Abstract
The purpose of this study was to synthesize biocompatible polyvinylpyrrolidone (PVP)-coated iron oxide (PVP-IO) nanoparticles and to evaluate their efficacy as a magnetic resonance imaging (MRI) contrast agent. The PVP-IO nanoparticles were synthesized by a thermal decomposition method and characterized by x-ray diffraction (XRD), transmission electron microscopy (TEM), dynamic light scattering (DLS), and a superconducting quantum interface device (SQUID). The core size of the particles is about 8–10 nm and the overall size is around 20–30 nm. The measured r2 (reciprocal of T2 relaxation time) and (reciprocal of relaxation time) are 141.2 and 338.1 (s mM)−1, respectively. The particles are highly soluble and stable in various buffers and in serum. The macrophage uptake of PVP-IO is comparable to that of Feridex as measured by a Prussian blue iron stain and phantom study. The signal intensity of a rabbit liver was effectively reduced after intravenous administration of PVP-IO. Therefore PVP-IO nanoparticles are potentially useful for T2-weighted MR imaging.
1. Introduction
Macrophages play an important role in inflammation and are a major producer of inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukins. MRI-compatible iron oxide-based methods for studying macrophage trafficking in arthritis models have been introduced [1, 2]. With regard to cancer, macrophages form a significant proportion of the total cell population in the vast majority of tumor tissues, and there is abundant evidence that, under certain conditions, macrophages are capable of cytotoxic or cytostatic activity against malignant cells [3]. The iron oxide nanoparticles are typically taken up by macrophages through an endocytosis pathway. Endocytosis is a very complex process and can be divided into pinocytosis, which is a constitutive and non-inducible process, and phagocytosis, which is the predominant pathway for endocytosis of large particles [4].
Feridex (ferumoxides injectable solution) has been widely used in clinics to detect and evaluate liver lesions associated with an alteration in the reticuloendothelial system (RES). The same superparamagnetic iron oxide (SPIO) nanoparticle agent has also allowed ex vivo labeling of macrophages for magnetic resonance imaging (MRI) visualization of macrophage homing. Since the bolus injection of ferumoxides is not recommended because of possible side effects, dynamic contrast-enhanced imaging has not been possible so far. Recently, Ferucarbotran (Resovist; Schering, Berlin, Germany) became available as a new SPIO agent for liver imaging in most European countries, as well as in some countries in Asia. Ferucarbotran is an SPIO agent that can be injected as a bolus [5], at a rate of 2 ml s−1 for example, which enables dynamic MRI to be performed during different vascular phases as we are accustomed to in liver imaging with extracellular contrast agents. In the accumulation phase, when the SPIO particles are taken up by the Kupffer cells of normal liver parenchyma or by Kupffer cells located in benign liver lesions, T2 (spin–spin relaxation) and (spin–spin relaxation and local magnetic field non-uniformity) effects and, less frequently, T1 (spin–lattice relaxation) effects, are used in lesion detection and characterization [6].
The main parameters that need to be considered for the effective macrophage uptake of polymer-coated iron oxide nanoparticles to detect inflammatory diseases by MRI are the particle size and magnetization value. Feridex has been evaluated as an MRI contrast agent for diagnosis of inflammatory and degenerative disorders associated with high macrophages phagocytic activity [4, 7]. However, Feridex has a broad size distribution that requires filtration before use. Furthermore, the synthesis of SPIO by the common co-precipitation method involves several sequential steps followed by a tedious centrifugation separation process in order to obtain uniform size, and large aggregates still occur during storage. The typical thermal decomposition method to synthesize Feridex also requires a high temperature (over 200 °C). Although the macrophage uptake of the bigger particles is higher than that of the smaller ones, overly large particles like Feridex with mean diameter of 150 nm are quickly phagocytosed, mainly by means of hepatic Kupffer cells and the mononuclear phagocyte system [8]. Therefore, the development of a polymer-coated iron oxide with size smaller than Feridex is required to allow uptake by migrating macrophages or macrophages in lymph nodes.
We are aiming to develop new biocompatible iron oxide agents that are relatively small and uniform in size for T2 and weighted MR contrast agents. We have previously prepared colloidal ferrofluid containing iron oxide nanoparticles coated with PVP via a thermal decomposition method [9]. We used PVP instead of other commonly used coating materials such as dextran, starch, albumin, silicones and poly(ethyleneglycol) (PEG) because PVP is water-soluble, non-charged, non-toxic, and is often used in various medical applications [10]. The proof-of-principle study resulted in PVP-IO nanoparticles with small core size and magnetization value. The polymer coating is also rather thick, so the hydrodynamic particle size falls in the range of SPIO (50–200 nm). Furthermore, the small core PVP-IO nanoparticles had poor monodispersity. In the current study, we improved the chemistry and prepared large core PVP-coated iron oxide nanoparticles as an MRI contrast agent.
2. Experimental details
Fe(CO)5 used as precursor and dimethylformamide (DMF) were obtained from Aldrich (St Louis, MO). Polyvinylpyrrolidone (PVP, average MW: 7000–11 000) was purchased from BASF (Germany). Feridex® (40 μmol Fe/kg) was obtained from Berlex Laboratories (Wayne, NJ). Mouse macrophage cell line RAW 264.7 was obtained from the American type culture collection (ATCC, Manassas, VA) and cultured following the manufacturer’s instructions.
2.1. Preparation of large core PVP-coated iron oxide nanoparticles
The polyvinylpyrrolidone (PVP)-coated iron oxide nanoparticles were synthesized according to a previously described method [9] with modifications. Briefly, 1 g of PVP was dissolved in 3 ml of DMF and the mixture was purged with nitrogen gas to remove oxygen. The resulting solution was heated up to 160 °C under vigorous mechanical stirring and 80 μl of Fe(CO)5 was injected into the mixture. During this process, the initial orange color of the solution gradually turned into a brownish-black colloidal solution. The mixture was reacted for 5 h at 160 °C. The final dark-brown colloid solution was then cooled to room temperature and precipitated with acetone. The supernatant was removed, and the remaining particles were washed with excess acetone to remove any unbound PVP and DMF residue. The washed particles were dried at 70 °C for further use.
2.2. Characterization of PVP-coated iron oxide nanoparticles
The particle size and morphology were examined by using a JEOL JEM-2010 analytical transmission electron microscope. The hydrodynamic diameter of the PVP-coated iron oxide nanoparticles was examined by using an electrophoretic light scattering spectrophotometer (Otsuka electronics, Photal ELS-8000). The crystallinity of PVP-IO was measured by using Rigaku D/MAX-IIIB x-ray diffractometer operating at 40 kV and 40 mA with Ni-filtered Cu Kα radiation (λ = 1.540 56 Å). The XRD pattern was recorded between 10 and 100° (2Θ value) at 5° min−1. Investigation of the magnetic property of PVP-IO was carried out at room temperature with |H| ≤ 20 kOe using a Quantum Design MPMS 5 superconducting quantum interface device (SQUID) magnetometer. The Fe content of the PVP-IO was determined with a Jobin-Yvon Ultima-C inductively coupled plasma-atomic emission spectrometer (ICP-AES).
2.3. Prussian blue staining
The principle of this method is that the ferric iron (Fe3+) in the presence of ferrocyanide ion is precipitated as the highly colored and highly water-insoluble complex, potassium ferric ferrocyanide, Prussian blue. The macrophages were cultivated for 48 h in six-well chamber slides in the presence of Feridex or PVP-coated iron oxide nanoparticles at different Fe concentrations (20, 50, and 100 μg ml−1). The cells were then washed three times with PBS and dried at 37 °C for 4 h, and then the attached cell monolayer was incubated with 10% potassium ferrocyanide in 20% hydrochloric acid for 20 min and washed with distilled water three times. For counterstaining with nuclear fast red, the cells were incubated with 1% eosin solution for 10 min and washed with distilled water. The cells were dehydrated by incubation with absolute ethanol for 3–5 min. The iron particles in the cells were observed as blue dots using an optical microscope with phase contrast.
2.4. In vitro MRI test
To confirm the feasibility of PVP-IO as an MRI contrast agent we first prepared the ferrofluid phantom, consisting of PVP-IO and Feridex with Fe concentrations varying from 0.125 to 1.25 mM in distilled water. Every sample was filled into an arrangement of 600 μl tubes without air in a plastic rack. The tubes containing samples were embedded in a phantom which consisted of tanks filled with 1% agarose gel to obtain appropriate images [11]. MRI was performed using a GE 1.5 T MRI system. The -weighted image of the phantom was obtained with a turbo spin echo (TSE) technique. The sequence parameters of -weighted MRI were 100 ms of repetition time (TR), 10 ms of echo time (TE), 3 mm thickness, and 14 cm field of view (FOV).
In order to measure the relaxivity of the newly synthesized PVP-IO, transverse T2-weighted spin echo images were acquired using a 3 T Siemens Tim Trio MR scanner. Gel preparations in 2 ml vials were placed in a holder for insertion into the eight-channel volume head resonator. The long axis of the vials was parallel to the static magnetic field, and a transverse tomographic plane orientation was used. A gradient echo acquisition was used with a TR of 2000 ms, TE of 1.8 ms, slice thickness of 12 mm, and a flip angle of 20°. The in-plane resolution was 0.88 mm. The normal first-order shim process was applied, and the phantoms were imaged at room temperature (20 °C).
To confirm the macrophage uptake of Feridex and PVP-IO, 1 × 106 macrophages in 2 ml culture media were added to each well of a six-well plate. Each well contained a different amount of Feridex or PVP-IO at different iron concentrations of 20, 50, and 100 μg ml−1. After incubation for 48 h, the cells were washed thoroughly with distilled water, harvested using a cell scraper, and subjected to -weighted MRI.
2.5. In vivo MRI test
To observe the in vivo MRI effect, -weighted MRI was performed in normal healthy New Zealand white rabbits weighing 3 kg. The rabbits were anesthetized with an intramuscular injection of a mixture of ketamine hydrochloride (50 mg kg−1) and xylazine hydrochloride (5 mg kg−1). The PVP-IO (6 mg kg−1 Fe) was administered into a marginal vein of the rabbit ear with a 26G-needle syringe. Feridex (6 mg/Fe) was injected via the ear vein through an inline 5 μm specific filter with a rapid bolus, immediately followed by a 5 ml saline solution flush. The T2-weighted MR images of the liver were obtained with a TSE technique using a 3 T MR machine (GE Excite). The sequence parameters were TR 4300 ms, TE 83.2 ms, 1 mm thickness, and 18 × 14 cm FOV (256 × 192 matrix, NEX (number of excitations) = 4). The MRI signals were serially obtained at the baseline, 3, 7, and 20 min after injection of PVP-IO.
3. Results and discussion
In this study, PVP-coated iron oxide nanoparticles were synthesized by an one-step thermal decomposition method adapted to make homogeneous nanoparticles more suitable for intravenous injection. We [9] have previously attempted to synthesize PVP-IO nanoparticles with small core size and thick coating and found that the overall size of the particles is dependent on the PVP/Fe(CO)5 reaction ratio and that the core size of the nanoparticles is dependent on the reaction time. For stable PVP-IO nanoparticles with large core size and thin coating for macrophage uptake, we found the optimal molar ratio of PVP/Fe(CO)5 to be 0.16 and the reaction time to be 5 h; in addition, more uniform PVP-IO nanoparticles were obtained after precipitation by acetone than by dialysis. The comparison of physical properties of our previously reported PVP-IO nanoparticles with small core (small core PVP-IO) [9], PVP-IO nanoparticles with large core developed in this study (large core PVP-IO), and Feridex is summarized in table 1. PVP-IO is highly soluble in water and various buffer solutions including saline, PBS and serum. The solubility of large core PVP-IO in PBS can be as high as 100 mg ml−1 without precipitation. The particles are also biocompatible, as no obvious cellular toxicity was observed through cell proliferation assay (data not shown).
Table 1.
Summary of physical properties of small core PVP-IO, large core PVP-IO, and Feridex.
| Nanoparticle | Core size (nm) | Hydrodynamic size (nm) | Magnetization value (emu g−1 Fe) |
|---|---|---|---|
| Small corea | 3–4 nm | 100–120 | 35 |
| Large coreb | 8–10 nm | 20–30 | 110 |
| Feridex | 4.8 ± 1.9 | 58.5 ± 185.8 | 70 |
Previously synthesized PVP-coated iron oxide nanoparticles [16].
PVP-coated iron oxide nanoparticles prepared in this study.
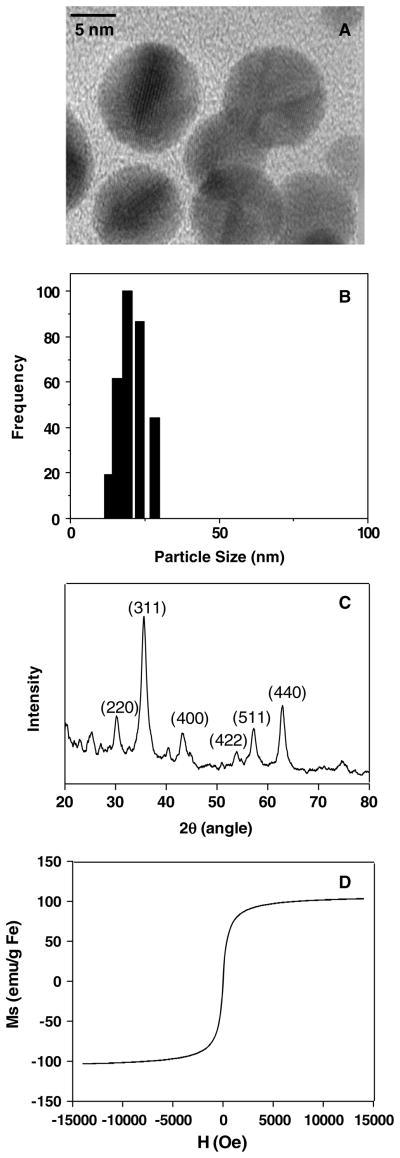
Transmission electron microscopy (TEM) revealed that the average size of large core iron oxide nanoparticles is about 8–10 nm, as illustrated in figure 1(A). In aqueous solution, the colloidal particles have a hydrodynamic diameter of about 20–30 nm, determined by dynamic light scattering (DLS) measurement (figure 1(B)). The small core PVP-IO previously made in our laboratory [9] had a core size of 3–4 nm with a broad hydrodynamic size distribution of 90–150 nm. Figure 1(C) shows the x-ray diffraction (XRD) data of large core PVP-IO. The peaks appear at 30.0°, 35.3°, 43.0°, 53.3°, 56.9°, and 62.5°, which are well indexed to reflections from the (220), (311), (400), (422), (511), and (440) crystal planes of spinel ferrite, respectively. The hysteresis loop of PVP-coated iron oxide nanoparticles shown in figure 1(D) had no coercive force, featuring superparamagnetic behavior. The large core PVP-IO nanoparticles were characterized by an augmented magnetic moment on increasing the magnetic field. The saturation magnetization of PVP-coated iron oxide nanoparticles is around 110 emu g−1 Fe, which is much higher than that of previously reported small core PVP-IO (about 35 emu g−1 Fe) [9] as well as Feridex (about 70 emu g−1 Fe) [12]. This trend matches with the report that the magnetism increases with the size and crystallization of nanocrystals [13]. A high saturation magnetization is preferred for T2-weighted MRI since the spin–spin relaxation process of protons in the water molecules surrounding the nanoparticles is facilitated by a large magnitude of magnetic spins in nanoparticles: large core iron oxide nanoparticles with high mass magnetization values may result in strong T2-weighted MR signal intensity decrease as measured by MRI [13].
Figure 1.
Physical characterization of large core PVP-IO nanoparticles. (A) The TEM image shows monodisperse PVP-IO with core size of 8–10 nm; (B) the DLS measurement indicates that the colloidal particles in aqueous solution have a hydrodynamic diameter of about 20–30 nm; (C) the XRD pattern is well indexed to reflections from the crystal planes of spinel ferrite, and (D) the magnetization curve finds the saturation magnetization of PVP-coated iron oxide nanoparticles to be around 110 emu g−1 Fe.
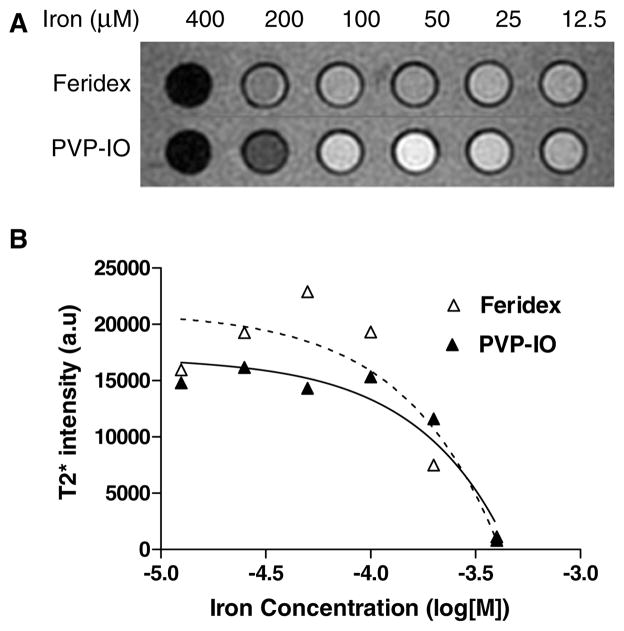
-weighted MRI was obtained with a 1.5 T MR machine (GE Excite) for the comparison of the MR contrast effect of the phantom. Figure 2(A) shows images of the synthesized large core PVP-IO colloids and Feridex in the same concentration gradient in distilled water. Figure 2(B) shows the signal intensity values converted by the image analysis tool for quantitative measurement. The results indicate that PVP-IO is slightly better than Feridex as a negative contrast agent for MRI.
Figure 2.
(A) Phantom image acquired from -weighted MR images of Feridex and large core PVP-IO at different iron concentrations. (B) The MR signal intensity is affected by the iron concentrations of Feridex and PVP-IO. PVP-IO is slightly better than Feridex as a negative contrast agent for MRI.
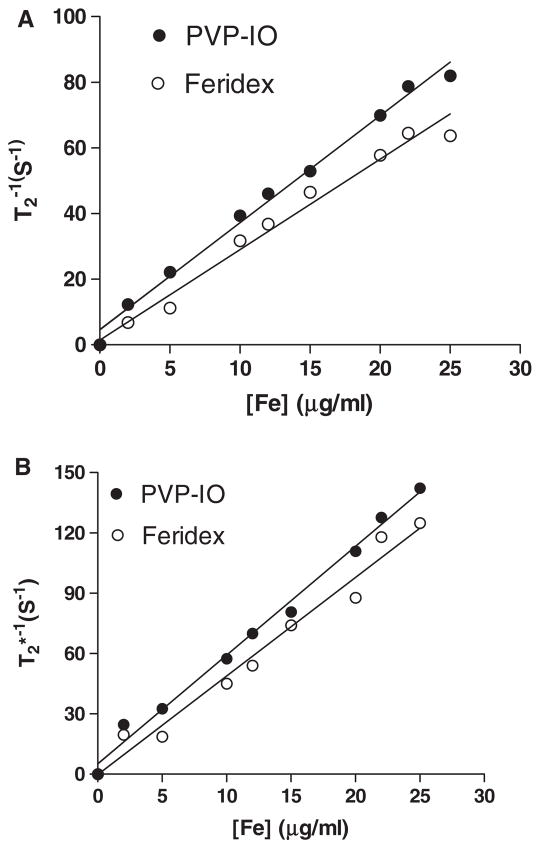
Transverse T2-weighted spin echo images were acquired using a 3 T Siemens Tim Trio MR scanner. The measured r2 (reciprocal of T2 relaxation time) and (reciprocal of relaxation time) values were 174.8 and 294.3 mM−1 s−1, respectively (figure 3). Remarkably, the large core PVP-IO show higher r2 and than Feridex (r2 = 151.9 mM−1 s−1, ). The higher relaxivity of PVP-coated magnetic nanoparticles is likely attributed to the high magnetic moment of PVP-IO (110 emu g−1 Fe) and effective magnetic relaxations from the proton spins around PVP-IO.
Figure 3.
(A) 1/T2 and (B) versus Fe concentration for PVP-IO (●) and Feridex (○). The relaxivity values r2 and were obtained from the slopes of the linear fits of experimental data.
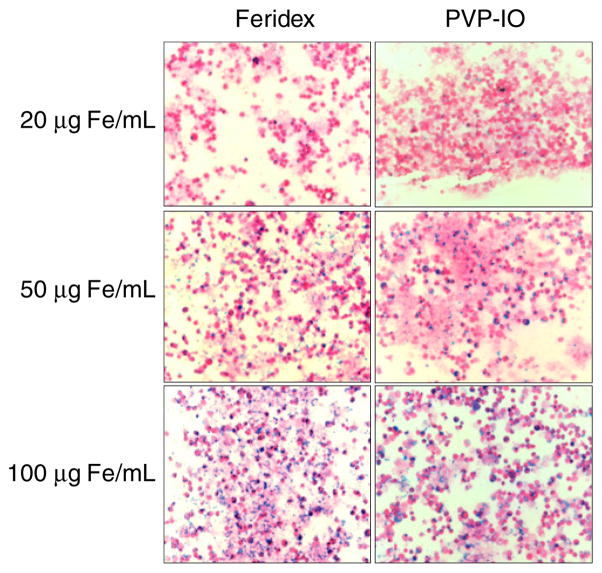
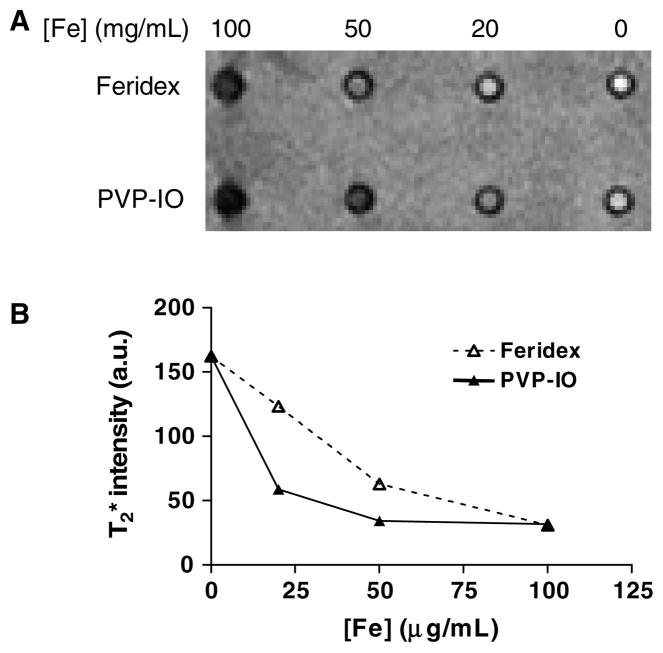
To detect inflammatory disease by MRI, it would be ideal for PVP-IO nanoparticles to possess high and persistent uptake by macrophages. To investigate this property, in vitro cell uptake experiments were carried out using a mouse macrophage cell line RAW 264.7. The uptake of PVP-IO by macrophages was compared to that of Feridex, which is currently used clinically for MRI and is known to be taken up by macrophages due to its size. To detect the presence of iron oxide nanoparticles in cells, Prussian blue staining was carried out after 48 h incubation of Feridex and large core PVP-IO nanoparticles with macrophages at 20, 50, and 100 μg ml−1. As shown in figure 4, the macrophage uptake of large core PVP-IO is comparable to or slightly higher than that of Feridex at all the concentrations examined, even though the overall size of PVP-IO is smaller than that of Feridex. Following the incubation, -weighted MR images were acquired to further confirm the ability of macrophages to take up the newly synthesized large core PVP-IO. As shown in figure 5, PVP-IO exhibited somewhat more negative contrast enhancement as compared to Feridex at relatively low concentrations of Fe. Such difference was diminished when a large amount of Fe were used (e.g. 100 mg Fe ml−1).
Figure 4.
Mouse macrophage cells were incubated with Feridex or large core PVP-IO for 48 h at different iron concentrations (20, 50, and 100 μmol ml−1), stained by Prussian blue and then counterstained with nuclear fast red. Similar or slightly higher macrophage uptake of large core PVP-IO as compared to Feridex is observed at all concentrations examined.
Figure 5.
(A) -weighted MR images and (B) MR signal intensity of gelatin gels containing macrophages (1 × 106 cells/well) incubated with Feridex and PVP-coated iron oxide nanoparticles at the different iron concentration of 20, 50, and 100 μg ml−1. PVP-IO exhibited more negative contrast enhancement as compared to Feridex at relatively low concentrations of Fe.
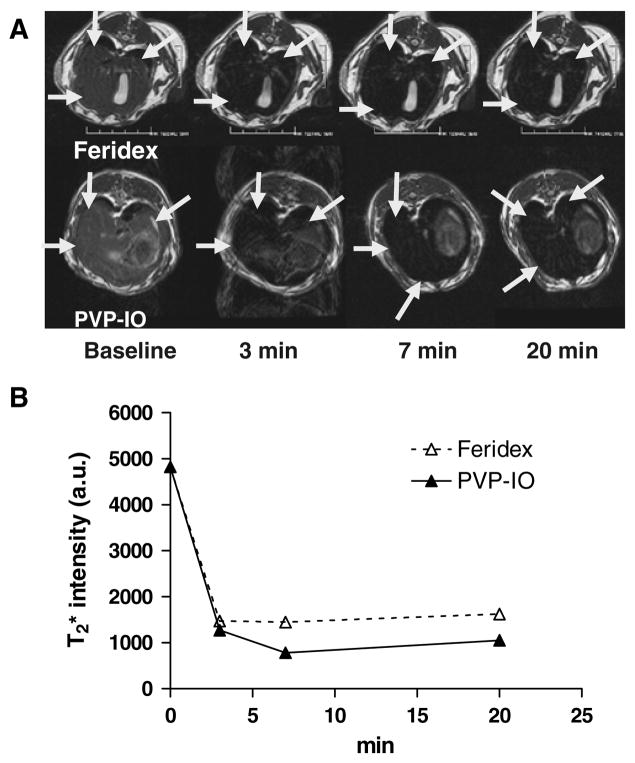
After successful demonstration of the uptake of large core PVP-IO nanoparticle by macrophages in culture, we tested the same particles in vivo for liver imaging. Due to the high solubility and monodispersity of the PVP-IO in aqueous buffer, we are able to perform bolus injection of PVP-IO without the presence of a filter, which is required by Feridex. Both Feridex and large core PVP-IO were able to detect a lower signal intensity in the rabbit liver parenchyma but the effect of PVP-IO is more obvious than that of Feridex (figure 6).
Figure 6.
-weighted MR images before, 3, 7, and 20 min after intravenous injection of Feridex (up) and PVP-coated iron oxide nanoparticles (down). Both Feridex and large core PVP-IO were able to lower signal intensity in the rabbit liver parenchyma but the effect of PVP-IO is more obvious than Feridex.
4. Conclusions
This paper reports the synthesis of superparamagnetic PVP-coated iron oxide nanoparticles with iron oxide diameter 8–10 nm, similar to those in the Feridex. The PVP-IO has a significantly smaller hydrodynamic diameter than that of Feridex. In vitro and in vivo MRI studies show that the reduction of MRI intensity is comparable to or more obvious for the PVP-IO as compared to Feridex at the same concentration of Fe. The newly synthesized PVP-IO can also be effectively ingested by macrophages in cell culture and in vivo after intravenous injection into rabbits. Our studies reveal that the PVP-IO nanoparticles are biocompatible and safe for in vivo administration. Further studies of PVP-IO for tumor targeting and inflammatory diseases are currently underway.
Acknowledgments
This work was supported, in part, by the Department of Defense (DOD) Breast Cancer Research Program (BCRP) Concept Award (W81XWH-06-1-0665), the National Cancer Institute (NCI) Center of Cancer Nanotechnology Excellence (CCNE) (U54 CA119367), and the Korea Research Foundation MOEHRD (KRF-2006-352-D00061).
References
- 1.Buskens E, Nederkoorn PJ, Buijs-Van Der Woude T, Mali WP, Kappelle LJ, Eikelboom BC, Van Der Graaf Y, Hunink MG. Imaging of carotid arteries in symptomatic patients: cost-effectiveness of diagnostic strategies. Radiology. 2004;233:101–12. doi: 10.1148/radiol.2331030863. [DOI] [PubMed] [Google Scholar]
- 2.Lutz AM, Gopfert K, Jochum W, Nanz D, Frohlich JM, Weishaupt D. USPIO-enhanced MR imaging for visualization of synovial hyperperfusion and detection of synovial macrophages: preliminary results in an experimental model of antigen-induced arthritis. J Magn Reson Imaging. 2006;24:657–66. doi: 10.1002/jmri.20667. [DOI] [PubMed] [Google Scholar]
- 3.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 4.Raynal I, Prigent P, Peyramaure S, Najid A, Rebuzzi C, Corot C. Macrophage endocytosis of superparamagnetic iron oxide nanoparticles: mechanisms and comparison of ferumoxides and ferumoxtran-10. Invest Radiol. 2004;39:56–63. doi: 10.1097/01.rli.0000101027.57021.28. [DOI] [PubMed] [Google Scholar]
- 5.Reimer P, Rummeny EJ, Daldrup HE, Balzer T, Tombach B, Berns T, Peters PE. Clinical results with Resovist: a phase 2 clinical trial. Radiology. 1995;195:489–96. doi: 10.1148/radiology.195.2.7724772. [DOI] [PubMed] [Google Scholar]
- 6.Reimer P, Muller M, Marx C, Wiedermann D, Muller R, Rummeny EJ, Ebert W, Shamsi K, Peters PE. T1 effects of a bolus-injectable superparamagnetic iron oxide, SH U 555A: dependence on field strength and plasma concentration–preliminary clinical experience with dynamic T1-weighted MR imaging. Radiology. 1998;209:831–6. doi: 10.1148/radiology.209.3.9844683. [DOI] [PubMed] [Google Scholar]
- 7.Stark DD, Weissleder R, Elizondo G, Hahn PF, Saini S, Todd LE, Wittenberg J, Ferrucci JT. Superparamagnetic iron oxide: clinical application as a contrast agent for MR imaging of the liver. Radiology. 1988;168:297–301. doi: 10.1148/radiology.168.2.3393649. [DOI] [PubMed] [Google Scholar]
- 8.Kaim AH, Wischer T, O’Reilly T, Jundt G, Frohlich J, von Schulthess GK, Allegrini PR. MR imaging with ultrasmall superparamagnetic iron oxide particles in experimental soft-tissue infections in rats. Radiology. 2002;225:808–14. doi: 10.1148/radiol.2253011485. [DOI] [PubMed] [Google Scholar]
- 9.Lee HY, Lim NH, Seo JA, Yuk SH, Kwak BK, Khang G, Lee HB, Cho SH. Preparation and magnetic resonance imaging effect of polyvinylpyrrolidone-coated iron oxide nanoparticles. J Biomed Mater Res B. 2006;79:142–50. doi: 10.1002/jbm.b.30524. [DOI] [PubMed] [Google Scholar]
- 10.Le Garrec D, Gori S, Luo L, Lessard D, Smith DC, Yessine MA, Ranger M, Leroux JC. Poly(N-vinylpyrrolidone)-block-poly(D,L-lactide) as a new polymeric solubilizer for hydrophobic anticancer drugs: in vitro and in vivo evaluation. J Control Release. 2004;99:83–101. doi: 10.1016/j.jconrel.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Cheng FY, Su CH, Yang YS, Yeh CS, Tsai CY, Wu CL, Wu MT, Shieh DB. Characterization of aqueous dispersions of Fe(3)O(4) nanoparticles and their biomedical applications. Biomaterials. 2005;26:729–38. doi: 10.1016/j.biomaterials.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Jung CW, Jacobs P. Physical and chemical properties of superparamagnetic iron oxide MR contrast agents: ferumoxides, ferumoxtran, ferumoxsil. Magn Reson Imaging. 1995;13:661–74. doi: 10.1016/0730-725x(95)00024-b. [DOI] [PubMed] [Google Scholar]
- 13.Jun YW, et al. Nanoscale size effect of magnetic nanocrystals and their utilization for cancer diagnosis via magnetic resonance imaging. J Am Chem Soc. 2005;127:5732–3. doi: 10.1021/ja0422155. [DOI] [PubMed] [Google Scholar]