Abstract
Many investigators have hypothesized that brain response to cortisol is altered in depression. However, neural activation in response to exogenously manipulated cortisol elevations has not yet been directly examined in depressed humans. Animal research shows that glucocorticoids have robust effects on hippocampal function, and can either enhance or suppress neuroplastic events in the hippocampus depending on a number of factors. We hypothesized that depressed individuals would show 1) altered hippocampal response to exogenous administration of cortisol, and 2) altered effects of cortisol on learning. In a repeated-measures design, 19 unmedicated depressed and 41 healthy individuals completed two fMRI scans. Fifteen mg oral hydrocortisone (i.e., cortisol) or placebo (order randomized and double-blind) was administered one hour prior to encoding of emotional and neutral words during fMRI scans. Data analysis examined the effects of cortisol administration on 1) brain activation during encoding, and 2) subsequent free recall for words. Cortisol affected subsequent recall performance in depressed but not healthy individuals. We found alterations in hippocampal response to cortisol in depressed women, but not in depressed men (who showed altered response to cortisol in other regions, including subgenual prefrontal cortex). In both depressed men and women, cortisol’s effects on hippocampal function were positively correlated with its effects on recall performance assessed days later. Our data provide evidence that in depressed compared to healthy women, cortisol’s effects on hippocampal function are altered. Our data also show that in both depressed men and women, cortisol’s effects on emotional memory formation and hippocampal function are related.
Keywords: hippocampus, cortisol, memory, depression, fMRI, emotion
1. Introduction
Animal models suggest that variation in neural response to corticosteroids is of utmost importance to depression (Pittenger & Duman, 2008). Pharmacological manipulation of corticosteroid receptors in humans provides indirect evidence of alterations in corticosteroid neural signaling in depression (DeBattista et al., 2000; DeBattista & Belanoff, 2006; Otte et al., 2010). Some sources suggest that depression may be associated with overactivity of cortisol in neural tissues (Schatzberg & Lindley, 2008), while other sources implicate reduced capacity of cortisol to modulate neural activity (Pariante, 2009). However, human studies of depression have not yet directly examined effects of cortisol on brain activity.
Human neuroimaging studies have implicated a number of stress- and cortisol-sensitive brain regions in the pathophysiology of depression (Gold et al., 2002). For instance, in depressed humans altered volume and activity have been consistently observed in the hippocampus and anterior cingulate (especially subgenual prefrontal cortex), and less consistently in the amygdala (MacQueen, 2009). To date, it is unclear the extent to which neural response to cortisol elevations plays a role in impaired functioning in these regions in depression. Furthermore, data are equivocal with regard to whether cognitive aspects of depression (e.g., mild memory impairment, mood congruent memory bias) can be partially attributed to variation in cortisol (Vythilingam et al., 2004). However, basic research in humans shows that cortisol has robust effects on memory, which depend on the emotional state of the individual (Abercrombie et al., 2006; Wolf, 2009).
Glucocorticoids (GCs; cortisol is the primary GC in primates, and corticosterone is primary in most rodents) enter the brain readily and have potent effects on neuroplasticity. Most well studied are the effects of GCs on the hippocampus, which is a brain region densely populated with corticosteroid receptors (Patel et al., 2000). GCs have potent effects on many aspects of hippocampal neuroplasticity, including effects on neurogenesis, synaptic plasticity, dendritic growth, and neurotrophic factors (Pittenger & Duman, 2008). Consistent with animal data showing robust effects of GCs on learning and hippocampal neuroplasticity, studies in both healthy and depressed humans show that hippocampal-dependent learning appears to be more sensitive to the effects of cortisol than other forms of learning (Kirschbaum et al., 1996; Hinkelmann et al., 2009).
Moderately-elevated GCs tend to enhance synaptic strength in the hippocampus (de Kloet et al., 1999; Joëls & Krugers, 2007). Conversely, extreme GC elevations tend to impair learning and weaken synaptic contacts (de Kloet et al., 1999; Joëls & Krugers, 2007). However, these statements are oversimplifications. A number of important factors determine whether the effects of GCs will potentiate, depress, or have no effect on learning and synaptic strength (Fuchs et al., 2006; Joëls & Krugers, 2007; Leuner & Gould, 2010). For instance, levels of emotional arousal and variation in the neural milieu (e.g., noradrenergic activation) at the time of GC elevations alter GC’s effects on neuroplasticity (de Kloet et al., 1999; Okuda et al., 2004; Roozendaal et al., 2006a; Joëls & Krugers, 2007).
Also of potential import for depression are data in rodents showing that the effects of GCs on hippocampal plasticity depend on the history of the organism (Alfarez et al., 2003). For instance, maternal care in rodent pups has dramatic effects into adulthood on stress-related learning and plasticity (Champagne et al., 2008; Bagot et al., 2009). Adult animals with a history of high levels of maternal care showed optimal learning under low stress conditions. Likewise, in hippocampal CA1 and dentate gyrus slices from adult rodents with a history of high levels of maternal care, corticosterone suppressed in vitro long-term potentiation (LTP). Conversely, animals with a history of low levels of maternal care showed enhanced learning under high stress conditions (Champagne et al., 2008). A history of lower rates of maternal care was also associated with an enhancement in hippocampal LTP in the presence of corticosterone (Champagne et al., 2008; Bagot et al., 2009). Although the implications of the animal data for humans are difficult to determine, the animal data provide “proof of concept” that effects of GCs on neuroplasticity vary depending on identifiable factors. In summary, both the animal and human literatures show that the effects of GCs on learning vary depending on a number of factors (e.g., emotional state and/or the past history of the individual).
Based on data summarized above, we hypothesized that compared to controls depressed patients would show altered response to cortisol. Specifically, we hypothesized that during a learning task (encoding of emotional and neutral words) depressed patients would show altered response to administration of hydrocortisone (CORT; i.e., cortisol), particularly with regard to hippocampal function. In addition, we hypothesized that the effects of CORT on emotional memory formation would be altered in depressed compared to healthy individuals. We also hypothesized that effects of CORT on the hippocampus would be related to effects of CORT on memory for words encoded during CORT administration. In addition, it is well-known that the effects of stress and GCs on memory vary by sex (Wolf et al., 2001; Shors, 2006). Thus, we hypothesized that effects would depend on sex.
2. Materials and Methods
2.1. Participants
Twenty unmedicated depressed (11 women) and 45 (24 women) never-depressed healthy subjects completed the study. Participants were recruited from the community and screened with the 17-item Hamilton Rating Scale for Depression (HDRS; Hamilton, 1960) and the SCID (First et al., 2002). In addition, a physician (JH) took a medical history as well as a drug screen and pregnancy test for women. Participants were right-handed and in good physical health. Depressed participants met criteria for DSM-IV Major Depressive Disorder and scored ≥14 on the 17-item HDRS. Depressed participants did not meet DSM-IV criteria for any other current Axis I diagnosis, with the exception of one subject who met criteria for dysthymia (double depression) and a number of subjects who had subthreshold diagnoses of an Anxiety Disorder. Depressed participants had no history of bipolar or psychotic disorder, and were not receiving treatment for depression. Control subjects had no history of Axis I diagnosis in themselves or first-degree relatives. Additional exclusion criteria included: Daily tobacco use, history of seizures, diabetes, hypertension, neurological problems, cardiac problems, any medication that directly affects CNS function (i.e., most medications were exclusionary, but a small number of subjects were taking antibiotics for acne), any steroid medication, metallic implants, electronic implants, pregnant or trying to become pregnant, women who are sexually active but not using a method of birth control, claustrophobia, working the “night shift” (e.g., between the hours of 11 pm and 7 am). We were unable to control for menstrual phase because of a National Institutes of Health stipulation that we scan depressed subjects within 2 weeks of screening, so as not to require more than 2 weeks off medication. Thus, we were not able to control for menstrual phase within the depressed group. Both the depressed and the control samples are mixed with regard to menstrual phase and hormonal contraceptive use. The University of Wisconsin Institutional Review Board approved study procedures, and participants provided written informed consent after the nature of the procedures had been fully explained. Participants were paid for their participation.
Two controls were excluded from analysis due to experimenter error involving re-exposure to a previously administered set of stimuli; one control subject was excluded due to abnormally high salivary cortisol levels following hydrocortisone administration (possibly due to chewing the orally administered capsule); and one control and one depressed subject were excluded due to structural brain abnormalities. Analyses of fMRI data excluded an additional female control and a depressed male because movement exceeded 4 mm. Please see Table 1 for sample characteristics and final n’s.
Table 1.
Mean (SD) descriptive data
| Controls |
Depressed |
|||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| N | 18 | 23 ‡ | 9 ‡ | 10 |
| Age | 28.28 (8.48) | 25.48 (7.42) | 25.22 (8.21) | 28.70 (11.5) |
| Racial Category | ||||
| American Indian/Alaska Native | 0% | 0% | 0% | 0% |
| Asian | 11.1% | 4.3% | 11.1% | 10% |
| Native Hawaiian or Pacific Islander | 0% | 0% | 0% | 0% |
| Black or African American | 5.6% | 0% | 11.1% | 10% |
| White | 77.8% | 82.6% | 66.7% | 80% |
| Multiple Races | 0% | 4.3% | 0% | 0% |
| Unknown/Unreported | 5.6% | 8.7% | 11.1% | 0% |
| Body Mass Index | 26.36 (4.43) | 24.41 (3.95) | 27.07 (3.95) | 27.38 (8.11) |
| Measures of Depression & Affect | ||||
| HDRS-17 | 0.22 (0.43) | 0.70 (1.02) | 17.22 (2.95) | 16.80 (2.62) |
| Number of lifetime episodes | N/A | N/A | 3.33 (1.66) | 4.11 (1.83) |
| Duration of current episode (in months) | N/A | N/A | 5.11 (6.45) | 5.28 (7.30) |
| PANAS Negative Affect (trait) | 12.8 (2.7) | 12.4 (2.4) | 23.6 (2.7) | 24.7 (10.2) |
| PANAS Negative Affect (state: CORT) | 10.9 (1.6) | 10.5 (0.85) | 13.2 (2.6) | 15.4 (7.2) |
| PANAS Negative Affect (state: PLACEBO) | 10.5 (1.1) | 10.5 (1.2) | 13.6 (2.0) | 15.4 (7.8) |
| PANAS Positive Affect (trait) | 37.7 (4.2) | 37.8 (4.5) | 22.6 (6.4) | 19.5 (6.8) |
| PANAS Positive Affect (state: CORT) | 31.0 (6.5) | 27.0 (6.9) | 18.6 (4.2) | 19.7 (7.2) |
| PANAS Positive Affect (state: PLACEBO) | 30.3 (7.6) | 27.0 (7.0) | 18.4 (4.2) | 20.3 (7.8) |
| Education (in years) | 15.33 (2.28) | 14.43 (2.19) | 14.67 (2.40) | 15.45 (2.63) |
| Estimated Verbal IQ | 112.0 (5.26) | 111.4 (5.17) | 111.7 (9.08) | 107.0 (11.68) |
Note:
fMRI analyses excluded two additional participants because of movement > 4 mm. Thus, n’s for analyses including fMRI data are 22 for female controls and 8 for depressed males, rather than the numbers reported in the table, which reflect n’s available for analyses of effects of CORT on affect and recall.
With the exception of the expected differences in measures of depression and affect, no significant differences emerged between genders or groups in descriptive data. HDRS-17 refers to the 17-item Hamilton Rating Scale for Depression (Hamilton, 1960). PANAS refers to the Positive and Negative Affect Schedule (Watson et al., 1988). Verbal IQ was estimated with the North American Adult Reading Test (NAART; Blair & Spreen, 1989).
2.2. Procedure
Eligible participants returned for four visits: fMRI simulation session, two fMRI scanning sessions, and memory testing session. At the simulation session, participants were acclimated to the tasks and the scanning environment using a mock scanner. A repeated measures design was used in which subjects participated in two different scanning sessions, which began between 16:30 and 17:30 and were spaced two days apart. The sessions lasted approximately 2.75 hours. Participants did not exercise and consumed nothing but water within 90 minutes of the session. Participants were orally administered 15 mg hydrocortisone (CORT, i.e., cortisol) or placebo (order of administration randomized and double-blind) one hour prior to memory encoding during fMRI scanning. A relatively low dose of CORT was used with the intent that brain corticosteroid receptors would not become fully saturated, and that subtle differences between depressed and healthy subjects’ brain responses could be detected.
During each scan, subjects viewed one of two psychometrically-matched sets of 88 descriptive words, i.e., 32 of each positively and negatively valenced and 24 neutral words. See Supplemental Information for word sets. Each word was viewed twice (in two separate tasks) to enhance free recall performance assessed days later, which is typically quite low for words. During the first viewing of each word set brain activation was measured and participants judged whether or not each word was self-referent. During the second viewing of the word set participants provided valence and arousal ratings of each word.
Free recall for words presented during fMRI scanning was measured 4–6 days later (i.e., 4 days after scan 1; and 6 days after scan 2). Verbal IQ was estimated with the North American Adult Reading Test (NAART; Blair & Spreen, 1989).
2.3. fMRI procedures
fMRI data were collected during the first viewing of the words, using an event-related design in which words were presented for two seconds with an ITI of 4–8 seconds. Whole-brain fMRI data were collected on 3-Tesla GE Signa scanner (GE Medical Systems, Waukesha, WI) using a quadrature birdcage coil. T1 high resolution anatomical images were collected and used to assist with localization of function (three-dimensional spoiled gradient-recalled echo; 256×256 in-plane resolution; 240 mm FOV; 124×1.2 mm axial slices). Blood oxygen level dependent (BOLD) functional images with a sagital acquisition were collected; T2*-weighted gradient echo planar imaging pulse sequence (30 slices thickness/gap 4/1 mm; 64×64 matrix; 240 mm FOV; Flip Angle: 90°; TR/TE: 2000/30 ms). A fiber-optic goggle system (Avotec, Stuart, FL) was used for stimulus display. Individual subject data were analyzed in AFNI (Cox, 1996) using the following procedures: slice-time correction, motion correction, 6mm spatial smoothing, and normalization to Talairach space. Field map correction was also applied to reduce distortion. Time series were modeled using a least-squares general linear model fit with the Gamma Variate Function as an idealized hemodynamic response to each word presentation, with six motion covariates. A contrast of (negative + positive) - neutral words was computed, as previous research has found that cortisol often enhances memory for emotionally arousing information regardless of valence (Wolf, 2009).
2.4. Salivary cortisol measurement
Salivary cortisol was collected throughout the sessions and at home after the scans with the Salivette (Sarstedt, Newton, NC; Figure 1). Samples were assayed using the chemi-luminescence assay, which has a high sensitivity of 0.16 ng/mL (IBL-International, Hamburg, Germany). Both intra and interassay CV were below 6%. Because cortisol values are typically skewed, analyses using salivary cortisol were performed on log-transformed values.
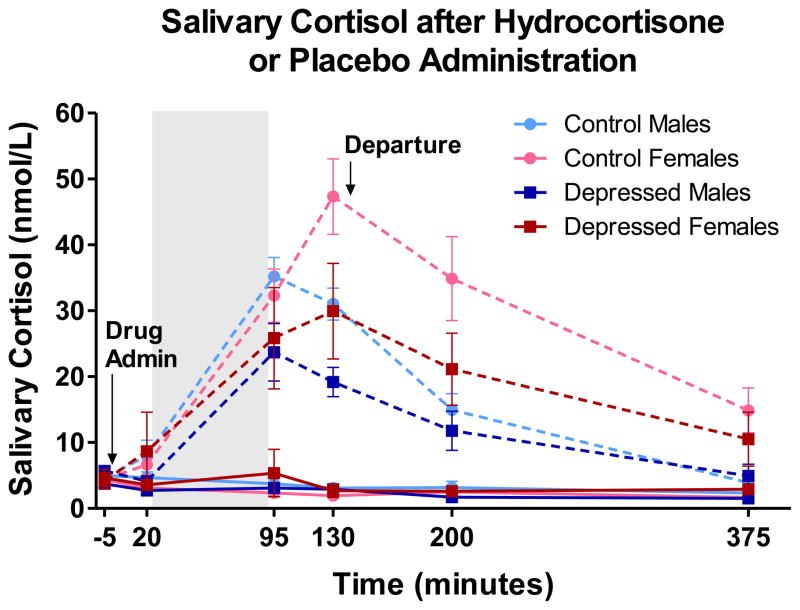
Figure 1.
Salivary cortisol levels in nmol/L. Stippled lines represent salivary cortisol levels during the session in which CORT was administered. Solid lines represent salivary cortisol levels during the session in which Placebo was administered. Shaded area represents duration of time in scanner. Drug administration occurred 20–30 minutes prior to placement in scanner, and approximately 60 minutes prior to functional scanning during word encoding task. Two saliva samples were collected in the evening at home after departure from the lab. Endogenous salivary cortisol levels (at baseline on CORT day and throughout Placebo session) did not differ between depressed and control subjects, F’s < 0.1. Despite small differences apparent in the figure, depressed and control subjects did not significantly differ in salivary cortisol levels following drug administration, F(1,57) = 0.09, n.s. However, salivary cortisol levels following drug administration differed by sex, F(1,57) = 4.38, p < .05. On average, women showed greater post-drug salivary cortisol, which do not appear to be solely due to differences in weight (see text).
2.5. Data analysis
Salivary cortisol levels were examined to confirm drug effects. We also examined effects of CORT on state affect measured with the PANAS (Watson et al., 1988). Recall assessed 4–6 days after scanning was examined using a mixed ANOVA with factors Group (depressed vs. controls) × Drug (CORT vs. placebo) × Valence (positive, neutral, negative) × Sex. Self-referent endorsement is not included in the model for the following reasons: CORT (vs. placebo, i.e. “Drug”) did not significantly affect rates of self-referent endorsement (p = .21), and more importantly, no interactive effects on self-referent endorsement emerged for Drug × Group or Drug × Group × Gender; F’s < 0.55, n.s. Furthermore, rates of recall measured days later are too low to examine effects of self-reference (floor effects would predominate). Studies examining self-referent encoding in depression examine memory soon after encoding when recall rates are higher.
fMRI data were analyzed for all subjects combined and separately for men and women. Contrast maps were entered into a random-effects, mixed-design group analysis for unbalanced designs (GroupANA) with subject as a random factor. A p-value threshold = .005 was used, and we applied volume correction for multiple comparisons using Monte Carlo simulations (AlphaSim, AFNI) to achieve mapwise correction at p < .05. To further examine our hypothesized region, the hippocampus, we applied a small volume correction resulting in minimum cluster size that can be considered significant at p < .05. Percent signal change was extracted for each significant cluster. Correlations were tested between recall performance and contrast data. Because we were interested in differences in the effects of CORT in depressed participants compared to controls, we report clusters emerging from Group × Drug interaction analyses. Main effects for Drug and for Group are reported in Supplemental Information.
3. Results
3.1. Salivary cortisol
Endogenous salivary cortisol levels (at baseline and throughout placebo session) did not differ between depressed and control subjects, F’s < 0.1 (Figure 1), which suggests that depressed participants did not show grossly dysregulated HPA functioning. Despite small differences apparent in Figure 1, depressed and control subjects did not significantly differ in salivary cortisol levels following drug administration, F(1,57)=0.09, n.s. However, salivary cortisol levels following drug administration differed by sex. Men and women showed similar salivary cortisol levels after drug administration throughout the scan, but diverged after the scan (Figure 1). Differences in weight do not appear to wholly account for sex differences in exogenous cortisol, as post-drug sex differences remain significant after statistical correction for either weight or BMI, F’s > 4.8, p’s < .05.
3.2. Emotional arousal
CORT had no effect on state positive (PA) or negative affect (NA; Watson et al., 1988) p’s > .85 (Table 1). In addition, no interactions of Drug (with Group and/or Sex) were found for PA or NA, p’s > .25. PANAS scores suggest that subjects were not emotionally aroused on the CORT administration day, compared to either Placebo day or trait affect scores (Table 1).
3.3. Recall for words
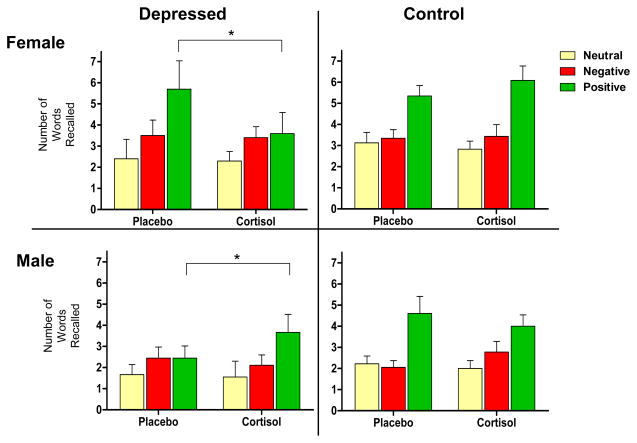
Recall performance is shown in Figure 2. Analysis revealed a Drug × Group × Sex × Valence interaction for free recall, F(2,112)=4.91, p<.01.1 Likewise, when examining memory bias (positive minus negative words remembered), a Drug × Group × Sex interaction is apparent, F(1,56)=5.84, p<.02.1 Posthoc analyses show that these interactions are due to effects of CORT on memory for positive words in the depressed group only, which are in opposite directions for men and women. In depressed women, CORT (compared to placebo) decreased the number of positive words subsequently recalled, t(9)=−2.47, p<.05; i.e., this sample of mildly-to-moderately depressed women showed a relatively positive memory bias for words encoded under placebo (similar to controls), but CORT abolished that positive bias (Figure 2). In depressed men, CORT (compared to placebo) increased the number of positive words subsequently recalled, t(8)=2.48, p<.05. For words encoded under placebo, depressed men showed no bias, but CORT administration instilled a relatively positive bias (Figure 2). CORT had no significant effects on memory formation in the control group. Also, no effects of CORT on memory for neutral words emerged in either group.
Figure 2.
*p < .05.
Free recall for words measured 4–6 days subsequent to encoding during fMRI. Analysis revealed a Drug × Group × Sex × Valence interaction, F(2,112) = 4.91, p < .01. Likewise, when examining memory bias (positive minus negative words recalled), a Drug × Group × Sex interaction is apparent, F(1,56) = 5.84, p < .02. Posthoc analyses show that interactions are due to effects of cortisol on memory for positive words in the depressed group only, which are in opposite directions for men and women. In depressed women (top left panel), CORT (compared to placebo) decreased the number of positive words subsequently recalled, t(9) = −2.47, p < .05; i.e., this sample of mildly depressed women showed a relatively positive memory bias for words encoded under placebo (similar to controls), but cortisol abolished that positive bias. In depressed men (bottom left panel), CORT (compared to placebo) increased the number of positive words subsequently recalled, t(8) = 2.48, p < .05. For words encoded under placebo, depressed men showed no bias, but CORT administration instilled a relatively positive bias.
3.4. Functional MRI results: Whole brain analysis
All subjects
No significant clusters emerged from the Group × Drug × Sex analysis or from the Group × Drug analysis including both men and women. Results for analyses completed separately for each sex follow:
Women
In depressed women, Group × Drug analysis revealed 3 clusters, all of which encompassed the hippocampus. A cluster encompassing the right posterior hippocampus, thresholded at F(1,30) = 9.18, survived the whole-brain cluster correction at p < .05 (Figure 3a). A cluster encompassing the left posterior hippocampus and parahippocampal gyrus, and another cluster in the left anterior hippocampus, also thresholded at F(1,30) = 9.18, survived the less stringent small volume correction at p < .05 for the hypothesized hippocampal region (Table 2).
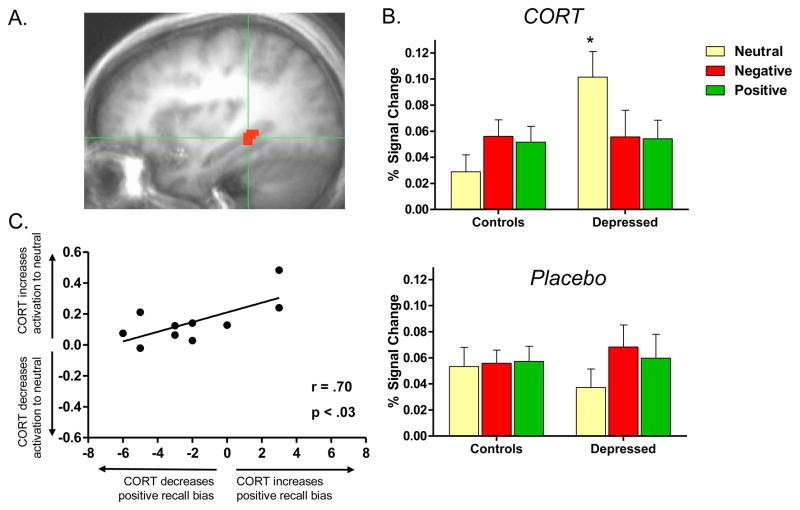
Figure 3.
Alterations in response to CORT administration in depressed women. Figure 3a: Right posterior hippocampus. Depressed compared to healthy women showed altered response to CORT in this region. Figure 3b: Altered response in this region was due to a CORT-related increase in hippocampal response to neutral words in depressed women compared to controls, F(1,30) = 9.63, p < .0051. Within the depressed group, response to neutral words was greater during CORT than placebo administration, t(9) = 5.51, p < .005. Figure 3c: Altered response to CORT was related to CORT’s effects on memory in depressed women, such that women who showed greater CORT-related increase in hippocampal response to neutral (relative to emotional) words showed greater preservation of positive recall bias, r(9) = .70, p < .03.
Table 2.
Brain regions showing altered response to CORT administration in depressed compared to healthy individuals
| Group × Drug Interaction | Talairach Coordinates |
Number of Voxels | ||
|---|---|---|---|---|
| x | y | z | ||
| Females | ||||
| R Posterior Hippocampus | 31 | −33 | −3 | 84 |
| L Posterior Hippocampus & Parahippocampal Gyrus | −36 | −29 | −8 | 49‡ |
| L Anterior Hippocampus | −27 | −12 | −14 | 41‡ |
| Males | ||||
| L Middle Occipital Gyrus | −39 | −91 | 17 | 252 |
| R Superior Frontal Gyrus | 23 | 67 | 19 | 128 |
| Midline Subgenual PFC (BA 25) | 3 | 12 | −5 | 100 |
| R Medial Frontal Gyrus (BA 10) | 7 | 66 | 4 | 99 |
| L Insula (BA 13) | −40 | −21 | 3 | 90 |
| L Superior Temporal Gyrus (BA 41) | −46 | −35 | 13 | 84 |
Note: L, Left; R, Right; PFC prefrontal cortex; Regions shown were significant in the Group (depressed vs. controls) × Drug (CORT vs. placebo) analyses, which were performed separately for females and males. All regions significant at p < .05 after whole brain correction, with the exception of clusters denoted with ‡, which identifies clusters significant at p < .05 after small volume correction (for hypothesized hippocampal region).
Posthoc analysis, where brain activation during encoding of words of each valence was extracted, showed a Group × Drug × Valence interaction, F(2,60) = 10.02, p < .0011 representing CORT’s effects on hippocampal response to neutral words (but not on emotionally arousing words; Figure 3b). The pattern shown in Figure 3b for the right posterior hippocampus was apparent for all three of the hippocampal clusters mentioned above, F’s > 6.8 (not shown). Thus, in depressed compared to healthy women CORT increased hippocampal response to neutral words (but had no effect on response to emotionally arousing words).
Men
Whole-brain analysis did not reveal the hypothesized Group × Drug interaction in the hippocampus in depressed compared to healthy men, but did show Group × Drug interactions in regions listed in Table 2. Contrary to the results reported above for women, in all these regions CORT increased activation to emotionally arousing words (relative to neutral words) in the depressed group, but not in the controls. Activation in these regions was not significantly correlated with memory performance.
3.5. Correlational analyses
Women
We extracted percent signal change for the three hippocampal clusters in which a Group × Drug interaction emerged. We found that CORT’s effects on percent signal change in both the left and the right posterior hippocampal clusters was related to recall measured 4–6 days later in depressed women, but not in controls (Table 3; Figure 3c). In depressed women, for both the left and the right posterior hippocampal clusters, the more CORT increased activation to neutral (compared to emotionally arousing) words, the more the positive bias in memory was preserved (Table 3; Figure 3c). Thus, greater CORT-related hippocampal response to neutral words was protective (i.e., protected against the overall tendency for CORT to reduce recall for positive words; Figure 2). No correlations were found between hippocampal activation and memory for neutral words.
Table 3.
The effects of CORT on hippocampal activation at encoding is related to effects of CORT on subsequent free recall performance in depressed women
| Healthy Women | Depressed Women | |||
|---|---|---|---|---|
| Recall Bias | Recall for Positive Words | Recall Bias | Recall for Positive Words | |
| R Posterior Hippocampus | r = −.16 | r = −.22 | r = .70* | r = .57 |
| L Posterior Hippocampus & Parahippocampal Gyrus | r = −.24 | r = −.34 | r = .66* | r = .85** |
p < .05;
p < .01
Note: The more CORT increased posterior hippocampal activation to neutral (compared to emotionally arousing) words, the more CORT preserved a positive bias for subsequently recalled words. Correlations between brain activation during encoding and subsequent recall were performed on CORT minus Placebo difference scores. See Figure 3c for representative scatter plot. “Recall Bias” refers to number of positive relative to negative words recalled (positive minus negative words recalled).
Men
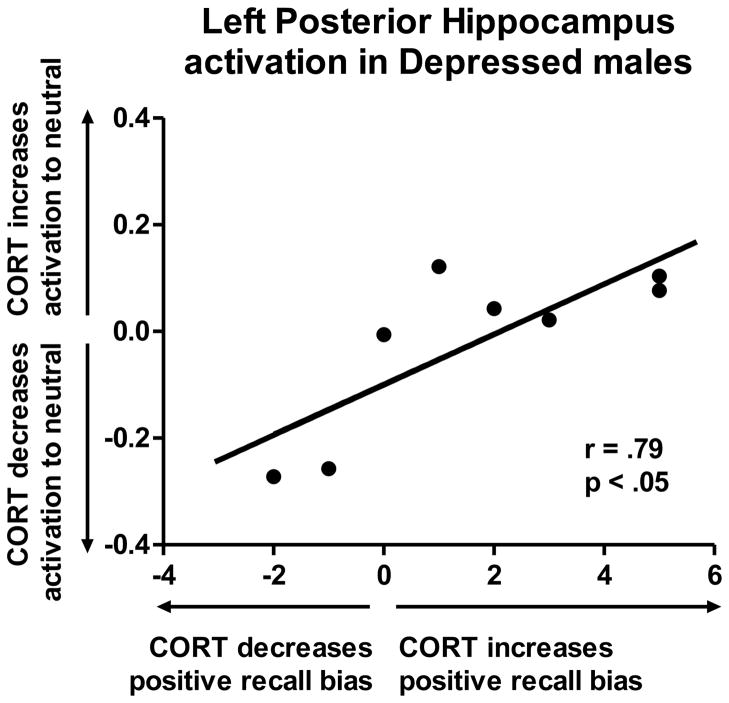
As mentioned above, the depressed men did not show a Group × Drug interaction in the hippocampus in the whole-brain analysis. However, for correlational analyses, we extracted hippocampal signal change using a mask based on the hippocampal clusters that emerged for depressed women. For the left posterior hippocampus, correlational findings were identical to the findings for women: the more CORT increased posterior hippocampal response to neutral (compared to emotionally arousing) words, the more CORT instilled a positive bias, r(7) = .79, p < 0.05 (Figure 4). Thus, increased hippocampal activation in response to neutral words during CORT elevation was protective in both male and female patients.
Figure 4.
For the left posterior hippocampus in depressed males, correlational findings were identical to the findings for women: the more CORT increased posterior hippocampal response to neutral (compared to emotionally arousing) words, the more CORT instilled a positive bias, r(7) = .79, p < 0.05.
4. Discussion
A low dose of hydrocortisone (CORT, i.e., cortisol) during encoding affected subsequent memory performance in mildly-to-moderately depressed but not healthy individuals. These effects varied by gender. In depressed women CORT abolished a relatively positive bias that was apparent for words encoded during placebo administration. However, in depressed men CORT instilled a relatively positive recall bias that was absent for words encoded during placebo. It should be noted that because of sex differences in cortisol levels during the memory consolidation period, it cannot be determined whether findings reflect true sex differences or a confound due to sex differences in exogenous cortisol levels.
CORT’s effects on regional brain activation
Compared to healthy subjects, depressed subjects showed alterations in the effects of CORT on brain activation during memory encoding.
Men
Compared to healthy men, CORT increased activation to emotionally arousing words (relative to neutral words) in depressed men in a number of regions including the following: right subgenual PFC (BA 25), right medial frontal cortex (BA 10), and left insula (BA 13). Activation in these regions was not correlated with subsequent memory performance. However, these findings are noteworthy given prior data showing relations between HPA regulation and activation in subgenual PFC and medial frontal regions (Diorio et al., 1993; Sullivan & Gratton, 1999; Herman et al., 2003; Kern et al., 2008; Jahn et al., 2010), and data showing the importance of the insula in neural processing of peripheral physiology (Craig, 2004) and HPA regulation during sadness (Ottowitz et al., 2004).
Women
Depressed women showed altered effects of CORT on hippocampal activation, as hypothesized. Compared to controls, depressed women showed increased hippocampal response to CORT while encoding neutral (but not emotionally arousing) words. We originally conceived of the neutral words as our “control condition,” and hypothesized that CORT would increase hippocampal activation in response to emotionally arousing (compared to neutral) words. However, in depressed women CORT increased hippocampal response to neutral words (but had no effect on response to emotionally arousing words).
Correlations between CORT’s effects on hippocampal activation and memory bias
In both depressed men and women, the effects of CORT on posterior hippocampal activation were correlated with the effects of CORT on memory formation. The individuals who showed the largest hippocampal response to CORT (compared to placebo) faired the best in terms of CORT’s effects on memory. More specifically, for depressed individuals who showed a CORT-related increase in hippocampal activation in response to neutral words (compared to emotionally arousing words), memory for positive words encoded during CORT administration was enhanced (or preserved). The relations between hippocampal activation and recall were in the same direction for depressed men and women despite sex differences in the direction of CORT-related effects on average recall performance. These correlations are not so-called “voodoo” correlations (Vul et al., 2009). Our method of selecting brain regions was “blind” to subsequent recall performance (Vul et al., 2009). Thus, these data provide compelling evidence that CORT-related increase in hippocampal response to nonarousing information may be protective in depressed men and women.
Null effects of CORT on memory in controls
Contrary to data in the depressed group, we found no effects of CORT on memory in the control group. Data from our lab and others has shown in healthy individuals that cortisol affects memory preferentially during emotional arousal (Abercrombie et al., 2006; Wolf, 2009). Our subjects were not emotionally aroused at the time of fMRI scanning as evidenced by PANAS scores. In addition, they were previously habituated to the scanning environment with mock scanning, and words are not particularly emotionally evocative stimuli. Thus, the lack of cortisol-related effects on memory in healthy controls are consistent with prior data showing that cortisol is unrelated to memory formation in healthy individuals who are not emotionally aroused (Abercrombie et al., 2006). Furthermore, it has been shown previously that cortisol activates the amygdala only under conditions of emotional arousal and noradrenergic activation (Roozendaal et al., 2006b; van Stegeren et al., 2010). Thus, the lack of an effect of cortisol on amygdala activation is also consistent with prior data showing no effects of cortisol under low arousal conditions.
Why is CORT-related enhancement of hippocampal activation to neutral stimuli related to enhancement (or preservation) of memory for positive words in depressed individuals?
Effects of CORT administration on hippocampal response to neutral words were not related to effects of CORT on recall of neutral words. Rather, in depressed subjects, CORT-related enhancement of hippocampal response to neutral words predicted CORT effects on memory for positive words (as described above). Prior research suggests the hippocampus and parahippocampal gyri are preferentially involved in memory for neutral information that is not emotionally arousing (Alkire et al., 1998). Data show that depressed subjects tend to view positively valenced information as less emotionally arousing than do never-depressed individuals (Sloan et al., 1997). Perhaps, the mild cortisol elevations in depressed subjects enhanced the hippocampal role in memory consolidation for information that is salient but not emotionally arousing. Thus, our data lead to the speculative conclusion that mild cortisol elevations may enhance hippocampal function that supports equanimity in cognitive processing of information. However, this speculation requires further study.
Altered neural signaling of cortisol in depression
As mentioned above, research from multiple sources implies that glucocorticoid signaling may be altered in the brain in depression (Raison & Miller, 2003; Schatzberg & Lindley, 2008; Pariante, 2009). Our data show regional specificity of altered effects of cortisol on brain functioning, as well as differences in the effects of cortisol depending on the valence of processed information. Thus, the current data suggest that research questions regarding global over- or under-sensitivity to cortisol may obfuscate important questions, such as 1) where in the brain is cortisol sensitivity altered in depression?, and 2) is cortisol sensitivity altered only during neural processing of certain types of information?
Endogenous glucocorticoids cross the blood-brain barrier and modulate activity in target tissues primarily via the two types of corticosteroid receptors, mineralocorticoid receptors (MR) and glucocorticoid receptors (GR). Prior data suggest that depression may involve imbalanced cortisol signaling at GR vs. MR, where increased functional activity of MR compensates for decreased GR functioning (Young et al., 2003; de Kloet et al., 2007; Otte et al., 2010). Given that the hippocampus is a region with dense population of both MR and GR (Seckl et al., 1991; Patel et al., 2000; but see Sanchez et al., 2000), the regional balance of MR/GR may be particularly important for hippocampal function (de Kloet et al., 2007). It cannot be determined whether signaling at GR or MR or both account for our results. However, one could speculate that the mildly increased cortisol levels enhanced MR functioning in hippocampal tissue, and possibly enhanced hippocampal processing of information that is not particularly emotionally arousing. Future research is needed to substantiate this speculation.
A number of studies have shown that both agonists and antagonists for corticosteroid receptors hold promise as therapeutic agents (DeBattista et al., 2000; Young et al., 2004; DeBattista & Belanoff, 2006; Otte et al., 2010). Our data suggest that the therapeutic efficacy of GC agonists or antagonists may depend on the regional effects of the pharmacological agents. In addition, variation in psychological context (associated with variation in the neural milieu, e.g., noradrenergic activation) during administration of the pharmacological agent may alter efficacy. Future studies might combine pharmacological agents that alter corticosteroid receptor functioning with psychotherapy that provides a positive learning context. Possibly, the pharmacological agent will enhance psychotherapeutic learning, but have no effect on symptoms if the context for positive learning and behavior change is not provided.
Limitations and Future Directions
This study was limited by the small sample size, and a group of depressed patients that may not be representative (i.e., unmedicated, non-smokers, low levels of comorbidity, relatively mildly depressed). It is possible that between-group differences would have differed or been more pronounced if more severely depressed patients had been examined. In addition, the study used a single dose of hydrocortisone that produced mild cortisol elevations. Future studies must examine the effects of different doses of CORT (and/or corticosteroid receptor antagonists or agonists) in larger samples of subjects to make firm conclusions regarding the beneficial vs. impairing effects of cortisol elevations on psychological functioning in depression. A dose of hydrocortisone that produces extreme cortisol elevations could have effects in the opposite direction of our results. Evidence suggests that psychotic symptoms, bipolar symptoms, and history of childhood trauma may be indicators of differing pathophysiologies and GC neural signaling in depression (Young et al., 2004; Heim et al., 2008; Schatzberg & Lindley, 2008). Possibly the effects of cortisol elevations vary depending on these or other factors.
Another limitation of the current study is that recall performance 4–6 days after scanning is too low to examine 1) hippocampal response to remembered vs. forgotten words, and 2) the effects of self-referent endorsement of words. Future studies should use more salient stimuli (such as pictures) that are better remembered over longer periods of time. Furthermore, the current study was not designed to examine the effects of cortisol on functional connectivity, which is certainly an important area for future study. Manual tracing of hippocampal volumes has not been conducted in this sample. Therefore, as of yet, our data are silent with regard to the question of whether variation in hippocampal volume accounts for variation in cortisol’s effects on hippocampal function.
In addition, pharmacologically manipulated cortisol levels differed in men and women. Thus, it is not possible to determine whether the observed sex differences are valid or a confound due to differing levels of cortisol. Consistent with prior research, sex differences in weight do not appear to wholly account for the sex differences in salivary cortisol levels achieved after pharmacological manipulation (Buchanan, 2000). In fact, data from another study in our laboratory show that even when intravenous hydrocortisone dose is adjusted for weight prior to administration, women reach higher plasma cortisol levels than men (unpublished data). In the future, studies with multiple doses will be required to tease apart whether gender differences are due to differing cortisol levels or true sex differences in neural response to cortisol. In addition, our sample size is too small to examine the effects of menstrual cycle and hormonal contraceptives on our effects, but this is undoubtedly an important area for future study (Andreano et al., 2008).
Summary
The current study is the first we know of to examine the effects of exogenously manipulated cortisol elevations on regional brain activity and learning in depression. Altered effects of cortisol on regional brain functioning and emotional memory were observed in depressed compared to healthy participants. Depressed men showed altered response to cortisol elevations in a number of frontal regions as well as the insula. However, cortisol’s effects on activation in these regions were not related to cortisol’s effects on memory. In depressed women, the predicted alterations in cortisol’s effects on hippocampal function emerged. Altered hippocampal response to CORT in depressed relative to healthy humans is consistent with animal models of depression implicating alterations in glucocorticoid effects on hippocampal function (Pittenger & Duman, 2008). In both depressed men and women, effects of cortisol on hippocampal activation were related to effects of cortisol on memory formation, such that cortisol-related enhancement of hippocampal activation was related to preservation (or instillation) of a relative positive memory bias. In light of previous data implicating neural insensitivity to cortisol (Raison & Miller, 2003; Pariante, 2009) and hippocampal dysfunction in depression (Hickie et al., 2005; Preston et al., 2010), these data suggest the speculative conclusion that mild elevations in cortisol may restore hippocampal function. Future research is required to firmly draw this conclusion.
Supplementary Material
Footnotes
The study was designed to minimize effects of order of administration of CORT vs. placebo (i.e., “Drug order”). For instance, subjects were habituated to fMRI scanning prior to the scanning days to minimize novelty. In addition, equal numbers of control (49%) and depressed (52%) participants received recall testing 4 vs. 6 days after CORT administration. Nonetheless, we tested effects of Drug order. Adding Drug order to the model did not alter the effects reported herein. For instance, the interactions presented for recall were unchanged, F’s > 4.9, p’s < .02. In addition, the finding for the right posterior hippocampus in women was unchanged when Drug order was entered in the model. The Group × Drug × Valence interaction remained, F(2,56) = 9.41, p < .001, as well as the difference between depressed and control subjects in CORT-related hippocampal response to neutral words, F(1,28) = 10.65, p < .005.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie HC, Speck NS, Monticelli RM. Endogenous cortisol elevations are related to memory facilitation only in individuals who are emotionally aroused. Psychoneuroendocrinology. 2006;31:187–196. doi: 10.1016/j.psyneuen.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Alfarez DN, Joëls M, Krugers HJ. Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. European Journal of Neuroscience. 2003;17:1928–1934. doi: 10.1046/j.1460-9568.2003.02622.x. [DOI] [PubMed] [Google Scholar]
- Alkire MT, Haier RJ, Fallon JH, Cahill L. Hippocampal, but not amygdala, activity at encoding correlates with long-term, free recall of nonemotional information. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14506–14510. doi: 10.1073/pnas.95.24.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano JM, Arjomandi H, Cahill L. Menstrual cycle modulation of the relationship between cortisol and long-term memory. Psychoneuroendocrinology. 2008;33:874–882. doi: 10.1016/j.psyneuen.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Bagot RC, van Hasselt FN, Champagne DL, Meaney MJ, Krugers HJ, Joels M. Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiology of Learning and Memory. 2009;92:292–300. doi: 10.1016/j.nlm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Blair JR, Spreen O. Predicting premorbid IQ: A revision of the national adult reading test. The Clinical Neuropsychologist. 1989;3:129–136. [Google Scholar]
- Buchanan TW. The effects of exogenous cortisol on emotion-modulated startle and cognition (Dissertation) Dissertation Abstracts International. 2000;61:114. [Google Scholar]
- Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, et al. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. The Journal of Neuroscience. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. Human feelings: why are some more aware than others? Trends in Cognitive Sciences. 2004;8:239–241. doi: 10.1016/j.tics.2004.04.004. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Derijk RH, Meijer OC. Therapy Insight: is there an imbalanced response of mineralocorticoid and glucocorticoid receptors in depression? Nature Clinical Practice Endocrinology & Metabolism. 2007;3:168–179. doi: 10.1038/ncpendmet0403. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joëls M. Stress and cognition: are corticosteroids good or bad guys? Trends in Neurosciences. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- DeBattista C, Belanoff J. The use of mifepristone in the treatment of neuropsychiatric disorders. Trends in Endocrinology and Metabolism. 2006;17:117–121. doi: 10.1016/j.tem.2006.02.006. [DOI] [PubMed] [Google Scholar]
- DeBattista C, Posener JA, Kalehzan BM, Schatzberg AF. Acute antidepressant effects of intravenous hydrocortisone and CRH in depressed patients: a double-blind, placebo-controlled study. American Journal of Psychiatry. 2000;157:1334–1337. doi: 10.1176/appi.ajp.157.8.1334. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. Journal of Neuroscience. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fuchs E, Flugge G, Czeh B. Remodeling of neuronal networks by stress. Frontiers in Bioscience. 2006;11:2746–2758. doi: 10.2741/2004. [DOI] [PubMed] [Google Scholar]
- Gold PW, Drevets WC, Charney DS. New insights into the role of cortisol and the glucocorticoid receptor in severe depression. Biological Psychiatry. 2002;52:381–385. doi: 10.1016/s0006-3223(02)01480-4. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Hemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Frontiers in Neuroendocrinology. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Hickie I, Naismith S, Ward PB, Turner K, Scott E, Mitchell P, et al. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. The British Journal of Psychiatry. 2005;186:197–202. doi: 10.1192/bjp.186.3.197. [DOI] [PubMed] [Google Scholar]
- Hinkelmann K, Moritz S, Botzenhardt J, Riedesel K, Wiedemann K, Kellner M, et al. Cognitive impairment in major depression: association with salivary cortisol. Biological Psychiatry. 2009;66:879–885. doi: 10.1016/j.biopsych.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Jahn AL, Fox AS, Abercrombie HC, Shelton SE, Oakes TR, Davidson RJ, et al. Subgenual prefrontal cortex activity predicts individual differences in hypothalamic-pituitary-adrenal activity across different contexts. Biological Psychiatry. 2010;67:175–181. doi: 10.1016/j.biopsych.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Krugers HJ. LTP after stress: up or down? Neural Plasticity. 2007;2007:93202. doi: 10.1155/2007/93202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33:517–529. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH. Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sciences. 1996;58:1475–1483. doi: 10.1016/0024-3205(96)00118-x. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E. Structural plasticity and hippocampal function. Annual Review of Psychology. 2010;61:111–140. C111–113. doi: 10.1146/annurev.psych.093008.100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM. Magnetic resonance imaging and prediction of outcome in patients with major depressive disorder. Journal of Psychiatry & Neuroscience. 2009;34:343–349. [PMC free article] [PubMed] [Google Scholar]
- Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte C, Hinkelmann K, Moritz S, Yassouridis A, Jahn H, Wiedemann K, et al. Modulation of the mineralocorticoid receptor as add-on treatment in depression: a randomized, double-blind, placebo-controlled proof-of-concept study. Journal of Psychiatric Research. 2010;44:339–346. doi: 10.1016/j.jpsychires.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Ottowitz WE, Dougherty DD, Sirota A, Niaura R, Rauch SL, Brown WA. Neural and endocrine correlates of sadness in women: implications for neural network regulation of HPA activity. The Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16:446–455. doi: 10.1176/jnp.16.4.446. [DOI] [PubMed] [Google Scholar]
- Pariante CM. Risk factors for development of depression and psychosis. Glucocorticoid receptors and pituitary implications for treatment with antidepressant and glucocorticoids. Annals of the New York Academy of Sciences. 2009;1179:144–152. doi: 10.1111/j.1749-6632.2009.04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PD, Lopez JF, Lyons DM, Burke S, Wallace M, Schatzberg AF. Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. Journal of Psychiatric Research. 2000;34:383–392. doi: 10.1016/s0022-3956(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Preston AR, Bornstein AM, Hutchinson JB, Gaare ME, Glover GH, Wagner AD. High-resolution fMRI of content-sensitive subsequent memory responses in human medial temporal lobe. Journal of Cognitive Neuroscience. 2010;22:156–173. doi: 10.1162/jocn.2009.21195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. American Journal of Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, de Quervain DJ, McGaugh JL. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006a;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2006b;103:6741–6746. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. Journal of Neuroscience. 2000;20:4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzberg AF, Lindley S. Glucocorticoid antagonists in neuropsychiatric disorders. European Journal of Pharmacology. 2008;583:358–364. doi: 10.1016/j.ejphar.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Dickson KL, Yates C, Fink G. Distribution of glucocorticoid and mineralocorticoid receptor messenger RNA expression in human postmortem hippocampus. Brain Research. 1991;561:332–337. doi: 10.1016/0006-8993(91)91612-5. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Stressful experience and learning across the lifespan. Annual Review of Psychology. 2006;57:55–85. doi: 10.1146/annurev.psych.57.102904.190205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DM, Strauss ME, Quirk SW, Sajatovic M. Subjective and expressive emotional responses in depression. Journal of Affective Disorders. 1997;46:135–141. doi: 10.1016/s0165-0327(97)00097-9. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. The Journal of Neuroscience. 1999;19:2834–2840. doi: 10.1523/JNEUROSCI.19-07-02834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Stegeren AH, Roozendaal B, Kindt M, Wolf OT, Joels M. Interacting noradrenergic and corticosteroid systems shift human brain activation patterns during encoding. Neurobiology of Learning and Memory. 2010;93:56–65. doi: 10.1016/j.nlm.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly High Correlations in fMRI Studies of Emotion, Personality, and Social Cognition. Perspectives on Psychological Science. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, et al. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biological Psychiatry. 2004;56:101–112. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wolf OT. Stress and memory in humans: twelve years of progress? Brain Research. 2009;1293:142–154. doi: 10.1016/j.brainres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology. 2001;26:711–720. doi: 10.1016/s0306-4530(01)00025-7. [DOI] [PubMed] [Google Scholar]
- Young AH, Gallagher P, Watson S, Del-Estal D, Owen BM, Ferrier IN. Improvements in neurocognitive function and mood following adjunctive treatment with mifepristone (RU-486) in bipolar disorder. Neuropsychopharmacology. 2004;29:1538–1545. doi: 10.1038/sj.npp.1300471. [DOI] [PubMed] [Google Scholar]
- Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, Akil H. Mineralocorticoid receptor function in major depression. Archives of General Psychiatry. 2003;60:24–28. doi: 10.1001/archpsyc.60.1.24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.