Abstract
Context
Cognitive-behavior therapy (CBT) and hypnotic medications are efficacious for short-term treatment of insomnia, but few patients achieve complete remission with any single treatment. It is unclear whether combined or maintenance therapies would enhance outcome.
Objectives
To evaluate the added value of medication over CBT alone for acute treatment of insomnia and the effects of maintenance therapies on long-term outcome.
Design, Setting, and Patients
Prospective, randomized, clinical trial involving two-stage therapy with 160 adults with persistent insomnia treated at a university hospital sleep center between January 2002 and April 2005.
Interventions
Participants received CBT alone or CBT plus zolpidem for an initial six-week therapy, followed by extended 6-month therapy. Patients treated with CBT initially attended monthly maintenance CBT or no additional treatment and those treated with combined therapy initially continued with CBT plus intermittent medication or CBT without medication (tapering).
Main Outcome Measures
Sleep onset latency, time awake after sleep onset, total sleep time, and sleep efficiency derived from daily diaries (primary) and response and remission rates derived from the Insomnia Severity Index (secondary).
Results
CBT used singly or in combination with medication produced significant improvements of sleep latency, time awake after sleep onset, and sleep efficiency during initial therapy (Ps < 0.001); a larger increase of sleep time was obtained with the combined approach (P = 0.04). Both CBT and combined therapies produced similar rates of treatment responders (60% [45/75] vs. 61% [45/74], P = 0.84) and remissions (39% [29/75] vs. 44% [33/74], P = 0.52) with acute treatment, but combined therapy produced a higher remission rate relative to CBT alone over the extended therapy and follow up period (56% [43/74, 32/59] vs. 43% [34/75, 28/68], P = 0.05). The best long-term outcome was obtained with patients treated with combined therapy initially, followed by CBT alone, as evidenced by higher remission rates at the 6-month follow-up relative to patients who remained on medication during extended therapy (67% [20/30] vs. 41% [12/29], P = 0.04)
Conclusions
In patients with persistent insomnia, the addition of medication to CBT produces added benefits during acute therapy, but long-term outcome is optimized when medication is discontinued during maintenance CBT.
Keywords: Insomnia, treatment, CBT, medication
Introduction
Insomnia is a prevalent public health problem affecting large segments of the population on a situational, recurrent, or chronic basis 1, 2. Persistent insomnia is associated with significant impairments of daytime functioning, reduced quality of life and, when untreated, it heightens the risks for major depression and hypertension 3–7. Despite its high prevalence, morbidity, and costs, insomnia often remains untreated; when treatment is initiated, it is often limited to self-help remedies (e.g., alcohol, over-the-counter drugs) of questionable efficacy and safety 8, 9.
Cognitive-behavior therapy (CBT) and pharmacotherapy (benzodiazepine-receptor agonists) are the only two treatments with adequate evidence supporting their use in the clinical management of insomnia 8. Numerous clinical trials have evaluated the efficacy of CBT and medication separately, but very few have conducted head-to-head comparisons contrasting their separate and combined effects for insomnia 10–17. Collectively, the limited evidence available indicates that both treatment modalities are effective in the short-term; medication produces rapid symptomatic relief 12, 13, but these benefits are typically not maintained after treatment discontinuation. Conversely, CBT may take longer to implement but it produces more sustained benefits over time 11, 14, 15, 17. Combined CBT and medication appears to have a slight advantage over single treatment modality during the initial course of treatment, but their long-term effects are more variable across patients 10, 11, 14, 17. Thus, although combined approaches may be preferable to drug therapy alone, an important question that warrants further investigation is whether adding medication to CBT has an additive effect on short- and long-term outcomes.
Insomnia therapies are typically implemented over brief intervals, averaging 10 days for medication 18 and 5 weeks for CBT 19–21. Given the recurrent or persistent nature of insomnia 22, 23, such short-term treatment may be inadequate for long-term management. To optimize short- and long-term outcomes, it is important to validate new treatment algorithms. The addition of an extended treatment phase, incorporating maintenance CBT booster sessions could optimize long-term outcomes. Likewise, using hypnotic medications on an intermittent rather than nightly schedule may prevent tolerance and maintain efficacy 24–26. Another model is to combine CBT with medication as initial therapy and, after a few weeks, discontinue medication while pursuing CBT so patients can integrate their newly learned self-management skills 16. Such maintenance strategies may enhance long-term outcomes relative to acute intervention alone.
The objectives of this study were to evaluate the short- and long-term effects of CBT, singly and combined with medication, for persistent insomnia and compare the efficacy of maintenance strategies to optimize long-term outcomes. The main research questions were: Is CBT combined with medication more effective than CBT alone for acute treatment of insomnia? When combining CBT and medication, is it preferable to discontinue medication after initial treatment or continue medication on an intermittent schedule in order to optimize outcome?
Methods
Participants
Patients were recruited from January 2002 to April 2005 through newspaper advertisements and referrals from health-care practitioners in the Quebec City area. Inclusion criteria were: (a) 30 years of age or older; (b) diagnosis of chronic insomnia based on a combination of criteria from the DSM-IV-TR 27 and the International Classification of Sleep Disorders, (ICSD-2) 28. These criteria were further operationalized as follows: (a) difficulties initiating and/or maintaining sleep, defined as a sleep onset latency and/or wake after sleep onset greater than 30 min, with a corresponding sleep time of less than 6.5 hours at least 3 nights per week (as measured by daily sleep diaries); (b) insomnia duration greater than 6 months; and (c) significant distress or impairment of daytime functioning (rating of at least 2 on item 5 of the Insomnia Severity Index [ISI]).
Exclusion criteria were: (a) presence of a progressive medical illness (e.g., cancer, dementia) directly related to the onset and course of insomnia; (b) use of medications known to alter sleep (e.g., steroids); (c) lifetime diagnosis of any psychotic or bipolar disorder; (d) current diagnosis of major depression, unless treated and in remission; (e) more than two past episodes of major depression; (f) history of suicide attempt; (g) alcohol or drug abuse within the past year; (h) sleep apnea (apnea/hypopnea index > 15), restless legs, or periodic limb movements during sleep (movement index with arousal > 15 per hour); (i) night-shift work or irregular sleep pattern. Patients with stable medical (e.g., hypertension) or psychiatric disorders (e.g., dysthymia, anxiety) were included in the study provided that these conditions were not the primary cause of insomnia. Patients using prescribed or over-the-counter sleep medications no more than twice weekly were enrolled after they withdrew from medications. Participants using alcohol as a sleep aid were required to discontinue this practice at least two weeks prior to baseline assessment.
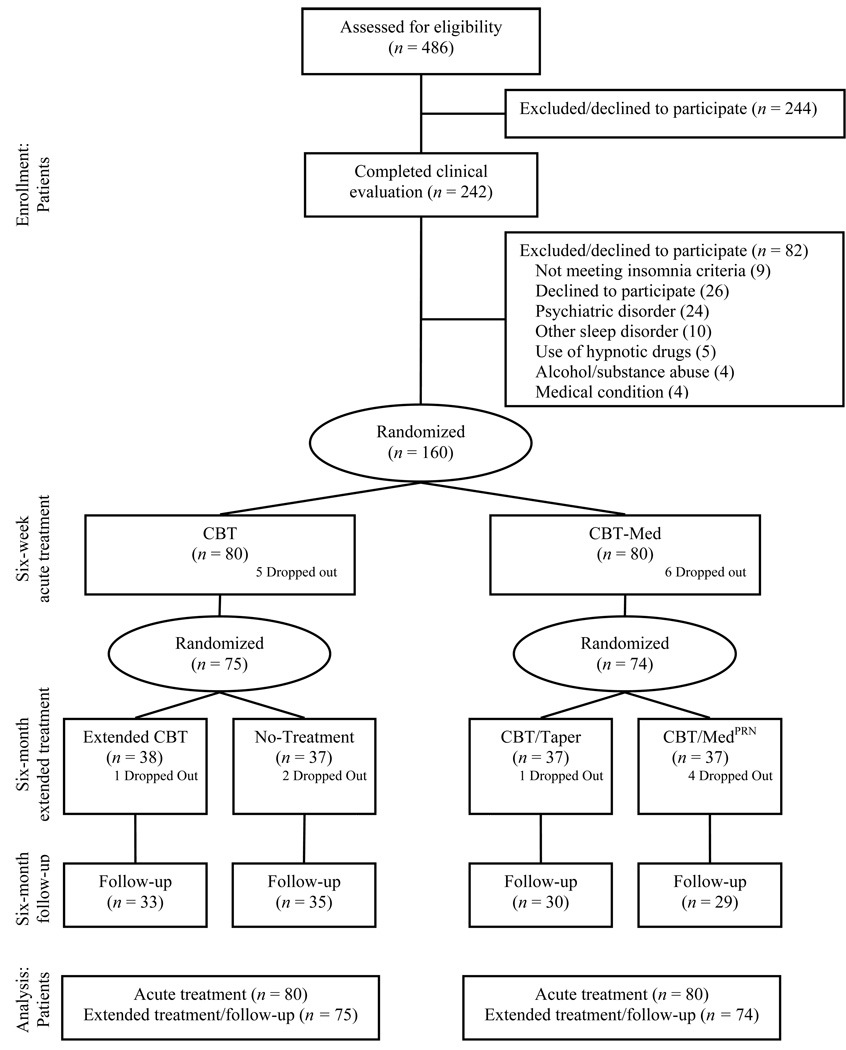
Of the 486 individuals who completed telephone screening for eligibility assessment, 242 completed second-stage screening consisting of (a) a clinical sleep/insomnia evaluation29 ; (b) a Structured Clinical Interview for DSM-IV (SCID-IV) 30 to rule out psychiatric disorders; (c) a medical history and physical examination; and (d) polysomnography (PSG). Eighty-two persons were excluded after this screening for various reasons (see Figure 1).
Figure 1.
Patients flow chart.
Study Design and Assessments
Participants were randomized to one of two acute treatments: (a) cognitive-behavior therapy (CBT; n = 80) or (b) combined cognitive-behavior therapy plus nightly zolpidem medication 10 mg (CBT/Med; n = 80). After completing this six-week initial treatment, they were randomized a second time to an extended treatment for the next six months. CBT patients were randomized to either extended CBT or a no-additional treatment. Patients treated with combined CBT/Med initially were randomized to extended CBT alone with no additional medication (CBT/Taper) or extended CBT and medication, to be used on an “as needed” schedule rather than every night as in the acute treatment phase (CBT/Medprn). Assignment to conditions for each period was determined by a computer-generated random allocation schedule. Assessments were conducted at baseline (pre), at the end of six-week acute (post I) and six-month extended treatment (post II), and six-month follow-up. The protocol was approved by the Université Laval Ethics Committee and all patients provided written informed consent.
Measures
Sleep diaries
Participants kept daily sleep diaries for a two-week baseline period, a six-week acute treatment phase, an for one week prior to each monthly therapy session during the extended treatment phase, and an additional two weeks at follow-up. The primary dependent variables derived from the diaries were sleep onset latency, time awake after sleep onset (including the last awakening prior to rising for the day), total sleep time, and sleep efficiency. The sleep diary is a standard assessment instrument in insomnia research 31, which allows for prospectively monitoring sleep patterns over extended periods of time in the patients' home.
Polysomnography (PSG)
Participants underwent seven nights of sleep laboratory evaluation, including three nights at baseline, two after acute treatment, and two at the end of extended treatment. Bedtime and arising times on those nights were based on usual sleep schedule at home, as determined by participants’ sleep diaries. Standard PSG montage was used 32. Respiration (air flow, tidal volume, and oxygen saturation) and anterior tibialis EMG were monitored during the first night to screen for sleep apnea and periodic limb movements during sleep. Sleep stages were scored visually by experienced technicians, blind to participants’ conditions, and according to standardized criteria 32. Primary dependent variables were sleep latency (lights out to first 5 minutes of consecutive sleep), time awake after sleep-onset, total sleep time, and sleep efficiency (ratio of sleep time to the actual time spent in bed). These variables were averaged over two PSG nights for each assessment phase.
Insomnia Severity Index (ISI)
The ISI 29, 33 is a 7-item patient-reported outcome assessing the severity of initial, middle, late insomnia; sleep satisfaction; interference of insomnia with daytime functioning; noticeability of sleep problems by others; and distress about sleep difficulties. A five-point scale is used to rate each item, yielding a total score ranging from 0 to 28. A higher score indicates more severe insomnia, within four severity categories: absence of insomnia (0–7); sub-threshold insomnia (8–14); moderate insomnia (15–21); severe insomnia (22–28). The ISI has adequate psychometric properties and is sensitive to measure treatment response 33. It was completed at each assessment phase. Patients were considered treatment responders if their ISI change score relative to baseline was greater than 7 and as clinical remitters if their absolute ISI score was smaller than 8.
Clinical Global Impression Scale (CGI)
An independent assessor, blind to participant’s condition, rated the degree of change at each assessment phase on a 0 (unchanged or worse) to 3 (marked improvement) scale.
Treatment Conditions
Cognitive-Behavior Therapy (CBT)
This multi-component intervention features behavioral, cognitive, and educational components 29, 34. The behavioral intervention included recommendations to restrict time in bed to the actual time slept and gradually increasing it back to an optimal sleep time 35. Each patient was prescribed an individualized “sleep window”, which was adjusted weekly. In addition, patients were instructed to: (a) go to bed only when sleepy at night; (b) use the bed and bedroom only for sleep and sex (i.e. no reading, TV watching, or worrying); (c) get out of bed and go in another room whenever unable to fall asleep or return to sleep within 20 minutes; return to bed only when sleepy again; (d) arise at the same time every morning 36. A short daytime nap before 3:00pm was optional in early treatment. Cognitive therapy aimed to alter faulty beliefs and misconceptions about sleep34. Examples of faulty beliefs that were targeted included unrealistic sleep expectations (e.g., the absolute need to sleep 8 hours every night) and amplification of the consequences of insomnia (e.g., all daytime impairments are due to poor sleep). Sleep hygiene education was provided about the effects of caffeine, alcohol, and exercise on sleep, and the effects of noise, light, and excessive temperature. CBT was administered by master’s level clinical psychologists using a treatment manual34.
During acute treatment, patients attended six, weekly, group 90-min therapy sessions. Patients assigned to extended CBT attended six additional monthly, individually-tailored CBT sessions. The focus of these maintenance sessions was on consolidating treatment strategies learned during initial therapy and developing methods for coping with residual insomnia. Their content was based on a case-by-case functional analysis and identification of remaining factors exacerbating sleep disturbances; techniques of relaxation, worry management, and problem-solving were used as needed. Patients assigned to the no additional treatment condition during the extended six-month phase did not have any follow up visits after acute treatment but were asked to complete the same assessment during this period.
Combined CBT plus medication (CBT/Med)
Patients assigned to this condition received CBT (as described above) and zolpidem (10 mg). Zolpidem is a non benzodiazepine receptor-agonist, with a rapid onset of action and a short half-life (mean of 2.5 hours); it is absorbed from the gastrointestinal tract and there is no accumulation during repeated administration; its therapeutic benefits are similar to benzodiazepine-hypnotics but there are fewer residual effects on daytime functioning and minimal rebound insomnia upon discontinuation; in addition, zolpidem produces little alteration of sleep architecture 37, 38. The medication was provided in the context of brief (15–20 min), weekly, consultation sessions with a general practitioner. These sessions focused on reviewing sleep diaries and changes in insomnia symptoms during the previous week, and monitoring potential side effects. Patients were encouraged to comply with the medication regimen but no cognitive-behavioral intervention was allowed during these sessions. The physician used a structured treatment manual with specific guidelines to deliver treatment according to study protocol. Pill count was conducted at each consultation visit.
During the six-week acute treatment, patients were instructed to take the medication nightly, 30 minutes before bedtime. During extended treatment, patients from the combined approach attended six additional, monthly, and individualized CBT sessions as described above. In addition, those assigned to extended CBT plus medication (CBT/Medprn) met with the physician monthly and received 10 zolpidem pills per month with the instruction to use their medication only when it was needed (as opposed to nightly during initial therapy). Unused medications were returned at each visit. At the end of the extended six-month therapy, medication was tapered as described below. Patients assigned to CBT without medication (CBT/Taper) received their last medication supply with a written withdrawal schedule. They were instructed to decrease the dose from 10 mg to 5 mg during the first week and then to take 5 mg every other night until they ran out of medication. Patients were informed of possible rebound insomnia during withdrawal and these concerns were addressed during extended CBT.
Data Management and Analysis
Sample size was based on a power analysis conducted for sleep efficiency and ISI scores. Effect sizes were estimated from previous studies14, 18, 39. Comparisons between CBT and CBT/Med yielded an effect size of 0.55 for sleep efficiency (difference of 5 points) and 0.82 for the ISI score (difference of 4 points). With a sample size of 80 subjects per condition during acute treatment and a 5% alpha (two-tailed), power was estimated at 94% and 98% to detect effect sizes of that magnitude40.
Descriptive and inferential statistics were completed using SAS 9.1.3 statistical software 41. Since subjects were randomized on two occasions, two sets of analyses were performed. The first set was based on a 2 (conditions) X 2 (times; baseline, posttreatment I) split-plot randomized design and the second set was based on a 4 (conditions) X 4 (times; baseline, posttreatment I and II, follow-up) split-plot randomized design. All analyzes were based on an intent-to-treat paradigm. To avoid imputation of missing data, linear mixed models 42 were used to test group, time, and interaction effects for continuous dependent variables and generalized linear mixed models 43 were used for binary dependent variables. A priori contrasts were used to investigate specific hypotheses, such as pre-post differences and maintenance of treatment gains at follow-up. To control for multiple comparisons, a “per family” error rate was adopted, where all comparisons for each dependent variable were performed within the nominal error rate. The simultaneous test procedure for factorial design44 was used to compute the appropriate corrected alpha level: 5% for main effects and interactions, 5% for temporal changes during acute treatment and 2% for temporal changes after extended treatment and follow-up. Confidence intervals were computed at 95% for all tests and temporal changes that failed to reach significance after correction were labelled accordingly.
Results
Participants
The sample included 160 adults (97 women, 63 men) with a mean age of 50.3 years old (SD = 10.1; range = 30 to 72) and a mean education of 14.7 years (SD = 3.5). All patients were Caucasian, predominantly married or in a common-law relationship (68.1%), and employed (73.3%). The majority (73.8%) reported mixed sleep-onset and maintenance insomnia. The average insomnia duration was 16.4 years (SD = 13.6). Although all patients were sleep-medication free prior to entering the study, 63 patients (39.4%) had previously used sleep medication. In terms of comorbidity, 24 patients (15.4% of sample) presented a comorbid psychiatric disorder (most commonly an anxiety disorder) and 92 patients (57.5%) presented at least one comorbid medical disorder (most commonly a cardiovascular condition). Descriptive data of demographic and clinical variables are summarized in Table 1.
Table 1.
Sociodemographic and Clinical Characteristics of Participants
| Characteristic | Cognitive Behavior Therapy (n = 80) |
Combined Cognitive Behavior Therapy and Medication (n = 80) |
Total Sample (N = 160) |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 51.73 | 10.75 | 48.79 | 9.74 | 50.26 | 10.13 |
| Education (years) | 14.74 | 3.64 | 14.67 | 3.47 | 14.70 | 3.54 |
| n | % | N | % | N | % | |
| Gender | ||||||
| Female | 50 | 62.50 | 47 | 58.75 | 97 | 60.63 |
| Male | 30 | 37.50 | 33 | 41.25 | 63 | 39.37 |
| Occupation (%) | ||||||
| Employed | 53 | 68.83 | 62 | 77.50 | 115 | 73.25 |
| Retired | 22 | 28.57 | 16 | 20.00 | 38 | 24.20 |
| Homemaker | 2 | 2.60 | 0 | 0.00 | 2 | 1.27 |
| Unemployed | 0 | 0.00 | 2 | 2.50 | 2 | 1.27 |
| Marital Status (%) | ||||||
| Single | 4 | 5.00 | 11 | 13.75 | 15 | 9.38 |
| Married/common law | 57 | 71.25 | 52 | 65.00 | 109 | 68.13 |
| Divorced / separated | 15 | 18.75 | 12 | 15.00 | 27 | 16.88 |
| Widowed | 4 | 5.00 | 5 | 6.25 | 9 | 5.63 |
| Mean | SD | Mean | SD | Mean | SD | |
| Insomnia duration (years) | 17.50 | 15.17 | 15.26 | 11.90 | 16.38 | 13.64 |
| n | % | N | % | N | % | |
| Type of insomnia | ||||||
| Initial | 3 | 3.75 | 1 | 1.25 | 4 | 2.50 |
| Middle | 17 | 21.25 | 19 | 23.75 | 36 | 22.50 |
| Late | 1 | 1.25 | 1 | 1.25 | 2 | 1.25 |
| Mixed | 59 | 73.75 | 59 | 73.75 | 118 | 73.75 |
| Comborbidity | ||||||
| Medical | 48 | 60.0 | 44 | 55.0 | 92 | 57.5 |
| Psychiatric | 11 | 14.1 | 13 | 16.7 | 24 | 15.4 |
| Prior hypnotic usage | 34 | 42.5 | 29 | 36.3 | 63 | 39.4 |
Treatment Attrition and Integrity
The overall attrition rate was 6.9% after acute treatment (n = 11), 11.9% after extended treatment (n = 19), and 20.6% at 6-month follow-up (n = 33). Attrition was not significantly different between groups and, except for more men than women dropping out of acute treatment, there was no significant difference between treatment completers and those who dropped out on demographic, clinical, or sleep/insomnia variables.
The mean number of therapy sessions attended during acute treatment was 5.6 for CBT and 5.8 for CBT/Med conditions and, during extended treatment, it was 5.5 for extended CBT alone, 5.4 for CBT/Taper, and 5.5 for CBT/Medprn. Records from pill counts revealed that compliance decreased over time during acute treatment, as 90.9% of pills were taken during the 1st week and 79.1% during the 6th week, F(5,361) = 6.28, P < .001. During extended treatment, patients in the CBT/Medprn used an average of 52.8% of available medication each month, with a decrease (non significant) from 58.6% in the first to 43.9% in the sixth month.
Sleep diary data
Means, change scores, and Cohen’s d values for sleep diary variables are displayed in Table 2. After the acute treatment phase, simple effects revealed significant reductions (from pre to post I) of sleep latency for both CBT (−19.9 min, CI= −26.1,−13.6) and CBT/Med groups (−11.9 min, CI= −18.2,−5.6). For time awake after sleep onset, significant and larger reductions were also observed for both CBT (−68.7 min, CI= −79.7,−57.8) and CBT/Med groups (−82.9 min, CI= −94.0, −71.9). A small and non significant decrease of total sleep time was observed for the CBT condition (−5.6 min) while an increase (10.3) was noted in the CBT/Med condition. For sleep efficiency, large and significant increases were observed in both CBT (14.4%, CI= 11.8, 16.9) and CBT/Med conditions (15.8%, CI= 13.2,18.4). Finally, tests for interactions showed that the combined condition produced a significantly greater increase of total sleep time relative to patients treated with CBT alone during acute treatment (15.9 min difference, CI= 1.0, 30.8).
Table 2.
Means and standard errors (SE) for sleep parameters as measured by daily sleep diaries
| Acute treatment |
Extended treatment |
Follow-up |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre |
Post I |
Post II |
6-months |
||||||||
| Condition |
Mean (SE) |
Mean (SE) |
Change (95% CI) |
d | Condition |
Mean (SE) |
Change (95% CI) |
d |
Mean (SE) |
Change (95% CI) |
d |
| Sleep onset latency (min) | |||||||||||
| CBT | 37.18 (2.69) |
17.32 (2.77) |
−19.9 (−26.1, −3.6) |
−0.83 | CBT | 18.91 (2.53) |
3.6 (−3.4, 10.5) |
0.17 | 16.43 (2.08) |
−1.8 (−9.1, 5.5) |
−0.09 |
| No txt | 22.41 (2.63) |
3.6 (−3.7, 10.8) |
0.17 | 18.30 (2.04) |
−4.1 (−11.5, 3.3) |
−0.20 | |||||
| Combined CBT/Med |
29.73 (2.69) |
17.80 (2.80) |
−11.9 (−18.2, −5.6) |
−0.50 | CBT/ Taper |
18.27 (2.58) |
0.2 (−7.0, 7.3) |
0.01 | 14.07 (2.12) |
−3.9 (−11.4, 3.7) |
−0.19 |
| CBT/ Medprn |
15.23 (2.66) |
−2.4 (−9.8, 5.0) |
−0.12 | 16.19 (2.18) |
1.1 (−6.8, 8.9) |
0.05 | |||||
| Wake after sleep onset (min) | |||||||||||
| CBT | 116.50 (4.93) |
47.79 (5.07) |
−68.7 (−79.7, −57.8) |
−1.56 | CBT | 61.63 (5.43) |
12.7* (0.4, 25.0) |
0.32 | 56.05 (5.53) |
−5.4 (−18.3, 7.5) |
−0.13 |
| No txt | 58.99 (5.61) |
13.6* (0.7, 26.5) |
0.34 | 62.68 (5.45) |
3.3 (−9.8, 16.3) |
0.08 | |||||
| Combined CBT+Med |
128.55 (4.93) |
45.62 (5.13) |
−82.9 (−94.0, −71.9) |
−1.88 | CBT/ Taper |
48.16 (5.52) |
6.3 (−6.4, 18.9) |
0.16 | 47.21 (5.64) |
−1.4 (−14.7, 12.0) |
−0.03 |
| CBT/ Medprn |
65.82 (5.68) |
15.9 (2.8, 28.9) |
0.39 | 63.90 (5.77) |
−2.1 (−16.0, 11.9) |
−0.05 | |||||
| Total sleep time (min) | |||||||||||
| CBT | 343.97 (7.06) |
338.38 (7.17) |
−5.6 (−16.1, 4.9) |
−0.09 | CBT | 363.41 (8.40) |
26.3 (11.2, 41.4) |
0.43 | 382.70 (10.25) |
21.0 (5.2, 36.7) |
0.34 |
| No txt | 385.34 (8.61) |
41.5 (25.7, 57.2) |
0.68 | 388.77 (10.14) |
5.0 (−11.0, 21.0) |
0.08 | |||||
| Combined CBT+Med |
348.87 (7.06) |
359.14 (7.22) |
10.3 (−0.3, 20.9) |
0.16 | CBT/ Taper |
390.82 (8.50) |
27.3 (11.9, 42.8) |
0.45 | 399.26 (10.41) |
9.5 (−6.9, 25.9) |
0.16 |
| CBT/ Medprn |
372.54 (8.69) |
18.1* (2.1, 34.1) |
0.30 | 390.81 (10.59) |
17.8* (0.8, 34.8) |
0.29 | |||||
| Sleep efficiency (%) | |||||||||||
| CBT | 68.99 (1.32) |
83.36 (1.35) |
14.4 (11.8, 16.9) |
1.22 | CBT | 81.43 (1.48) |
−2.0 (−5.0, 1.1) |
−0.18 | 83.81 (1.48) |
2.3 (−1.0, 5.5) |
0.21 |
| No txt | 82.38 (1.53) |
−1.6 (−4.8, 1.6) |
−0.15 | 82.50 (1.46) |
0.2 (−3.0, 3.5) |
0.02 | |||||
| Combined CBT/Med |
68.64 (1.32) |
84.46 (1.37) |
15.8 (13.3, 18.4) |
1.34 | CBT/ Taper |
85.49 (1.51) |
0.1 (−3.1, 3.2) |
0.01 | 86.68 (1.50) |
1.2 (−2.1, 4.6) |
0.11 |
| CBT/ Medprn |
82.04 (1.55) |
−1.4 (−4.7, 1.8) |
−0.13 | 82.68 (1.54) |
0.6 (−2.8. 4.1) |
0.06 | |||||
Note. * These comparisons were no longer significant after applying a correction (P values between 0.02 and 0.05).
After the extended phase of treatment, simple effects revealed no significant change of sleep latency for all four conditions from the end of acute (post I) to the end of extended treatment (post II). For time awake after sleep onset, three conditions exhibited small increases but only one was significant after applying alpha corrections (CBT/Medprn group, 15.9 min, CI=2.8, 28.9); change for the CBT/Taper group (6.4 min) for this same period was not significant. For total sleep time, significant increases were observed for three of the four conditions: extended CBT (26.3 min, CI=11.2, 41.4), CBT/no additional treatment (41.5 min, CI=25.7, 57.2), CBT/Taper (27.3 min, CI=11.9, 42.8). For sleep efficiency, no significant change was observed. Tests for interactions revealed no significant group differences in the magnitude of changes occurring on any of these variables during extended treatment.
Follow-Up Analyses. Comparisons of 6-month follow-up data with Post II data showed no further changes for any variable except for total sleep time which was further increased in the extended CBT (21 min, CI=5.2, 36.7). Thus, sleep improvements achieved with treatment were well maintained over time.
Polysomnography data
Means, change scores and Cohen’s d values for PSG sleep variables are displayed in Table 3. With acute treatment, there was a significant, albeit modest, reduction of sleep latency for both the CBT (−6.4 min, CI= −9.1,−3.8) and CBT/Med groups (−2.8 min, CI= −5.5, −0.2). Significant and larger reductions of time awake after sleep onset were observed for both CBT (−27.2 min, CI= −34.9, −19.5) and CBT/Med groups (−27.1 min, CI= −34.8, −19.3). For total sleep time, significant decreases were observed for both CBT (−25.9 min, CI= −34.9, −16.8) and CBT/Med conditions (−18.5 min, CI= −27.6, −9.4). For sleep efficiency, significant increases were observed in both CBT (5.5%, CI= 3.8, 7.2) and CBT/Med conditions (5.0%, CI= 3.3, 6.7). Tests for interactions revealed no differential treatment effects during acute treatment.
Table 3.
Means and standard errors (SE) for sleep parameters as measured by polysomnography.
| Acute treatment |
Extended treatment |
|||||||
|---|---|---|---|---|---|---|---|---|
| Pre |
Post I |
Post II |
||||||
| Condition |
Mean (SE) |
Mean (SE) |
Change (95% CI) |
d | Condition |
Mean (SE) |
Change (95% CI) |
d |
| Sleep onset latency (min) | ||||||||
| CBT | 17.22 (1.11) |
10.80 (1.15) |
−6.4 (−9.1, −3.8) |
−0.64 | CBT | 10.91 (1.57) |
−0.40 (−4.1, 3.3) |
−0.04 |
| No txt | 15.56 (1.62) |
5.22 (1.4, 9.0) |
0.56 | |||||
| Combined CBT/Med |
14.03 (1.12) |
11.21 (1.16) |
−2.8 (−5.5, −0.2) |
−0.28 | CBT/ Taper |
9.31 (1.57) |
−1.8 (−5.5, 1.9) |
−0.19 |
| CBT/ Medprn |
12.80 (1.69) |
1.4 (−2.5, 5.3) |
0.15 | |||||
| Wake after sleep onset (min) | ||||||||
| CBT | 64.51 (3.57) |
37.34 (3.68) |
−27.2 (−34.9, −19.5) |
−0.85 | CBT | 48.93 (4.80) |
10.6* (0.0, 21.3) |
0.34 |
| No txt | 43.42 (4.91) |
7.1 (−3.9, 18.1) |
0.23 | |||||
| Combined CBT/Med |
61.21 (3.59) |
34.15 (3.71) |
−27.1 (−34.8, −19.3) |
−0.85 | CBT/ Taper |
47.79 (4.81) |
15.4 (4.8, 26.1) |
0.50 |
| CBT/ Medprn |
53.11 (5.10) |
16.9 (5.6, 28.2) |
0.54 | |||||
| Total sleep time (min) | ||||||||
| CBT | 371.42 (4.77) |
345.58 (4.90) |
−25.9 (−34.9, −16.8) |
−0.61 | CBT | 348.40 (7.40) |
8.7 (−5.8, 23.1) |
0.20 |
| No txt | 361.84 (7.57) |
10.4 (−4.6, 25.3) |
0.24 | |||||
| Combined CBT/Med |
377.62 (4.80) |
359.10 (4.93) |
−18.5 (−27.6, −9.4) |
−0.43 | CBT/ Taper |
362.78 (7.41) |
2.3 (−12.2, 16.8) |
0.05 |
| CBT/ Medprn |
343.64 (7.86) |
−14.2 (−29.6, 1.1) |
−0.33 | |||||
| Sleep efficiency (%) | ||||||||
| CBT | 82.30 (0.86) |
87.76 (0.88) |
5.5 (3.8, 7.2) |
0.71 | CBT | 85.12 (1.16) |
−2.1 (−4.6, 0.4) |
−0.28 |
| No txt | 86.22 (1.19) |
−2.1 (−4.6, 0.4) |
−0.28 | |||||
| Combined CBT/Med |
83.72 (0.86) |
88.71 (0.89) |
5.0 (3.3, 6.7) |
0.65 | CBT/ Taper |
86.41 (1.17) |
−2.7* (−5.2, −0.2) |
−0.36 |
| CBT/ Medprn |
84.12 (1.24) |
−4.2 (−6.8, −1.5) |
−0.56 | |||||
Note. * These comparisons were no longer significant after applying a correction (P values between 0.02 and 0.05).
After the extended phase of treatment, simple effects revealed a significant increase of sleep latency for the CBT/no additional treatment group (5.2 min, CI= 1.4, 9.0), and no significant changes for the two combined conditions. For time awake after sleep onset, there was no further significant change for the CBT with or without additional treatment. However, both extended conditions of the CBT/Med group reported similar significant increases of time awake after sleep onset for CBT/Taper (15.4 min, CI=4.8, 26.6) and for CBT/Medprn (16.9 min, CI=5.6, 28.2). For total sleep time, no significant changes were observed for any of the group. For sleep efficiency, only the CBT/Medprn condition showed a significant worsening over time (−4.2%, CI= −6.8, −1.5). Tests for interactions revealed a greater increase of sleep latency in the CBT with no additional treatment compared to the extended CBT group (5.6% difference, CI= 0.3, 10.9).
Treatment response and remission rates
Insomnia Severity Index
ISI descriptive statistics and Cohen’s d values are displayed in Table 4. Significant decreases in insomnia severity were observed in both CBT (−8.3 units, CI= −9.4, −7.2) and CBT/Med conditions (−8.8 units, CI= −9.9, −7.7) during acute treatment. No additional change was observed during extended treatment or follow up for any of the group. Mean baseline ISI scores (17.5) were in the moderate severity range before treatment, fell below clinical threshold (8.9) after acute treatment, and either remained within that range after extended treatment or fell further down within the no insomnia category (ISI score < 8) for CBT/Taper.
Table 4.
Insomnia Severity Index means and standard errors (SE) and percentages of treatment responders and remitters
| Acute treatment |
Extended treatment |
Follow-up |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre |
Post-I |
Post-II |
6-months |
||||||||
| Condition |
Mean (SE) |
Mean (SE) |
Change (95% CI) |
d | Condition |
Mean (SE) |
Change (95% CI) |
d |
Mean (SE) |
Change (95% CI) |
d |
| ISI (patient) raw score | |||||||||||
| CBT | 17.26 (0.47) |
8.94 (0.48) |
−8.3 (−9.4, −7.2) |
−2.00 | CBT | 8.68 (0.73) |
−1.0 (−2.5, 0.6) |
−0.23 | 8.94 (0.71) |
0.3 (−1.3, 1.9) |
0.06 |
| No txt | 8.11 (0.75) |
−0.2 (−1.8, 1.4) |
−0.04 | 8.85 (0.69) |
0.7 (−0.8, 2.3) |
0.18 | |||||
| Combined CBT/Med |
17.55 (0.47) |
8.76 (0.49) |
−8.8 (−9.9, −7.7) |
−2.11 | CBT/ Taper |
6.95 (0.74) |
−0.4 (−2.0, 1.2) |
−0.09 | 5.82 (0.74) |
−1.1 (−2.8, 0.6) |
−0.26 |
| CBT/ Medprn |
8.71 (0.76) |
−1.4 (−3.0, 0.2) |
−0.32 | 8.77 (0.75) |
0.0 (−1.7, 1.7) |
0.01 | |||||
|
% (n) |
Condition |
% (n) |
OR (95% CI) |
% (n) |
OR (95% CI) |
||||||
| Treatment responders (ISI reductions ≥ 8 compared to baseline ISI) | |||||||||||
| CBT | -- | 59.46* (45/75) |
-- | CBT | 62.87 (24/38) |
1.23 (0.62,2.45) |
62.80 (21/33) |
1.00 (0.45,2.20) |
|||
| No txt | 55.01 (20/37) |
0.76 (0.35,1.67) |
57.15 (20/35) |
1.09 (0.54,2.22) |
|||||||
| Combined CBT/Med |
-- | 61.11 (45/74) |
-- | CBT/ Taper |
73.88 (27/37) |
1.26 (0.44,3.49) |
80.86 (24/30) |
1.49 (0.62,3.61) |
|||
| CBT/ Medprn |
69.56 (26/37) |
1.94 (0.78,4.85) |
64.87 (19/29) |
0.81 (0.31,1.91) |
|||||||
| Treatment remitters (ISI < 8) | |||||||||||
| CBT | 0.0 | 39.19 (29/75) |
-- | CBT | 43.87 (17/38) |
1.92 (0.78,4.74) |
43.89 (14/33) |
1.00 (0.51,1.97) |
|||
| No txt | 45.29 (17/37) |
0.85 (0.34,2.14) |
40.28 (14/35) |
0.81 (0.37,1.79) |
|||||||
| Combined CBT/Med |
0.0 | 44.44 (33/74) |
-- | CBT/ Taper |
56.89 (21/37) |
0.85 (0.43,1.66) |
67.78 (20/30) |
1.59 (0.78,3.27) |
|||
| CBT/ Medprn |
59.76 (22/37) |
3.50 (1.53,8.04) |
41.73 (12/29) |
0.48 (0.25,0.93) |
|||||||
Note. These “estimated” percentages, derived from generalized linear mixed models, are adjusted for missing data and may not correspond exactly to the actual frequencies.
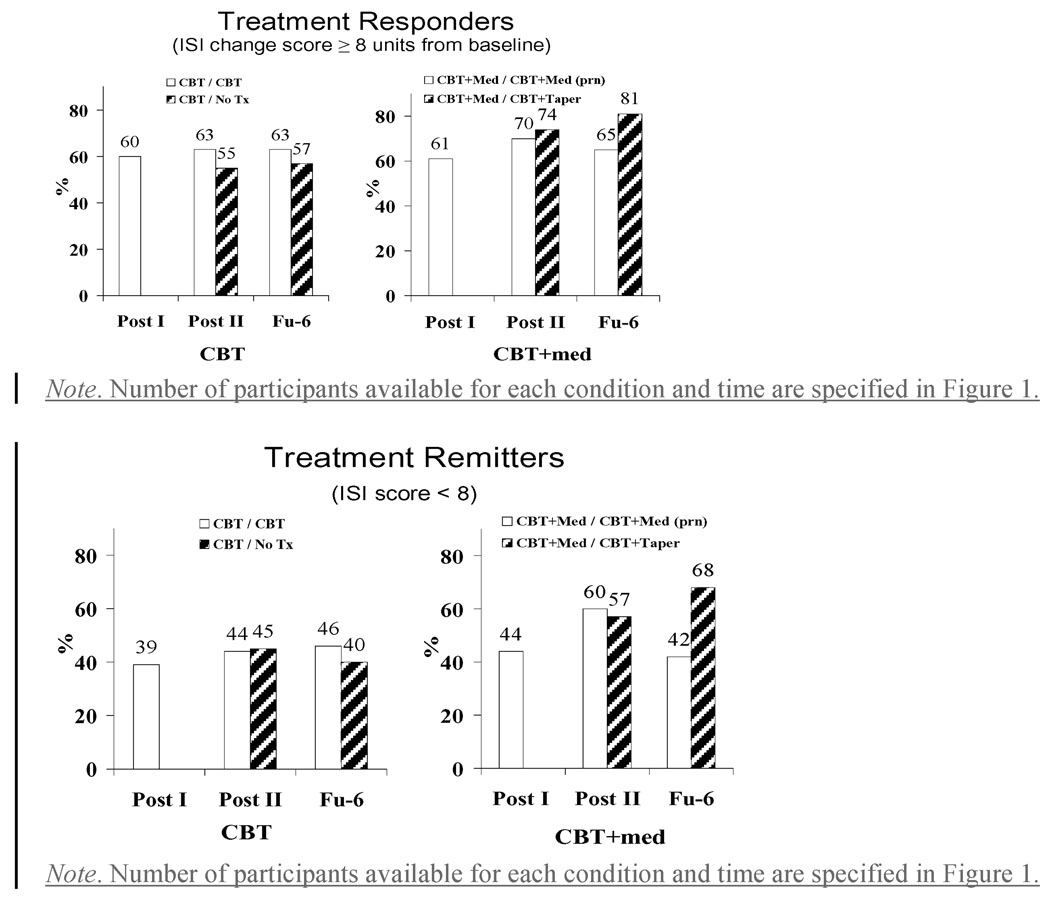
A treatment responders’ analysis was conducted on response and remission rates, which were compared across conditions and time using generalized liner mixed models. Patients were considered treatment responders if their ISI change score relative to baseline was greater than 7 (equivalent to one category on the ISI) and remitters if their absolute ISI score was smaller than 8 (i.e., no insomnia category). The proportions of responders (59.5% for CBT and 61.1% for CBT/Med, P = .84) and remitters (39.2% for CBT and 44.4% for CBT/Med, P = .52) were equivalent in the two groups after the acute treatment phase (see Figure 2). After the extended phase, no significant time effect was found but a significant group X time interaction was obtained for remission, F(6,253) = 2.16, P = .05. Simple effects revealed that remission rates increased steadily over time for the CBT/Taper condition, from 44.4% after acute treatment to 56.9% after extended treatment, to 67.8% at 6-month follow-up, while the increased remission rates observed for the CBT/Medprn condition after extended treatment (from 44.4% to 59.8%) disappeared at follow-up (41.7%), resulting in a different trajectory of change between both conditions of the CBT/Med treatment arm at 6-month follow-up, F(1,253) = 4.19, P = .04. No other contrasts between treatment arms were significant (Ps ranging from 0.15 to 0.91).
Figure 2.
Proportions of treatment responders and remitters according to treatment condition and assessment phase.
Clinical Global Improvement
After the acute treatment phase, 89.2% (66/74) of CBT patients and 83.3% (60/72) of the CBT/Med patients were rated as moderately or markedly improved by an independent assessor; this group difference was not significant. After extended treatment, the number of patients rated as moderately or markedly improved were as follows: CBT (29/34; 85%), CBT/no additional treatment (29/33; 87.9%), CBT/Taper (28/32; 87.5%) and CBT/Medprn (19/26; 73.1%). No significant group differences were found among the four groups.
Discussion
The findings indicate that CBT, used singly or in combination with medication, was effective for treating persistent insomnia. The addition of medication produced some added benefits, albeit modest, to outcomes during the acute treatment phase, mostly in terms of increased sleep time. Overall, 60% of patients achieved a treatment response and 42% were in remission after the acute treatment phase and these rates increased to 65% and 51% after extended treatment. Patients treated with a combined approach during acute treatment achieved better long-term outcomes when medication was discontinued after the initial six-week trial. In general, sleep improvements were well sustained over time. Generalization of the present findings should be cautious as all patients were Caucasians and less than 10% were older than 65 years of age. Otherwise, gender, education, insomnia severity, and presence of medical or psychiatric comorbidity did not moderate treatment response.
Previous studies combining CBT with medication11, 14, 17 have reported either no added value or only a slight advantage over single therapy alone. The present findings suggest that combining medication with CBT may provide an added benefit during the initial course of therapy, but the clinical significance of such added benefit is unclear. Although a gain of 15 min in sleep time is marginal, it may nonetheless be important given that CBT typically involves restricting time spent in bed and produces an initial reduction of sleep time. When combined with medication as in the present study, CBT did not reduce sleep time and may actually have enhanced compliance with the CBT regimen. When outcome was measured in terms of treatment response and remission rates, there was no added value in combining medication with CBT, at least during initial treatment.
Examination of different maintenance treatment regimens showed that extended, individualized CBT, did not add significant benefits to the initial group CBT. Although this finding was unexpected, it is plausible that some patients had already reached a ceiling effect with the initial CBT trial and there was probably no need for additional therapy. Conversely, for patients who still presented residual insomnia, a more intense treatment regimen (bi-monthly CBT visits), or a switch to a different therapy (medication) could have enhanced outcome. For the combined treatment arm, patients who discontinued their medication after the initial course of therapy did better than those who continued using medication on an intermittent schedule. This result is not consistent with some evidence 24, 25 suggesting that intermittent medication use fosters long-term maintenance of therapeutic benefits. On the other hand, following combined therapy, it makes good clinical practice to discontinue medication while patients are still in CBT. Such practice would minimize drug exposure and risk for dependence with long-term medication and would provide patients with more time to integrate newly learned psychological and behavioral skills to overcome insomnia 16. Thus, this sequential regimen would seem preferable to a combined approach in which both therapies are initiated and discontinued at the same time, a common practice in most previous studies 14, 15, 17.
The present study evaluated only three maintenance strategies and it is plausible that other treatment sequences might prove more effective. For instance, patients who fail to achieve an adequate response (or remission) during initial therapy could receive a second level therapy that might involve either adding or switching to a different treatment modality. A patient treated with CBT initially would be switched to medication whereas someone treated with medication initially would switch to CBT or to another class of hypnotic medication. When selecting initial treatment in clinical practice, it may also be necessary to take into account practical (availability and acceptability of different treatment options) and clinical considerations (acute vs. chronic insomnia, prior treatment exposure, comorbidity), as well as evidence of efficacy 45–47.
Although the present findings are promising, there is currently no treatment that works for every patient with insomnia and additional studies are needed to develop treatment algorithms to guide practitioners in the clinical management of insomnia48. Questions of clinical and scientific interest for further investigations include whether CBT or medication should be the first line therapy for persistent insomnia and how best to proceed with second line treatment for those who do not respond to initial treatment. Additional studies are also needed to examine how best to integrate CBT and medication as a function of insomnia severity, prior treatment exposure, psychological and medical comorbidity, and patient’s preference.
Acknowledgements
Research supported by a grant from the National Institute of Mental Health (MH#60413). This was not an industry-supported study. Dr. Morin has served as a consultant to Actelion, Lundbeck, Sanofi-Aventis, Sepracor, and Schering-Plough. Thanks to all therapists (Véronique Mimeault, Sonia Boivin, Geneviève Belleville, Catherine Guay) and the staff of the Sleep Research Center (Manon Lamy, Sonia Petit, Claude Tremblay, Denis Chapdelaine, Amélie Bernier) for their contribution to this study.
Footnotes
Trial registration: www.clinicaltrials.gov (#NCT 00042146)
References
- 1.Morin CM, LeBlanc M, Daley M, Gregoire JP, Merette C. Epidemiology of insomnia: Prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Medicine. 2006 Mar;7(2):123–130. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Ohayon MM. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Medicine Reviews. 2002;6(2):97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 3.Daley M, Morin CM, Leblanc M, Gregoire JP, Savard J, Baillargeon L. Insomnia and its relationship to health-care utilization, work absenteeism, productivity and accidents. Sleep Medicine. 2008 Aug 25; doi: 10.1016/j.sleep.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? Journal of the American Medical Association. 1989;262(11):1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 5.Ozminkowski RJ, Wang S, Walsh JK. The direct and indirect costs of untreated insomnia in adults in the United States. Sleep. 2007 Mar 1;30(3):263–273. doi: 10.1093/sleep/30.3.263. [DOI] [PubMed] [Google Scholar]
- 6.Simon GE, VonKorff M. Prevalence, burden, and treatment of insomnia in primary care. American Journal of Psychiatry. 1997;154(10):1417–1423. doi: 10.1176/ajp.154.10.1417. [DOI] [PubMed] [Google Scholar]
- 7.Suka M, Yoshida K, Sugimori H. Persistent insomnia is a predictor of hypertension in Japanese male workers. Journal of occupational health. 2003 Nov;45(6):344–350. doi: 10.1539/joh.45.344. [DOI] [PubMed] [Google Scholar]
- 8.National Institutes of Health. National Institutes of Health State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults, June 13–15, 2005. Sleep. 2005 Sep 1;28(9):1049–1057. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- 9.Daley M, Morin CM, Leblanc M, Gregoire JP, Savard J. The economic burden of insomnia: Direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms and good sleepers. Sleep. In press. [PMC free article] [PubMed] [Google Scholar]
- 10.Hauri PJ. Can we mix behavioral therapy with hypnotics when treating insomniacs? Sleep. 1997;20(12):1111–1118. doi: 10.1093/sleep/20.12.1111. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs GD, Pace-Schott EF, Stickgold R, Otto MW. Cognitive behavior therapy and pharmacotherapy for insomnia: A randomized controlled trial and direct comparison. Archives of internal medicine. 2004 Sep 27;164(17):1888–1896. doi: 10.1001/archinte.164.17.1888. [DOI] [PubMed] [Google Scholar]
- 12.McClusky HY, Milby JB, Switzer PK, Williams V, Wooten V. Efficacy of behavioral versus triazolam treatment in persistent sleep-onset insomnia. American Journal of Psychiatry. 1991;148(1):121–126. doi: 10.1176/ajp.148.1.121. [DOI] [PubMed] [Google Scholar]
- 13.Milby JB, Williams V, Hall JN, Khuder S, McGill T, Wooten V. Effectiveness of combined triazolam-behavioral therapy for primary insomnia. American Journal of Psychiatry. 1993;150(8):1259–1260. doi: 10.1176/ajp.150.8.1259. [DOI] [PubMed] [Google Scholar]
- 14.Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: A randomized controlled trial. Journal of the American Medical Association. 1999 Mar 17;281(11):991–999. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 15.Sivertsen B, Omvik S, Pallesen S, et al. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: A randomized controlled trial. Journal of the American Medical Association. 2006 Jun 28;295(24):2851–2858. doi: 10.1001/jama.295.24.2851. [DOI] [PubMed] [Google Scholar]
- 16.Vallieres A, Morin CM, Guay B. Sequential combinations of drug and cognitive behavioral therapy for chronic insomnia: An exploratory study. Behaviour research and therapy. 2005 Dec;43(12):1611–1630. doi: 10.1016/j.brat.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Wu R, Bao J, Zhang C, Deng J, Long C. Comparison of sleep condition and sleep-related psychological activity after cognitive-behavior and pharmacological therapy for chronic insomnia. Psychotherapy and psychosomatics. 2006;75(4):220–228. doi: 10.1159/000092892. [DOI] [PubMed] [Google Scholar]
- 18.Nowell PD, Mazumdar S, Buysse DJ, Dew MA, Reynolds CF, 3rd, Kupfer DJ. Benzodiazepines and zolpidem for chronic insomnia: A meta-analysis of treatment efficacy. Journal of the American Medical Association. 1997;278(24):2170–2177. [PubMed] [Google Scholar]
- 19.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998–2004) Sleep. 2006 Nov 1;29(11):1398–1414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 20.Edinger JD, Wohlgemuth WK, Radtke RA, Coffman CJ, Carney CE. Dose-response effects of cognitive-behavioral insomnia therapy: A randomized clinical trial. Sleep. 2007 Feb 1;30(2):203–212. doi: 10.1093/sleep/30.2.203. [DOI] [PubMed] [Google Scholar]
- 21.Espie CA, Fleming L, Cassidy J, et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. Journal of clinical oncology. 2008 Oct 1;26(28):4651–4658. doi: 10.1200/JCO.2007.13.9006. [DOI] [PubMed] [Google Scholar]
- 22.Morin CM, Belanger L, Leblanc M, et al. The natural history of insomnia: A population-based, three-year longitudinal study. Archives of Internal Medicine. doi: 10.1001/archinternmed.2008.610. In press. [DOI] [PubMed] [Google Scholar]
- 23.Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rossler W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008 Apr 1;31(4):473–480. doi: 10.1093/sleep/31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajak G, Cluydts R, Allain H, et al. The challenge of chronic insomnia: is non-nightly hypnotic treatment a feasible alternative? European psychiatry. 2003 Aug;18(5):201–208. doi: 10.1016/s0924-9338(03)00089-0. [DOI] [PubMed] [Google Scholar]
- 25.Perlis ML, McCall WV, Krystal AD, Walsh JK. Long-term, non-nightly administration of zolpidem in the treatment of patients with primary insomnia. The Journal of clinical psychiatry. 2004 Aug;65(8):1128–1137. doi: 10.4088/jcp.v65n0816. [DOI] [PubMed] [Google Scholar]
- 26.Roth T, Franklin M, Bramley TJ. The state of insomnia and emerging trends. American Journal of Managed Care. 2007 Nov;13(5 Suppl):S117–S120. [PubMed] [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 28.American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manuel. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 29.Morin CM. Insomnia : Psychological assessment and management. New York: Guilford Press; 1993. [Google Scholar]
- 30.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition (SCID-I/P, Version 2.0) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1997. [Google Scholar]
- 31.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006 Sep 1;29(9):1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 32.Rechtschaffen A, Kales A. Washington DC: US Government Printing Office. National Institute of Health Publication; A manual of standarized terminology, techniques and scoring system for sleep stages in human subjects. 1968
- 33.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 34.Morin CM, Espie CA. Insomnia: A clinical guide to assessment and treatment. New York: Kluwer Academic/Plenum; 2003. [Google Scholar]
- 35.Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10(1):45–56. [PubMed] [Google Scholar]
- 36.Bootzin RR, Epstein D, Wood JM. Stimulus control instructions. In: Hauri P, editor. Case studies in insomnia. New York: Plenum Press; 1991. pp. 19–28. [Google Scholar]
- 37.Buysse DJ. Chronic insomnia. The American journal of psychiatry. 2008 Jun;165(6):678–686. doi: 10.1176/appi.ajp.2008.08010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curry DT, Eisenstein RD, Walsh JK. Pharmacologic management of insomnia: past, present, and future. Psychiatr Clin North Am. 2006 Dec;29(4):871–893. doi: 10.1016/j.psc.2006.09.006. abstract vii–viii. [DOI] [PubMed] [Google Scholar]
- 39.Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: A meta-analysis of treatment efficacy. American Journal of Psychiatry. 1994;151(8):1172–1180. doi: 10.1176/ajp.151.8.1172. [DOI] [PubMed] [Google Scholar]
- 40.Cohen . Statistical power analysis in the behavioral sciences. 2nd ed. Hillsdale, NJ: Erlbaurm; 1988. [Google Scholar]
- 41.SAS Institute. SAS/STAT 9.1 User's Guide, Volumes 1–7. Cary, NC: SAS Institute; 2004. [Google Scholar]
- 42.Brown H, Prescott R. Applied Mixed Models in Medicine. New York: J. Wiley & Sons; 1999. [Google Scholar]
- 43.SAS Institute. SAS/STAT 9.1.3 Generalized Linear Mixed Model (GLIMMIX) Procedure. Cary, NC: SAS Institute; 2006. [Google Scholar]
- 44.Kirk RE. Experimental Desing: Procedures for the Behavioral Sciences. 3rd ed. Pacific Grove, CA: Brooks/Cole Publishing Company; 1995. [Google Scholar]
- 45.Morin CM. Combined therapeutics for insomnia: Should our first approach be behavioral or pharmacological? Sleep medicine. 2006 Aug;7 Suppl 1:S15–S19. doi: 10.1016/j.sleep.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Lichstein KL, Wilson NM, Johnson CT. Psychological treatment of secondary insomnia. Psychology and Aging. 2000;15(2):232–240. doi: 10.1037//0882-7974.15.2.232. [DOI] [PubMed] [Google Scholar]
- 47.Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008 Apr 1;31(4):489–495. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harvey AG, Tang NK. Cognitive behaviour therapy for primary insomnia: can we rest yet? Sleep medicine reviews. 2003 Jun;7(3):237–262. doi: 10.1053/smrv.2002.0266. [DOI] [PubMed] [Google Scholar]