post-treatment (data not shown).
Fig. 3. Pattern of T cell infiltration in low-dose irradiated, vector-injected muscle is significantly different from non-irradiated, vector-injected muscle.
Infiltrating CD4+ and CD8+ T cells were examined by immunohistochemistry in treated and control muscles at week 4, 8, and 12 post-treatment. This figure shows a representative image of week 8 analysis. Arrows indicate single or multiple muscle fibers surrounded with infiltrating T cells.
High-dose irradiation delays anti-dystrophin humoral response
We next examined the effect of removing central and peripheral immune cells of mdx mice by 900 rads irradiation prior to intramuscular dystrophin gene delivery (1.5×1010 genome copies in each TA muscle). The immune systems of irradiated mice were then reconstituted by transfer of wild-type BM from C57BL/10 (B10) donors. Mice were analyzed at 4, 8, or 12 weeks post-treatment. We had two control groups: one that underwent only gene delivery without receiving other treatments and one that was irradiated and received adoptive transfer of B10 BM without gene transfer (Table 1).
We considered what would be an expected time course to clear the initial load of vector capsid proteins. To explore this experimentally, C2C12 myoblast cells were infected in vitro with Ad vector. Harvested cell samples at various time points for 48 hours were assayed for Ad vector capsid proteins by Western blot. Nearly all Ad capsid proteins were degraded in vitro by 12 to 24 hours post-infection (data not shown). Despite likely differences between viral protein degradation in vitro and in vivo, the in vitro results gave us an approximate time point for progressing to our in vivo studies. Balancing the desire to maximize the time for viral proteins to degrade prior to BM transfer with a need to minimize mouse loss due to high dose irradiation, we performed BM transfer at about 15 hours post-gene transfer. BM transfer consisted of tail vein intravascular (IV) injections of 4.0-5.0 × 106 B10 BM cells.
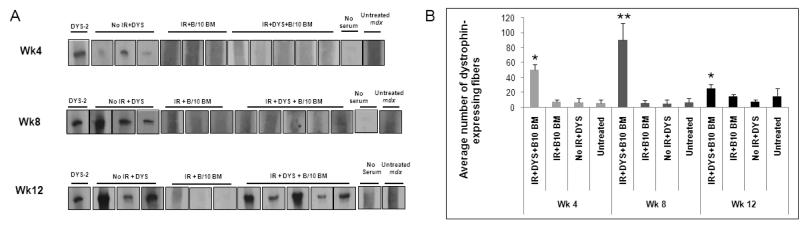
At 4 weeks post-gene transfer none of the mice that had been irradiated and received B10 BM produced anti-dystrophin antibodies. However, the vector-injected mice that had not been irradiated produced anti-dystrophin antibodies at this time point, similar to the control group in the low-dose irradiation study (Fig. 4A). Unlike the low-dose study group, in which a few mice produced anti-dystrophin antibodies at week 8 post-treatment, at this time-point none of the irradiated, vector-injected mice that received B10 BM produced an anti-dystrophin humoral response (Fig. 4A). By 12 weeks post-treatment, however, we observed anti-dystrophin humoral response in all of these mice (Fig. 4A).
Fig. 4. The anti-dystrophin humoral response and loss of muscle dystrophin expression was delayed in mdx mice treated with high dose irradiation followed by dystrophin gene transfer using a high-capacity adenoviral vector.
(A) Sera from mdx mice that 1) were irradiated at 900 rads and received an intramuscular injection of dystrophin vector (IR + DYS+B10 BM), 2) were irradiated and received B10 BM only (IR+B10 BM), 3) received an intramuscular injection of dystrophin vector without irradiation (no IR + DYS), or 4) received neither irradiation nor dystrophin vector (untreated mdx) were incubated with membrane-immobilized dystrophin protein. Sera were collected from treated and control mice at 4, 8, and 12 weeks (Wk) after treatment (n=5 for IR+DYS+B10 BM and n=3 for all control groups). No serum indicates a negative control without mouse serum labeling. DYS-2 indicates a positive immunoblotting control using a monoclonal anti-dystrophin antibody as the primary antibody. All experimental samples and representative control samples are presented here. (B) Dystrophin-expressing fibers per TA muscle section were counted for all experimental mice shown in (A) and all untreated mdx control mice. Dystrophin-expressing fibers were labeled on muscle cryosections using an anti-dystrophin monoclonal antibody and were counted per cryosection of treated TA muscles. The percentages on the bars for each time point indicate the average percentage of dystrophin-expressing fibers per total fibers per section. Muscle cross-sections of each vector-injected and uninjected TA muscles were analyzed and the mean of the fibers counted in sections from treated and control mice were calculated. Dystrophin-expressing fibers were counted to be 10%, 20%, and 5% of total muscle fibers per section at weeks 4, 8, and 12, respectively. Data is expressed as mean ± standard error (SE). *(P<0.05) and **(P<0.001) indicate significant differences from untreated mdx control.
HC-Ad vector-mediated dystrophin expression in high-dose irradiated mdx muscles
Dystrophin protein expression in the muscles of treated mdx mice was assessed by immunohistological assay, as before. In the high-dose irradiation group we first determined that adoptive transfer of B10 BM alone did not give rise to dystrophin-expressing fibers above the level in untreated age-matched mdx muscles, for at least 12 weeks post-treatment (Fig. 4B). At all time-points post-treatment, dystrophin expression was significantly higher in the muscles of the irradiated mice that had received vector and B10 BM compared to the control groups (Fig. 4B, wk4). Compared to week 4, at week 8 post-treatment, the level of dystrophin expression increased in B10 BM recipient mice that received dystrophin vector (Fig. 4B; wk8). The subsequent decline in dystrophin expression level at week 12 post-treatment correlated with the production of anti-dystrophin antibodies by these mice.
Lymphocytes in the host lymph nodes and vector-injected muscles after high-dose irradiation and BM transfer
The lymph nodes draining vector-treated muscles of the high-dose irradiated mice were analyzed to examine peripheral lymphocyte levels. The percentage of B cells in the draining lymph nodes was similar among all groups at 4 and 8 weeks post-treatment (Fig. 5A). By week 12 post-treatment the B cell levels in all groups had decreased (Fig. 5A, wk12).
Fig. 5. Infiltrating T cells and peripheral lymphocytes from vector-injected, muscle-draining lymph nodes in high dose irradiated mdx mice differ from untreated mdx mice.
(A) The B cell population in draining lymph nodes was analyzed by flow cytometry in high-dose irradiated and non-irradiated mdx mice at 4, 8, and 12 weeks post-treatment. Infiltrating CD4+ (B) and CD8+ (C) T cells were examined by immunohistochemistry in the treated muscles of high-dose irradiated and non-irradiated mdx mice at the three time points mentioned above. Number of mice in each group is the same as in Fig. 4. Data is expressed as mean ± standard error (SE). * (P<0.05) indicates significant difference from untreated mdx control.
We also looked at T cell infiltration in the treated muscles at the same time points. At week 4 post-treatment, the number of CD4+ T cells was significantly lower in the muscles of the irradiated mice that received dystrophin vector and B10 BM compared to the non-irradiated, vector-injected mice (Fig. 5B and 5C). CD8+ T cells were at comparable levels in the irradiated and non-irradiated, vector-injected mice. Starting at week 8, however, muscle infiltrating CD4+ and CD8+ T cells increased in the irradiated, vector-injected mice at comparable levels to the non-irradiated, vector-injected mice (Fig. 5B and 5C). The levels of infiltrating CD4+ and CD8+ T cells remained stable at week 12 post-treatment compared to week 8 in the irradiated, vector-injected group that received B10 BM (Fig. 5B and 5C).
Infiltrating T cells were observed scattered throughout in the high-dose irradiated, vector-injected muscles at all time points (data not shown) in contrast to clustering around specific muscle fibers in non-irradiated, vector-injected muscle (Fig. 3). These observed differences in the pattern of muscle T cell infiltration was similar to that observed in the low-dose irradiation study.
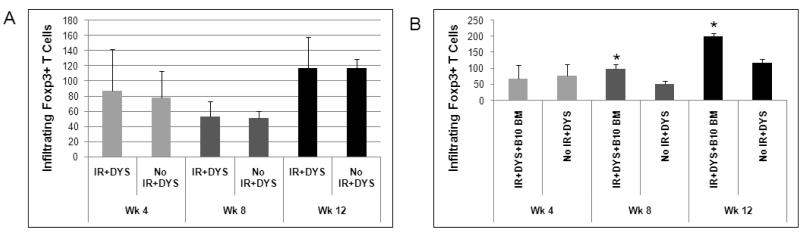
Regulatory T cells in treated muscles
An important cell population in modulating immunity is a regulatory subset of CD4+ T cells expressing the fork head box 3 (Foxp3) transcription factor, commonly referred to as Foxp3+ regulatory T (Treg) cells (30;32;33). We compared the infiltration of Treg cells in treated muscles at each time point to explore a possible role of these cells in modulating the immunity that we observed in treated mdx mice. In the low-dose irradiation study, the number of muscle-infiltrating Treg cells was comparable at each time point for the vector-treated groups with and without irradiation (Fig. 6A). In our treatment groups there was a decrease from week 4 to week 8 followed by an increase from week 8 to week 12, a pattern that was also observed in untreated mdx mouse muscle, suggesting a contribution of the dystrophic disease progression in muscle. In the high-dose irradiation study there was a significant increase in Treg cell infiltration starting at week 8 post-treatment in the irradiated, vector-injected mice, but not in the non-irradiated, vector-injected mice (Fig. 6B). The number of these cells further increased at week 12 and remained significantly higher in the irradiated, vector-injected B10 BM recipients as compared to the non-irradiated, vector-injected mice (Fig. 6B).
Fig. 6. Regulatory T cell infiltration in treated mdx muscles.
Infiltrating Foxp3+ regulatory T cells in treated muscles of both low-dose (A) and high-dose (B) irradiated and control groups were analyzed by immunohistochemistry at 4, 8, and 12 weeks post-treatment. Number of mice in each group is the same as in Fig. 1 and Fig.4. Data is expressed as mean ± standard error (SE). * (P<0.05) indicates significant difference from untreated mdx control.
We also examined the level of muscle infiltration of programmed death-1 (PD-1) expressing cells, that have been shown to have immune regulatory effects (34-36). There was no significant difference in the number of PD-1+ muscle-infiltrating cells in different treatment groups at different time points in mice receiving either low-dose or high-dose irradiation (data not shown).
Discussion
In studies on therapeutic gene replacement for DMD, the immune response of the dystrophic host to vector-mediated dystrophin protein expression is an important limiting factor with the potential to cause rejection of recombinant dystrophin. Further understanding of the immunological reactions against dystrophin, immune mechanisms that may modulate anti-dystrophin immunity, and manipulations that may reduce anti-dystrophin immune responses in a dystrophic host provides the potential to develop approaches designed to prolong dystrophin expression in the recipient of therapeutic gene transfer.
A temporal analysis of the effect of irradiation alone or irradiation combined with BM transfer on the immunity against vector-mediated full-length dystrophin expression in mdx muscle has not been previously reported. Previous studies have examined the effects on dystrophic muscle of adoptive transfer of wild-type BM to irradiated mdx mice (37), muscle precursor cells to irradiated mdx mice (38), or BM-derived stem cells to non-irradiated mdx mice (39). In the current study we limited the dose of irradiation to a maximum of 900 rads, as in other studies (40), to avoid damage to muscle stem cells (41). The use of the HC-Ad vector for gene delivery allowed exposure to all epitopes of an expressed full-length murine dystrophin cDNA.
In our studies, BM transfer alone to mdx mice did not increase the level of muscle dystrophin expression over the background level in untreated mdx mice at any time-point, which confirmed the findings of others (37;42). Therefore, the dystrophin expression observed after dystrophin vector delivery following irradiation and BM transfer is vector-mediated.
Our findings demonstrate that irradiation of the dystrophic mdx host prior to intramuscular vector injection led to a delayed, reduced, or absent humoral response against dystrophin protein. The changes in anti-dystrophin immunity in treated mice allowed us to study the progression of host immunity against dystrophin and its effect on dystrophin expression over a period of 12 weeks. The observation that both low-dose and high-dose irradiation delayed the development of an anti-dystrophin antibody response is significant compared to the previous findings of more rapid host immunity against dystrophin (21).
The delay in development of an anti-dystrophin humoral response was greater in the high-dose irradiated group compared to the low-dose irradiated group, as no anti-dystrophin humoral response was observed at 8 weeks post-treatment. Our results suggest that the more complete immune cell depletion (both central and peripheral immune cells) and reconstitution with dystrophin-tolerant immune cells results in greater suppression of anti-dystrophin immunity induced by dystrophin gene transfer to the dystrophin-deficient mdx host.
The level of CD4+ and CD8+ T cell infiltration in untreated dystrophic mdx muscle (31;43) and in dystrophin vector-injected mdx muscle (19;44) has been previously investigated. The dystrophic pathology of mdx mouse muscle includes a high level of T cell infiltration in the damaged tissue that is thought to promote the pathology associated with the disease (31;43). It has been shown that specific immune modulations that lead to absent or reduced T cells in the mdx mouse can ameliorate the disease process in muscle (31;43;45) and, in a gene transfer setting, reduce immunity against dystrophin protein in the host (18;44). Our present study compared the levels of infiltrating CD4+ and CD8+ T cells in vector-injected muscles to untreated mdx mouse muscle at different time points. Thus, we evaluated the role that these T cell sub-populations may play in a gene therapy setting as both disease and the immunity against recombinant dystrophin progressed. In contrast to the observed correlation with the anti-dystrophin humoral response, irradiation prior to gene transfer does not seem to affect levels of infiltrating T cells in vector-injected muscles of the mdx host during 12 weeks post-treatment in either low- or high-dose irradiation groups. Interestingly, however, the pattern of T cell infiltration in muscle tissue did appear to be affected by irradiation.
In the early stages after gene transfer, the finding of muscle fibers surrounded and invaded by T cells was only observed in the non-irradiated, vector-injected muscles. This focal pattern of T cell infiltration was not observed in the irradiated vector-injected and untreated mdx mice at this time point. Double-staining of T cells and dystrophin in the attacked fibers was not feasible because only necrotic fiber remnants remained. Nonetheless, the distinctive pattern of T cell infiltration plus the absence of dystrophin-expressing fibers in non-irradiated, vector-injected muscles at early stages post-gene transfer suggested that transduced fibers were targeted for immune attack. The same pattern of T cell infiltration has also been observed in previous studies in which transfer of muscle precursor cells from dystrophin-normal donors to irradiated mdx mice, that had received mixed BM from muscle cell-donors and mdx donors, resulted in infiltration of CD4+ and CD8+ T cells around dystrophin-expressing muscle fibers at 3-4 months post-treatment (46).
Overall, our analysis of T cell infiltration in treated mdx muscle indicates that even though CD4+ and CD8+ T cells infiltrates are found in irradiated, vector-treated mdx muscles, this host immune response does not lead to an immediate rejection of dystrophin protein in the vector-injected muscles of irradiated mice for at least 12 weeks post-treatment. In contrast, T cell infiltration in the vector-injected muscles of non-irradiated mice appeared to reject dystrophin-expressing fibers, suggested by the pattern of T cell infiltration surrounding muscle fibers and the absence of dystrophin expression as early as 4 weeks post-treatment. One limitation of the analysis of cellular immunity observed in treated or untreated mdx muscle was that the antigens inducing immunity cannot be specifically determined.
To decipher the mechanism underlying the delayed and diminished host immune response observed in both low- and high-dose irradiated groups, we examined infiltration of Treg cells and PD-1+ T cells in treated and control mdx muscles. Only Treg cells were different among the groups. We have previously observed Foxp3+ Treg cells to be at slightly higher levels in untreated mdx mouse muscle compared to age-matched B10 muscle (unpublished data). In this study, we observed even higher levels of Treg cells in vector-injected muscle of mdx mice 8 and 12 weeks after receiving high-dose irradiation and reconstitution of the immune system with B10 BM. This increase in Treg cells suggested their role in the diminished anti-dystrophin immune response observed in the setting of central and peripheral immune cell depletion followed by reconstitution. In contrast, Treg cells did not appear to play a role in the delayed immunity associated with depletion of the peripheral immune cells alone followed by self-reconstitution (low-dose irradiation). Taken together, these results suggest a role for Treg cells in the suppression of immunity induced by dystrophin vector gene transfer followed by reconstitution of the central immune system. The adoptive transfer of whole BM from B10 mice may have contributed to the relatively high level of Treg cells in treated muscles since the transferred BM is likely to contain cells tolerant to B10 self-antigens, including muscle proteins. The exact role of Treg cells in the setting of dystrophin gene delivery to dystrophic muscle will require further study.
In summary, we show that manipulation of the host immune system by irradiation of the adult dystrophic mdx mouse prior to intramuscular HC-Ad dystrophin vector delivery results in a delayed and diminished humoral immune response to vector-mediated dystrophin protein expression in the adult mdx mouse. The delay in the response correlated with a significantly slower rate of elimination of vector-mediated dystrophin protein expression in treated mdx muscle compared to muscle of non-irradiated vector-injected mice. Although there are technical limitations to determining the antigen specificity of T cells infiltrating muscle, the pattern of T cells surrounding and invading muscle fibers suggests that viral vector-mediated dystrophin transduction contributes to the antigenic targets, particularly since this pattern of T cell infiltration is diminished and delayed by irradiation. The failure to eliminate anti-dystrophin immunity with the central and peripheral immune depletion achieved by irradiation supports the contention that dystrophin expression in dystrophin-deficient, dystrophic muscle is immune-stimulatory, a finding which has significant ramifications for dystrophin gene replacement therapy.
Materials and Methods
Mice
Wild type (C57BL/10J) and mdx (C57BL/10ScSnDmdmdx/J) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All mouse studies were performed under approved protocols by the University of Pittsburgh Institutional Animal Care and Use Committee.
Dystrophin high-capacity adenoviral vector
The HC-Ad vector contains a full-length murine dystrophin cDNA under the control of the muscle creatine kinase (MCK) promoter, the left and right viral inverted terminal repeats (ITRs) of adenovirus serotype 5 and hypoxanthine-guanine phosphoribosyl transferase (HPRT) ‘stuffer’ DNA. The construction of this vector has been fully described previously (18;47).
Mouse irradiation
Mice were irradiated at the age of 5-6 weeks in a LINAC irradiator at either a low dose (600 rads) or a high dose (900 rads). Specialized care included autoclaved cages, autoclaved food, and acidified water (pH 2.6) from the day before irradiation until the end of each study. In the low-dose irradiated group the immune system was allowed to spontaneously reconstitute following gene transfer and in the high-dose irradiated group the mice received whole BM from wild-type C57BL/10 mice at 12 hours post-gene transfer. BM was transferred by intravascular (IV) injections of 4.0-5.0 × 106 cells.
Intramuscular vector injections
Irradiated and non-irradiated mice received inhalational anesthesia with isoflurane and were injected with 1.0-2.0 ×1010 genome copies of HC-AdmDYS vector intramuscularly in the TA muscle bilaterally. Each muscle was injected with a volume of 20μl in PBS, using a 28G needle (B-D; Franklin Lakes, NJ).
Total muscle protein extract (TMPE) preparation
Freshly isolated mouse muscle was homogenized in TEES buffer (50mM Tris-HCl pH 8.0, 5mM EGTA pH 7.4, 5mM EDTA pH 8.0, 5% SDS) and incubated on ice for 45 minutes. Samples were then sonicated briefly and centrifuged at 14,000 rpm for 20 minutes at 4°C. Supernatant was collected and stored at −80°C.
Serum preparation
Blood was collected by cardiac puncture shortly after sacrifice and centrifuged at 10,000 rpm for 15 minutes at 4°C. Supernatant was collected and stored at 4°C.
Gel electrophoresis and Western blotting
The membranes with immobilized murine dystrophin protein used for the assay were generated from wild-type C57BL/10J (B10) total muscle protein extracts (TMPE) electrophoresed on SDS-PAGE. In brief, TMPE from B10 mice were run on 5% Acrylamide gel (Bio-Rad; Hercules, CA) for 3 hours at 110V. Protein samples were transferred from the gel to nitrocellulose membrane (Amersham biosciences) for 1.5 hours at 110V at 4°C. The membrane was blocked in 5% milk/1% sheep serum/TBST (10mM Tris pH 8.0, 0.15M NaCl, 0.5mM Tween-20) overnight at 4°C. The membrane was then cut in pieces with a dystrophin protein band in each piece. The pieces of membrane were then each incubated with a diluted serum sample (1:300) in TBST for 1.5 hours, then with HRP-conjugated sheep anti-mouse IgG (GE Healthcare; Piscataway, NJ) diluted in TBST for 45 minutes at room temperature. ECL detection reagent (GE Healthcare; Piscataway, NJ) was used to detect the chemiluminescent signal and Kodak film was used for visualizing the signal. Presence or absence of a dystrophin band was recorded. The initial exposure time was 15-30 minutes and to confirm the absence of a band in irradiated mice an additional film was exposed to the membranes overnight.
Bone marrow (BM) preparation
Tibia and femur of adult wild-type mice were dissected with complete removal of surrounding muscle. Syringes with 26G needles were used to flush the BM out of the bones onto a cell strainer using RPMI-1460 medium supplemented with 2% fetal bovine serum (FBS), 5% penicillin/streptomycin, and 10U/ml heparin (Sigma-Aldrich; St. Louis, MO). BM was then washed with a serum-free RPMI-1460 medium (5% penicillin/streptomycin, 20mM HEPES) and counted. For adoptive transfer, cells were re-suspended in serum-free medium at a concentration of 2.0×107 cells/ml. Cell suspension (200 μl) was injected I.V. (4-5×106 cells/mouse) using a 28G needle (B-D).
Flow cytometry
Single-cell suspensions were prepared from lymph nodes by mechanical disaggregation through a 40μm cell strainer (Fisher) into PBS. Cells were incubated with rat anti-CD4 (FITC), CD25 (APC), and Foxp3 (PE) (ebiosciences; San Diego, CA; 11-0042-82, ,17-0251-81, 12-5773-80, respectively) and rat anti-CD8 (PE), CD19 (FITC), and CD11c (FITC) (Pharmingen; San Jose, CA; 553032, 557398, and 557400, respectively) in FACS staining buffer (ebiosciences; San Diego, CA) as indicated by the manufacturer’s instructions. The stained cells were then analyzed by a B-D SLR II flow cytometer.
Muscle Tissue Processing
Freshly dissected muscle was incubated in 2% paraformaldehyde/PBS on ice for 2 hours, then transferred into 30% sucrose overnight at 4°C. The next day the muscle tissue was snap-frozen in 2-methylbutane cooled with dry ice and stored at −80°C.
Immunohistochemistry of inflammatory cells
10μm cryo-sections of muscle samples were prepared. Cross-sections (4-5 sections per TA per mouse) were made from different parts of each TA muscle for this staining. Sections were rehydrated in PBS, blocked in peroxidase blocking reagent (DAKO Cytomation; Carpinteria, CA) for 5 minutes, and then blocked in 10% goat serum/PBS for 1 hour at room temperature. The primary antibody incubations using rat anti-CD4, Foxp3, and PD-1 (ebiosciences; San Diego, CA; 16-0041-81, 14-4771-80, and 13-9985-81, respectively) and rat anti-CD8 (Pharmingen; San Jose, CA) purified antibodies diluted in 10% goat serum/PBS were done for 1.5 hours at room temperature. Sections (except for PD-1 sections because PD-1 primary antibody was biotinylated) were incubated for 1 hour with secondary biotinylated goat anti-rat IgG (Pharmingen; San Jose, CA) diluted in DAKO antibody diluent (DAKO Cytomation; Carpinteria, CA). Sections were incubated in ABC Vectastain avidin-HRP detection solution (Vector Laboratories; Burlingame, CA) for 30 minutes at room temperature and DAB peroxidase substrate solution (Vector Laboratories; Burlingame, CA) for 4 minutes. Eosin counterstaining was performed for visualization of muscle fibers. To analyze infiltrating cells in each group, the total number of cells per cross-section of vector-injected TA muscles was counted and the average number of cells from sections from different mice was calculated.
Immuno-fluorescence detection of dystrophin
10μm cryo-sections, as mentioned above, were rehydrated with PBS, blocked first with avidin and biotin block (Vector Laboratories; Burlingame, CA), and then with mouse IgG block (Vector Laboratories; Burlingame, CA), according to the manufacturer’s instructions. Incubation with primary anti-DYS was done for 3 hours. Sections were then incubated with biotinylated goat anti-mouse IgG (Pharmingen; San Jose, CA) and tertiary FITC-conjugated donkey anti-goat IgG.
Statistical Analysis
The statistical analysis was performed by student’s t-test, in which a treatment group and a control group, or two treatment groups were compared as unpaired sets. Values of variables were presented as the mean with standard deviation (SD). In all experiments, P values <0.05 were considered significant.
Acknowledgements
We thank Daniel P. Reay for providing valuable technical support and breeding mice used in these studies. We also thank Penelope A. Morel for her constructive critique of this manuscript. This work was supported by F31-NS056780-01A2 (SE) from the NIH grant W81XWH-05-1-0334 (PRC) from the US Army Medical Research and Materiel Command. The authors take full responsibility for the and contents of this paper, which do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
Conflict of Interest There are no competing financial interests in relation to the work described.
Reference List
- (1).Spowart G, Buckton KE, Skinner R, Emery AE. X chromosome in Duchenne muscular dystrophy. Lancet. 1982 May 29;1(8283):1251. doi: 10.1016/s0140-6736(82)92380-7. [DOI] [PubMed] [Google Scholar]
- (2).Drousiotou A, Ioannou P, Georgiou T, Mavrikiou E, Christopoulos G, Kyriakides T, et al. Neonatal screening for Duchenne muscular dystrophy: a novel semiquantitative application of the bioluminescence test for creatine kinase in a pilot national program in Cyprus. Genet Test. 1998;2(1):55–60. doi: 10.1089/gte.1998.2.55. [DOI] [PubMed] [Google Scholar]
- (3).Emery AE. Population frequencies of inherited neuromuscular diseases--a world survey. Neuromuscul Disord. 1991;1(1):19–29. doi: 10.1016/0960-8966(91)90039-u. [DOI] [PubMed] [Google Scholar]
- (4).Parsons EP, Bradley DM, Clarke AJ. Newborn screening for Duchenne muscular dystrophy. Arch Dis Child. 2003 Jan;88(1):91–2. doi: 10.1136/adc.88.1.91-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H, Kunkel LM. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988 Jan;2(1):90–5. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- (6).Hoffman EP, Brown RH, Jr., Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–28. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- (7).Love DR, Davies KE. Duchenne muscular dystrophy: the gene and the protein. Mol Biol Med. 1989 Feb;6(1):7–17. [PubMed] [Google Scholar]
- (8).Koenig M, Monaco AP, Kunkel LM. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–28. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- (9).Groh S, Zong H, Goddeeris MM, Lebakken CS, Venzke D, Pessin JE, et al. Sarcoglycan complex: implications for metabolic defects in muscular dystrophies. J Biol Chem. 2009 Jul 17;284(29):19178–82. doi: 10.1074/jbc.C109.010728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Satz JS, Philp AR, Nguyen H, Kusano H, Lee J, Turk R, et al. Visual impairment in the absence of dystroglycan. J Neurosci. 2009 Oct 21;29(42):13136–46. doi: 10.1523/JNEUROSCI.0474-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Yoshida M, Ozawa E. Glycoprotein complex anchoring dystrophin to sarcolemma. J Biochem. 1990 Nov;108(5):748–52. doi: 10.1093/oxfordjournals.jbchem.a123276. [DOI] [PubMed] [Google Scholar]
- (12).Bonilla E, Samitt CE, Miranda AF, Hays AP, Salviati G, DiMauro S, et al. Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell. 1988 Aug 12;54(4):447–52. doi: 10.1016/0092-8674(88)90065-7. [DOI] [PubMed] [Google Scholar]
- (13).Emery AE. Dystrophin function. Lancet. 1990 May 26;335(8700):1289. doi: 10.1016/0140-6736(90)91364-g. [DOI] [PubMed] [Google Scholar]
- (14).Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989 Jun 30;244(4912):1578–80. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- (15).Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, Kota J, Coley BD, Galloway G, et al. Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann Neurol. 2009 Sep;66(3):290–7. doi: 10.1002/ana.21732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Gilbert R, Nalbantoglu J, Howell JM, Davies L, Fletcher S, Amalfitano A, et al. Dystrophin expression in muscle following gene transfer with a fully deleted (“gutted”) adenovirus is markedly improved by trans-acting adenoviral gene products. Hum Gene Ther. 2001 Sep 20;12(14):1741–55. doi: 10.1089/104303401750476249. [DOI] [PubMed] [Google Scholar]
- (17).Alba R, Bosch A, Chillon M. Gutless adenovirus: last-generation adenovirus for gene therapy. Gene Therapy. 2005 Jun 1;12:18–27. doi: 10.1038/sj.gt.3302612. [DOI] [PubMed] [Google Scholar]
- (18).Jiang Z, Schiedner G, Gilchrist S, Kochanek S, Clemens PR. CTLA4Ig delivered by high-capacity adenoviral vector induces stable expression of dystrophin in mdx mouse muscle. Gene Therapy. 2007 Oct;11(19):1453–61. doi: 10.1038/sj.gt.3302315. [DOI] [PubMed] [Google Scholar]
- (19).Acsadi G, Lochmuller H, Jani A, Huard J, Massie B, Prescott S, et al. Dystrophin expression in muscles of mdx mice after adenovirus-mediated in vivo gene transfer. Hum Gene Ther. 1996 Jan 20;7(2):129–40. doi: 10.1089/hum.1996.7.2-129. [DOI] [PubMed] [Google Scholar]
- (20).Ferrer A, Wells KE, Wells DJ. Immune responses to dystropin: implications for gene therapy of Duchenne muscular dystrophy. Gene Ther. 2000 Sep;7(17):1439–46. doi: 10.1038/sj.gt.3301259. [DOI] [PubMed] [Google Scholar]
- (21).Gilchrist S, Ontel M, Kochanek S, Clemens PR. Immune response to full-length dystrophin delivered to Dmd muscle by a high-capacity adenoviral vector. Mol Ther. 2002 Sep;6(3):359–68. doi: 10.1006/mthe.2002.0675. [DOI] [PubMed] [Google Scholar]
- (22).Danko I, Chapman V, Wolff JA. The frequency of revertants in mdx mouse genetic models for Duchenne muscular dystrophy. Pediatr Res. 1992 Jul;32(1):128–31. doi: 10.1203/00006450-199207000-00025. [DOI] [PubMed] [Google Scholar]
- (23).Hoffman EP, Morgan JE, Watkins SC, Partridge TA. Somatic reversion/suppression of the mouse mdx phenotype in vivo. J Neurol Sci. 1990 Oct;99(1):9–25. doi: 10.1016/0022-510x(90)90195-s. [DOI] [PubMed] [Google Scholar]
- (24).Sahashi K, Ibi T, Suoh H, Nakao N, Tashiro M, Marui K, et al. Immunostaining of dystrophin and utrophin in skeletal muscle of dystrophinopathies. Intern Med. 1994 May;33(5):277–83. doi: 10.2169/internalmedicine.33.277. [DOI] [PubMed] [Google Scholar]
- (25).Thanh LT, Nguyen TM, Helliwell TR, Morris GE. Characterization of revertant muscle fibers in Duchenne muscular dystrophy, using exon-specific monoclonal antibodies against dystrophin. Am J Hum Genet. 1995 Mar;56(3):725–31. [PMC free article] [PubMed] [Google Scholar]
- (26).Appleby MW, Ramsdell F. Scurfy, the Foxp3 locus, and the molecular basis of peripheral tolerance. Curr Top Microbiol Immunol. 2008;321:151–68. doi: 10.1007/978-3-540-75203-5_7. [DOI] [PubMed] [Google Scholar]
- (27).Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000 May 26;101(5):455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- (28).Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006 Aug;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- (29).Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008 May 30;133(5):775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- (30).Eghtesad S, Morel PA, Clemens PR. The companions: regulatory T cells and gene therapy. Immunology. 2009 May;127(1):1–7. doi: 10.1111/j.1365-2567.2009.03069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Spencer MJ, Tidball JG. Do immune cells promote the pathology of dystrophin-deficient myopathies? Neuromuscul Disord. 2001 Sep;11(6-7):556–64. doi: 10.1016/s0960-8966(01)00198-5. [DOI] [PubMed] [Google Scholar]
- (32).Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003 Apr;4(4):330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- (33).Yagi H, Nomura T, Kakamura K, Yamazaki S, Kitawaki T, Hori S, et al. Crucial role of Foxp3 in the development and function of human CD4+CD25+ regulatory T cells. The Japanese Society for Immunology. 2004 Apr 10;16(11):1643–56. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- (34).Cheng X, Dai H, Wan N, Moore Y, Vankayalapati R, Dai Z. Interaction of programmed death-1 and programmed death-1 ligand-1 contributes to testicular immune privilege. Transplantation. 2009 Jun 27;87(12):1778–86. doi: 10.1097/TP.0b013e3181a75633. [DOI] [PubMed] [Google Scholar]
- (35).Dai H, Zhu H, Lei P, Yagita H, Liu J, Wen X, et al. Programmed death-1 signaling is essential for the skin allograft protection by alternatively activated dendritic cell infusion in mice. Transplantation. 2009 Oct 15;88(7):864–73. doi: 10.1097/TP.0b013e3181b6ea74. [DOI] [PubMed] [Google Scholar]
- (36).Folkl A, Bienzle D. Structure and function of programmed death (PD) molecules. Vet Immunol Immunopathol. 2009 Oct 14; doi: 10.1016/j.vetimm.2009.10.006. [DOI] [PubMed] [Google Scholar]
- (37).Hagiwara H, Ohsawa Y, Asakura S, Murakami T, Teshima T, Sunada Y. Bone marrow transplantation improves outcome in a mouse model of congenital muscular dystrophy. FEBS Lett. 2006 Aug 7;580(18):4463–8. doi: 10.1016/j.febslet.2006.07.015. [DOI] [PubMed] [Google Scholar]
- (38).Camirand G, Stephan L, Rousseau J, Sackett MK, Caron NJ, Mills P, et al. Central tolerance to myogenic cell transplants does not include muscle neoantigens. Transplantation. 2008 Jun 27;85(12):1791–801. doi: 10.1097/TP.0b013e31817726bc. [DOI] [PubMed] [Google Scholar]
- (39).Feng SW, Lu XL, Liu ZS, Zhang YN, Liu TY, Li JL, et al. Dynamic distribution of bone marrow-derived mesenchymal stromal cells and change of pathology after infusing into mdx mice. Cytotherapy. 2008;10(3):254–64. doi: 10.1080/14653240802020381. [DOI] [PubMed] [Google Scholar]
- (40).Camirand G, Stephan L, Rousseau J, Sackett MK, Caron NJ, Mills P, et al. Central tolerance to myogenic cell transplants does not include muscle neoantigens. Transplantation. 2008 Jun 27;85(12):1791–801. doi: 10.1097/TP.0b013e31817726bc. [DOI] [PubMed] [Google Scholar]
- (41).Quinlan JG, Lyden SP, Cambier DM, Johnson SR, Michaels SE, Denman DL. Radiation inhibition of mdx mouse muscle regeneration: dose and age factors. Muscle Nerve. 1995 Feb;18(2):201–6. doi: 10.1002/mus.880180209. [DOI] [PubMed] [Google Scholar]
- (42).Chretien F, Dreyfus PA, Christov C, Caramelle P, Lagrange JL, Chazaud B, et al. In vivo fusion of circulating fluorescent cells with dystrophin-deficient myofibers results in extensive sarcoplasmic fluorescence expression but limited dystrophin sarcolemmal expression. Am J Pathol. 2005 Jun;166(6):1741–8. doi: 10.1016/S0002-9440(10)62484-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Spencer MJ, Montecino-Rodriguez E, Dorshkind K, Tidball JG. Helper (CD4(+)) and cytotoxic (CD8(+)) T cells promote the pathology of dystrophin-deficient muscle. Clin Immunol. 2001 Feb;98(2):235–43. doi: 10.1006/clim.2000.4966. [DOI] [PubMed] [Google Scholar]
- (44).Jiang Z, Schiedner G, van Rooijen N, Liu CC, Kochanek S, Clemens PR. Sustained Muscle Expression of Dystrophin from a High-Capacity Adenoviral Vector with Systemic Gene Transfer of T Cell Costimulatory Blockade. Mol Ther. 2004 Oct;10(4):688–96. doi: 10.1016/j.ymthe.2004.07.020. [DOI] [PubMed] [Google Scholar]
- (45).Farini A, Meregalli M, Belicchi M, Battistelli M, Parolini D, D’Antona G, et al. T and B lymphocyte depletion has a marked effect on the fibrosis of dystrophic skeletal muscles in the scid/mdx mouse. J Pathol. 2007 Oct;213(2):229–38. doi: 10.1002/path.2213. [DOI] [PubMed] [Google Scholar]
- (46).Camirand G, Stephan L, Rousseau J, Sackett MK, Caron NJ, Mills P, et al. Central tolerance to myogenic cell transplants does not include muscle neoantigens. Transplantation. 2008 Jun 27;85(12):1791–801. doi: 10.1097/TP.0b013e31817726bc. [DOI] [PubMed] [Google Scholar]
- (47).Jiang Z, Schiedner G, van Rooijen N, Liu CC, Kochanek S, Clemens PR. Sustained Muscle Expression of Dystrophin from a High-Capacity Adenoviral Vector with Systemic Gene Transfer of T Cell Costimulatory Blockade. Mol Ther. 2004 Oct;10(4):688–96. doi: 10.1016/j.ymthe.2004.07.020. [DOI] [PubMed] [Google Scholar]