Abstract
Calcineurin inhibitors such as cyclosporin A (CsA) are the mainstay of immunosuppressive treatment for organ transplant recipients. Squamous cell carcinoma (SCC) of the skin is a major complication of treatment with these drugs, with a 65–100 fold higher risk than in the normal population1. By contrast, the incidence of basal cell carcinoma (BCC), the other major keratinocyte-derived tumour of the skin, of melanoma and of internal malignancies increases to a significantly lesser extent 1. Here we report that genetic and pharmacological suppression of calcineurin/NFAT function promotes tumour formation in mouse skin and in xenografts, in immune compromised mice, of H-rasV12 expressing primary human keratinocytes or keratinocyte-derived SCC cells. Calcineurin/NFAT inhibition counteracts p53-dependent cancer cell senescence thereby increasing tumourigenic potential. ATF3, a member of the “enlarged” AP-1 family, is selectively induced by calcineurin/NFAT inhibition, both under experimental conditions and in clinically occurring tumours, and increased ATF3 expression accounts for suppression of p53-dependent senescence and enhanced tumourigenic potential. Thus, intact calcineurin/NFAT signalling is critically required for p53 and senescence-associated mechanisms that protect against skin squamous cancer development.
Keywords: Squamous Cell Carcinoma, Cancer Stem cells, cyclosporine, calcineurin/NFAT, p53, ATF3
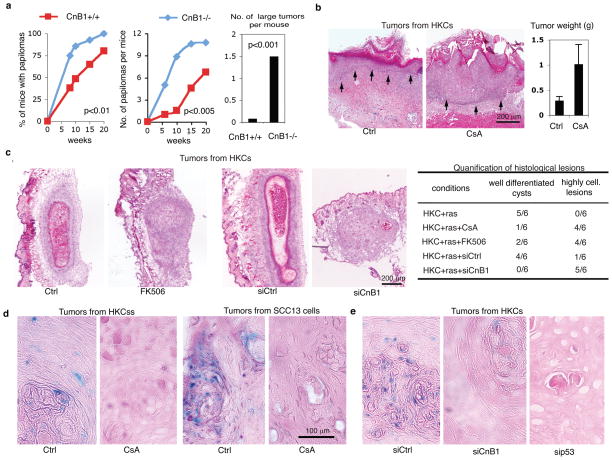
The selectively increased risk of SCC formation in CsA-treated patients1 suggested that that calcineurin signalling, central to keratinocyte growth/differentiation control 2–4, plays an intrinsic role in keratinocyte tumour suppression. Mice with keratinocyte-specific deletion of the calcineurin B1 gene (CnB1), essential for calcineurin activity 3, exhibited increased susceptibility to chemically-induced carcinogenesis, with decreased latency, higher incidence and size of tumours, and earlier malignant conversion (Fig. 1a, Suppl. Fig. 1).
Figure 1. Calcineurin/NFAT inhibition promotes keratinocyte tumour formation.
a, Multistep skin carcinogenesis of mice with keratinocyte-specific CnB1 deletion (CnB1 −/−)3 together with littermate controls (CnB1+/+). Numbers of total and large (>4.5mm diameter) tumours were determined weekly. For histology see Suppl. Fig. 1. b, Grafting of H-rasV12 expressing HKCs onto Scid mice plus/minus subsequent CsA treatment. Epidermal-dermal junction: black arrows. For higher magnification images and experimental conditions see Suppl. Fig. 2a. c, H-rasV12 expressing HKCs were injected at dermal-epidermal junction of Scid mice plus/minus subsequent CsA or FK506 treatment. Similar assays were performed with H-rasV12 expressing HKCs with siRNA-mediated CnB1 knockdown (siCnB1) versus control (siCtrl). Histological analysis was 10 days later, summarized on the right. For higher magnification images and experimental conditions: Suppl. Fig. 2b,c. d and e, Lesions formed by H-rasV12 expressing HKCs or SCC13 cells in mice plus/minus CsA-treatment (d) or plus/minus CnB1 and p53 knockdown (e) were analyzed by in situ chromogenic assay for senescence associated β-galactosidase activity17. For additional images, data quantification, experimental conditions see suppl. Figs. 3–5.
To assess the relevance of these findings to human skin, primary human keratinocytes (HKCs) were infected with an oncogenic H-rasV12-transducing retrovirus, followed by skin-reconstitution grafting assays onto immune compromised (Scid) mice. In control mice, H-rasV12-expressing HKCs produced an acanthotic epithelium with normal pattern of differentiation. In CsA-treated mice, grafted cells gave rise to tumours with disordered proliferation and histopathological features of moderately to poorly differentiated SCC (Fig. 1b; Suppl. Fig. 2a). Similar results were obtained by cell injection at the dermal-epidermal junction, i.e. a location approximating that of malignant skin tumour formation. Ras-expressing HKCs formed only differentiated epidermal cysts in control animals, while, in animals treated with CsA or the unrelated calcineurin inhibitor FK506, they produced highly cellular lesions with decreased differentiation (Fig. 1c, Suppl. Fig. 2b). Similar lesions were produced by H-rasV12-expressing HKCs with siRNA-mediated knock-down of the CnB1 gene (Fig. 1c, Suppl. Fig. 2c), and in mice treated with a calcineurin/NFAT inhibitory peptide (VIVIT)5, versus control scrambled peptide (VEET) (Suppl. Fig. 2d–f).
Cutaneous SCCs are characterized by mutation and/or decreased expression of p53, but only 10–20% have ras mutations6, raising the question of the relevance of the present findings for cancer cells without ras activation. Nucleotide sequencing showed that SCC12 and SCC13 cells, two independent lines from cutaneous SCC with poorly aggressive properties 7, have wild type ras genes and, as reported 8,9, single p53 missense mutations. Up-regulation of endogenous p53 in these cells still induces “canonical” effectors like p21WAF1/Cip1 10, possibly through an indirect mechanism like the reported ability of mutant p53 to bind and titrate p6311,12, a negative regulator of p21 expression and senescence in keratinocytes13,14. Injection of SCC12 and SCC13 cells at the dermal-epidermal junction resulted in differentiated cysts. By contrast, in mice treated with CsA or VIVIT, or as a consequence of p53 knockdown, SCC cells formed highly cellular and moderately differentiated infiltrating tumours (Suppl. Figs.3,4a).
Cancer cell senescence is a failsafe mechanism against tumour development15 which can be associated with increased expression of terminal differentiation markers16. Staining for senescence-associated β-galactosidase activity (SA-β-Gal)17 was positive in lesions formed by ras-expressing HKCs or SCC13 cells in control but not CsA-treated mice (Fig. 1d, Suppl. Fig. 5a). This was paralleled by differences in Ki67 and K1 differentiation marker expression (Suppl. Fig. 6). Similarly decreased SA-β-Gal staining was found in tumours formed by ras-expressing HKCs with knockdown of CnB1 or, as predicted from the literature, of p53, a key mediator of oncogene-induced senescence15 (Fig. 1e, Suppl. Fig. 5b). Senescence was also suppressed in tumours formed by SCC13 cells with p53 knockdown (Suppl. Fig. 4b,c).
Ras induced senescence of cultured cells was also counteracted by CsA or VIVIT treatment and p53 knockdown (Suppl. Fig. 7a–c). Induction of terminal differentiation markers was similarly suppressed (Suppl. Fig. 7d). Paralleling these changes and consistent with previous reports15, oncogenic ras expression caused increased p53 protein levels, without effects on transcription (Suppl. Fig. 7e). Importantly, calcineurin/NFAT inhibition counteracted the ras effects suppressing p53 expression not only at the protein but also mRNA level (Suppl. Fig. 7e).
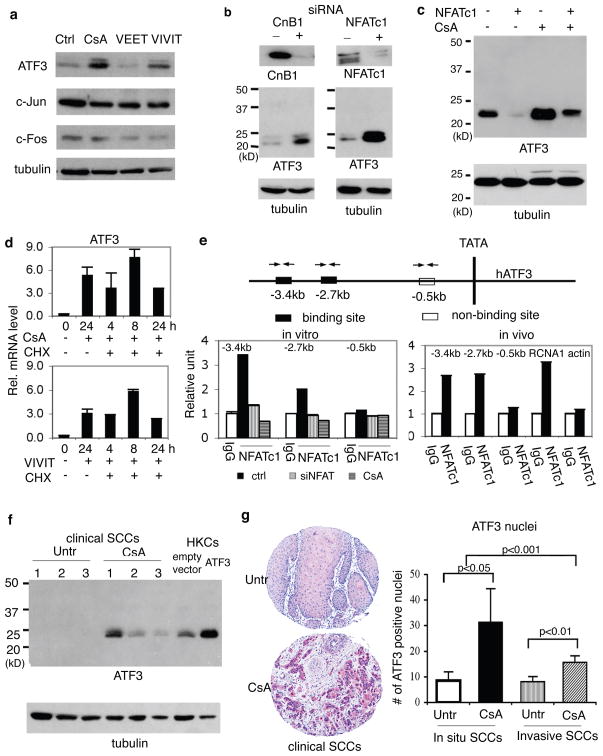
In HKCs and SCC cells, p53 gene transcription is under negative control of the AP-1 complex, specifically c-Jun and c-Fos10. Real time RT-PCR and immunoblotting showed that c-Jun and c-Fos levels were unaffected by CsA or VIVIT treatment of HKCs. By contrast, expression of ATF3, a member of the “enlarged” AP-1 family previously connected with SCC progression18, was sharply up-regulated (Fig. 2a, Suppl. Fig. 8a). ATF3 expression also increased after Calcineurin B1 or NFATc1 knockdown (Fig. 2b; Suppl. Fig. 8b,c), and in SCC13 cells treated with CsA or VIVIT (Suppl. Fig. 8d). Enhanced ATF3 expression in CsA- or VIVIT-treated keratinocytes was paralleled by increased binding of the ATF3 protein to specific oligonucleotide sequences of the p53 promoter containing intact, but not mutated, ATF3 binding sites (Suppl. Fig. 8e). Increased ATF3 expression in CsA-treated keratinocytes was suppressed by retrovirally expressed constitutively active NFATc119 (Fig. 2c, Suppl. Fig. 8f). The kinetics of NFATc1 knockdown and ATF3 up-regulation were highly correlated (Suppl. Fig. 8g), and induction of ATF3 by CsA or VIVIT occurred to similar or greater extent when protein synthesis was inhibited (Fig. 2d). Consistent with ATF3 being a direct target, chromatin immunoprecipitation assays showed binding of endogenous NFATc1 to two distinct regions of the ATF3 promoter harboring NFAT binding sites, such binding being abolished by NFATc1 knockdown or CsA treatment (Fig. 2e, left panel). In intact human epidermis, we also detected NFATc1 binding to the ATF3 promoter comparable to a well established NFATc1 target, the calcipressin gene (RCNA1)20 (Fig. 2e, right panel).
Figure 2. Calcineurin/NFAT signalling negatively controls ATF3 expression.
a and b, HKCs plus/minus CsA or VIVIT treatment (a) or CnB1 or NFATc1 knockdown (b) were analyzed in parallel with controls by immunoblotting. Similar results were obtained at mRNA level (Suppl. Fig. 8a–c). c, HKCs infected with retroviruses expressing constitutively active NFATc1 (+)19 or GFP control (−) plus/minus subsequent CsA treatment were analyzed for ATF3 expression. Similar results were obtained by real time RT-PCR (Suppl. Fig. 8f). d, HKCs plus/minus CsA/VIVIT treatment and cycloheximide exposure were analyzed at various times (hours) for ATF3 expression by real time RT-PCR. Error bars represent mean ± s.d (n = 3 replicates). e, Extracts of HKCs plus/minus CsA treatment or NFACTc1 knockdown (left panel) or intact human epidermis (right panel) were processed for Chip with anti-NFATc1 antibodies or non-immune IgGs, followed by real time PCR of ATF3 promoter regions containing and lacking high-affinity NFATc1 binding sites (black and white boxes in the map above). Chip assays of the NFAT binding region of the calcipressin (RCNA1) gene20, and a β-actin genomic region without NFAT binding sites were included. f, SCCs from 3 patients under CsA treatment and from untreated patients (Untr) from the general population were analyzed by immunoblotting for ATF3 expression, in parallel with HKCs infected with an ATF3 expressing retrovirus (ATF3) versus empty vector control. Similar differences in ATF3 mRNA expression were observed with an independent set of patients (Suppl. Fig. 9a). g, tissue arrays of cutaneous SCCs from patients under CsA treatment versus general untreated population (Untr) were analyzed for ATF3 expression by immunohistochemistry. Representative staining is shown along with quantification of ATF3 positive nuclei in each visual field of tumour tissues. Error bars represent mean ± s.d (n = 18, 16, 126 and 127 tumors per group, in the order).
CsA treatment caused ATF3 up-regulation and p53 down-modulation also in human skin explants and tumour xenografts (Suppl. Fig. 8h,i). Increased ATF3 and decreased p53 expression was also observed in SCCs from CsA-treated patients versus untreated patients, together with decreased senescence (Fig. 2f, Suppl. Fig. 9). Increased ATF3 expression in SCCs from CsA-treated patients was further confirmed by tissue array/immunohistochemical analysis of a cohort of tumour biopsies (Fig. 2g). Nucleotide sequence analysis of p53 in 5 SCCs from CsA-treated patients revealed missense mutations affecting the DNA binding domain and/or polymorphisms associated with cancer development (72 Pro/Arg substitution) similar to those in the general patient population (www-p53.iarc.fr).
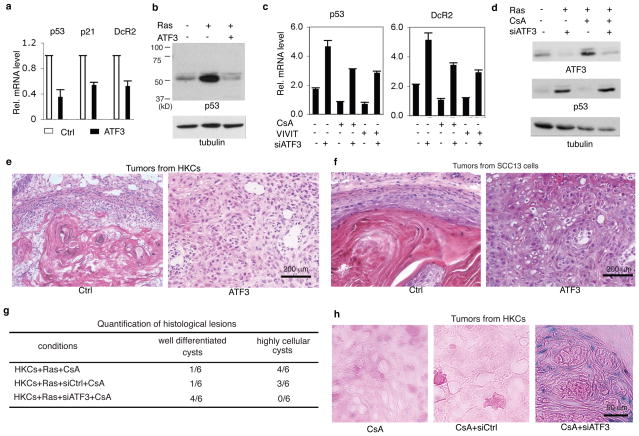
Functionally, ectopic ATF3 expression, at levels comparable to those occurring in SCCs from CsA-treated patients (Fig. 2f), blocked expression of p53 and senescence-associated genes (Fig. 3a,b). Conversely, ATF3 knockdown induced these genes, counteracting the CsA and VIVIT effects (Fig. 3c,d, Suppl. Fig. 10). In vivo, ectopic ATF3 promoted tumourigenicity of H-rasV12-expressing HKCs and SCC cells, produced aggressive tumours with reduced senescence as those caused by calcineurin/NFAT inhibitors (Fig. 3e,f; Suppl. Fig. 11a,b,e). Conversely, ATF3 knockdown overcame the tumour promoting effects of these compounds and restored senescence (Fig. 3g,h; Suppl. Fig. 11c,d).
Figure 3. ATF3 up-regulation enhances keratinocyte tumour formation and suppresses cancer cell senescence.
a, HKCs infected with ATF3 expressing (ATF3) and control retroviruses were analyzed by real time RT-PCR for indicated genes. Error bars represent mean ± s.d (n = 3 replicates). b, HKCs plus/minus infection with ATF3 and H-rasV12 expressing retroviruses were analyzed for p53 expression by immunoblotting. c, HKCs plus/minus ATF3 knockdown and CsA/VIVIT treatment were analyzed by real time RT-PCR for p53 and DcR2 expression. Additional results are shown in Suppl. Fig. 10a. d, HKCs plus/minus H-rasV12 expression, ATF3 knockdown and CsA treatment as indicated were analyzed for ATF3 and p53 expression by immunoblotting. For densitometric quantification and experimental conditions see Suppl. Fig. 10b. e and f, H-rasV12 expressing HKCs (e) or SCC13 cells (f) infected with ATF3 expressing and control retroviruses were injected at the dermal-epidermal junction of Scid mice. Shown are histological images of lesions recovered 6 weeks later. For quantification see Suppl. Fig. 11a,b. g, H-rasV12 expressing HKCs plus/minus ATF3 knockdown were injected at the dermal-epidermal junction of Scid mice, followed by CsA treatment. Mice were sacrificed 10 days later. For histological images see Suppl. Fig. 11c. h Lesions from the previous experiment were analyzed for SA-β-galactosidase activity. Low magnification images and quantification are shown in Suppl. Fig. 11d.
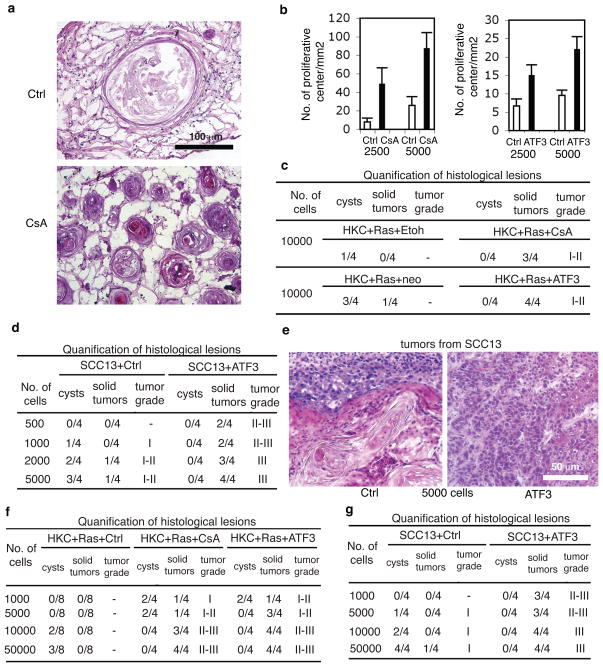
Senescence serves to restrict tumourigenic potential of cells15. To test whether calcineurin inhibition exerts opposite effects, H-rasV12-expressing HKCs were sorted for elevated integrin α6 and low CD71 levels (α6bri CD71dim cells), enriching for cells with high self renewal potential21. In culture, response of α6bri CD71dim cells to VIVIT and CsA treatment, in term of ATF3 and p53 levels, was similar to that of total unsorted populations (data not shown). To assess their in vivo behaviour, H-rasV12-expressing α6bri CD71dim cells were injected in serial dilutions, together with constant amount of normal HKCs, into NOD/SCID interleukin-2 receptor gamma chain null (Il2rg(−/−)) mice22. Addition of matrigel allowed retention of injected cells in well identifiable “nodules”. Histological analysis of nodules from control mice at 1 month after injection showed that H-rasV12-expressing HKCs injected in low number formed only a few keratinized cysts with limited cellularity. By contrast, in nodules recovered from CsA-treated mice there was a striking number of “proliferative centers”, composed of keratinocytes with elevated Ki67 positivity (Fig. 4a,b; Suppl. Fig. 12). When injected in higher number, H-rasV12-expressing HKCs formed highly keratinized and poorly proliferative cysts in control mice, while in CsA-treated animals they gave rise to overt tumours (Fig. 4c). Similar results were obtained with sorted H-rasV12- and ATF3- expressing HKCs versus controls (Fig. 4b,c).
Figure 4. Calcineurin inhibition and increased ATF3 enhance cancer initiating cell populations.
a–c, Ha-rasV12 expressing HKCs sorted for high α6 integrin and low CD71 expression (α6briCD71dim cells) were injected in the indicated numbers into NOD/SCID-Il2rg(−/−) mice22, plus/minus subsequent CsA treatment for 4 weeks. Similar experiments were also performed with sorted H-rasV12expressing HKCs plus/minus ATF3 overexpression. a: histological images of nodules formed by 2,500 Ha-rasV12-HKCs in mice plus/minus CsA treatment. b, quantification of proliferative centers and, c, cysts or solid tumours formed by the indicated cell numbers. Error bars represent mean ± s.d (n = 4 nodules per condition). For low magnification images, quantification criteria and immunofluorescence analysis see Suppl. Fig. 12a,b. d and e, sorted α6briCD71dim populations of SCC13 cells plus/minus ATF3 overexpression were injected in the indicated numbers into Scid mice. Shown are quantification of the results and representative histological images. Experimental conditions were the same as with CD133 sorted cells (Suppl. Fig. 13). f and g, Cells dissociated from tumours formed by Ha-rasV12-HKCs in mice plus/minus CsA treatment, or with ATF3 overexpression (f) or from tumours formed SCC13 cells plus/minus ATF3 overexpression (g) were injected in decreasing numbers into secondary recipient mice (Scid), with tissue retrieval 4 weeks later. For representative images and experimental conditions see Suppl. Fig. 14.
Established cancer cell lines, including SCC cells, also contain distinct sub-populations with different growth/tumourigenic potential23,24. SCC13 cells plus/minus increased ATF3 expression were sorted for elevated integrin α6 and low CD71 levels (cells) or for expression of CD133, a cell surface marker of putative cancer stem cell populations, including keratinocyte-derived24. Sorting resulted in >20 fold enrichment of tumourigenic cells. As few as 500 sorted SCC13 cells overexpressing ATF3 produced detectable tumours, while larger numbers of control cells were required (Fig. 4d; Suppl. Fig. 13a). Histologically, even the lowest number of ATF3-expressing cells produced highly cellular tumours with poor differentiation, while control cells formed differentiated cystic lesions (Fig. 4d,e, Suppl. Fig. 13a,b).
In further testing, as little as 1000 freshly dissociated cells from tumours formed by H-rasV12-expressing HKCs in CsA-treated mice gave rise to secondary tumours, while substantially higher cell numbers were required in control mice (Fig. 4f). Similar results were obtained with H-rasV12-expressing HKCs or SCC13 cells plus/minus increased ATF3 expression (Fig. 4f,g). Histologically, secondary tumours formed in CsA-treated mice or by ATF3-expressing cells were highly cellular and poorly differentiated, while those under control conditions consisted, as the primary tumours, of differentiated cysts (Fig. 4f,g, Suppl. Fig. 14).
Many regulatory pathways, including calcineurin/NFAT2–4,25, play both growth-promoting and -suppressing functions, depending on cell type and context. An impact on tumour growth has been recently attributed to calcineurin via indirect effects on angiogenesis26,27. The intrinsic double negative genetic pathway that we have uncovered here is based on calcineurin/NFAT suppression of ATF3, a negative regulator of p53. The resulting impact on keratinocyte cancer cell senescence versus growth is of likely clinical significance for the many patients under treatment with calcineurin inhibitors as immunosuppressants1, and may be of relevance for other cancer types where altered calcium signalling plays a role28.
Methods summary
Multistep skin carcinogenesis assays were performed with mice with keratinocyte-specific deletion of the CnB1 gene (CnB1loxP/loxPxK5-CrePR1)3 in parallel with Cre-negative controls (CnB1loxP/loxP). Keratinocyte grafting assays were performed as previously described29. Intradermal tumourigenicity assays were adapted from hair follicle reconstitution assays30. Detailed conditions for these assays as well as chromatin immunoprecipitation, immunoblotting, immunofluorescence, senescence β-galactosidase staining, biotinylated DNA pull down assays, and sorting can be found in the Method section and supplementary figure legends.
Supplementary Material
Acknowledgments
We thank Drs. P. Khavari, S. Kitajima, N. Clipstone, G. Crabtree for gift of retroviruses, W. Austen (MGH, Boston, MA) for human skin material, C. Brisken and C. Missero for careful reading of the manuscript, and E. Castillo for sequencing of the ras and p53 genes. This work was supported by grants from NIH (AR054856 and AR39190), the Swiss National Foundation (311003A-122281/1), Oncosuisse (OCS-02361-02-2009), the European Union (Epistem, Sixth Framework Program, LSHB-CT-2005-019067) and, in part, by a grant to S.C. by the Korean Government Foundation (KRF-2007-013-E00044) and to G.H. by the Olga-Mayenfisch-Stiftung.
Footnotes
Author Contributions: B-C. N., P.D, S.C, Y.B., K.L. and G.H. performed research and analysed data; X.W. and G.P.D. designed and performed research, analysed data and wrote the manuscript.
References
- 1.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681–1691. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 2.Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mammucari C, et al. Integration of Notch 1 and calcineurin/NFAT signaling pathways in keratinocyte growth and differentiation control. Dev Cell. 2005;8:665–676. doi: 10.1016/j.devcel.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Santini MP, Talora C, Seki T, Bolgan L, Dotto GP. Cross talk among calcineurin, Sp1/Sp3, and NFAT in control of p21(WAF1/CIP1) expression in keratinocyte differentiation. Proc Natl Acad Sci U S A. 2001;98:9575–9580. doi: 10.1073/pnas.161299698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aramburu J, et al. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–2133. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- 6.Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis. 2005;26:1657–1667. doi: 10.1093/carcin/bgi123. [DOI] [PubMed] [Google Scholar]
- 7.Rheinwald JG, Beckett MA. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultures from human squamous cell carcinomas. Cancer Res. 1981;41:1657–1663. [PubMed] [Google Scholar]
- 8.Brash DE, et al. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci U S A. 1991;88:10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns JE, et al. Gene mutations and increased levels of p53 protein in human squamous cell carcinomas and their cell lines. Br J Cancer. 1993;67:1274–1284. doi: 10.1038/bjc.1993.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolev V, et al. EGFR signalling as a negative regulator of Notch1 gene transcription and function in proliferating keratinocytes and cancer. Nat Cell Biol. 2008;10:902–911. doi: 10.1038/ncb1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adorno M, et al. A Mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell. 2009;137:87–98. doi: 10.1016/j.cell.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 12.Gaiddon C, Lokshin M, Ahn J, Zhang T, Prives C. A subset of tumor-derived mutant forms of p53 down-regulate p63 and p73 through a direct interaction with the p53 core domain. Mol Cell Biol. 2001;21:1874–1887. doi: 10.1128/MCB.21.5.1874-1887.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keyes WM, et al. p63 deficiency activates a program of cellular senescence and leads to accelerated aging. Genes Dev. 2005;19:1986–1999. doi: 10.1101/gad.342305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen BC, et al. Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev. 2006;20:1028–1042. doi: 10.1101/gad.1406006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448:767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- 16.Krizhanovsky V, et al. Implications of cellular senescence in tissue damage response, tumor suppression, and stem cell biology. Cold Spring Harb Symp Quant Biol. 2008;73:513–522. doi: 10.1101/sqb.2008.73.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimri GP, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang A, et al. Epidermal hyperplasia and oral carcinoma in mice overexpressing the transcription factor ATF3 in basal epithelial cells. Mol Carcinog. 2007;46:476–487. doi: 10.1002/mc.20298. [DOI] [PubMed] [Google Scholar]
- 19.Neal JW, Clipstone NA. A constitutively active NFATc1 mutant induces a transformed phenotype in 3T3-L1 fibroblasts. J Biol Chem. 2003;278:17246–17254. doi: 10.1074/jbc.M300528200. [DOI] [PubMed] [Google Scholar]
- 20.Cano E, Canellada A, Minami T, Iglesias T, Redondo JM. Depolarization of neural cells induces transcription of the Down syndrome critical region 1 isoform 4 via a calcineurin/nuclear factor of activated T cells-dependent pathway. J Biol Chem. 2005;280:29435–29443. doi: 10.1074/jbc.M506205200. [DOI] [PubMed] [Google Scholar]
- 21.Li A, Kaur P. FACS enrichment of human keratinocyte stem cells. Methods Mol Biol. 2005;289:87–96. doi: 10.1385/1-59259-830-7:087. [DOI] [PubMed] [Google Scholar]
- 22.Quintana E, et al. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Locke M, Heywood M, Fawell S, Mackenzie IC. Retention of intrinsic stem cell hierarchies in carcinoma-derived cell lines. Cancer Res. 2005;65:8944–8950. doi: 10.1158/0008-5472.CAN-05-0931. [DOI] [PubMed] [Google Scholar]
- 24.Prince ME, Ailles LE. Cancer stem cells in head and neck squamous cell cancer. J Clin Oncol. 2008;26:2871–2875. doi: 10.1200/JCO.2007.15.1613. [DOI] [PubMed] [Google Scholar]
- 25.Mancini M, Toker A. NFAT proteins: emerging roles in cancer progression. Nat Rev Cancer. 2009;9:810–820. doi: 10.1038/nrc2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baek KH, et al. Down’s syndrome suppression of tumour growth and the role of the calcineurin inhibitor DSCR1. Nature. 2009;459:1126–1130. doi: 10.1038/nature08062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryeom S, et al. Targeted deletion of the calcineurin inhibitor DSCR1 suppresses tumor growth. Cancer Cell. 2008;13:420–431. doi: 10.1016/j.ccr.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Roderick HL, Cook SJ. Ca2+ signalling checkpoints in cancer: remodelling Ca2+ for cancer cell proliferation and survival. Nat Rev Cancer. 2008;8:361–375. doi: 10.1038/nrc2374. [DOI] [PubMed] [Google Scholar]
- 29.Lefort K, et al. Notch1 is a p53 target gene involved in human keratinocyte tumor suppression through negative regulation of ROCK1/2 and MRCKalpha kinases. Genes Dev. 2007;21:562–577. doi: 10.1101/gad.1484707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng Y, et al. Organogenesis from dissociated cells: generation of mature cycling hair follicles from skin-derived cells. J Invest Dermatol. 2005;124:867–876. doi: 10.1111/j.0022-202X.2005.23716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.