Abstract
Purpose
The goal of the study was to assess individual differences in risk-taking behavior among adolescents in the laboratory. A second aim was to evaluate whether the laboratory-based risk-taking behavior is associated with other behavioral and psychological measures associated with risk-taking behavior.
Methods
Eighty-two adolescents with no personal history of psychiatric disorder completed a computerized decision-making task, the Wheel of Fortune (WOF). By offering choices between clearly defined probabilities and real monetary outcomes, this task assesses risk preferences when participants are confronted with potential rewards and losses. The participants also completed a variety of behavioral and psychological measures associated with risk-taking behavior.
Results
Performance on the task varied based on the probability and anticipated outcomes. In the winning sub-task, participants selected low probability-high magnitude reward (high-risk choice) less frequently than high probability-low magnitude reward (low-risk choice). In the losing sub-task, participants selected low probability-high magnitude loss more often than high probability-low magnitude loss. On average, the selection of probabilistic rewards was optimal and similar to performance in adults. There were, however, individual differences in performance, and one-third of the adolescents made high-risk choice more frequently than low-risk choice while selecting a reward. After controlling for sociodemographic and psychological variables, high-risk choice on the winning task predicted “real-world” risk-taking behavior and substance-related problems.
Conclusions
These findings highlight individual differences in risk-taking behavior. Preliminary data on face validity of the WOF task suggest that it might be a valuable laboratory tool for studying behavioral and neurobiological processes associated with risk-taking behavior in adolescents.
Keywords: adolescent, decision-making, risky behavior, reward, punishment
Introduction
Adolescence is a major transition period in life when individuals undergo significant physical, psychological and social changes to assume the roles and responsibilities of adulthood. Although these maturational transitions offer tremendous opportunities for youth, because the developing brain and behavioral and cognitive systems mature at different rates, this period also is marked by heightened vulnerability [1]. Relative to other age groups, adolescence is described as a period of increased risk-taking [2, 3]. For example, adolescents are more likely than adults to drive speedily and/or while intoxicated, to have casual sexual partners and/or unprotected sex, and to engage in violent and other criminal behavior [4]. Yet, not all adolescents are risk-takers and identifying individual differences in risk-taking is important for targeting those at greatest risk [5]. A wide range of vulnerability factors and life processes interact to determine adolescent risk-taking behavior. Multi-dimensional approaches are needed, ranging from behavioral processes and neural substrates underlying risk-taking behaviors to assessing risk-taking behaviors in the real world [6].
Several paradigms have been developed for the assessment of risk-taking behavior in the laboratory, and the Iowa Gambling Task (IGT) is most popular [7,8]. The IGT is a card game in which participants are told to accumulate as much play money as possible by picking one card at a time from any of four decks. Participants differ on their ability to avoid decks which have cards with higher immediate gains but overall long-term losses. Performance on the IGT relies on a complex set of cognitive processes, particularly integrating information about the outcome of choices into a continuously updated decision-making strategy under ambiguous conditions. Previous studies of the IGT in pediatric populations found that younger adolescents perform more poorly than older adolescents and adults [8,9,10]. It is not clear whether these developmental differences on the IGT are due to differences in learning or because younger adolescents are more risk-taking. The IGT also does not consider risk-taking along a continuum in which some degree of risk is adaptive and higher levels produce negative consequences [11].
A second task frequently used to study risk-taking behavior is the Balloon Analogue Risk Task (BART) [12]. Participants press on the mouse to puff up the computer image of a balloon, each press earns one cent, and all earnings are lost if the balloon pops. Pressing a separate ‘collect’ icon at any time saves earnings and leads to presentation of the next balloon. Balloons pop at different sizes, unpredictable to the participant. Thus, unlike the IGT, in which each trial involves a choice between two gambles with different risks, the BART involves variable number of choices in the context of increasing risk (i.e., the amount of money accrued and the probability of losing that money increase with each balloon pump). In both tasks, the probabilities are not known in advance; on the IGT participants have to figure it out as the task progresses and on the BART they are never sure.
The aim of the current investigation was to use a monetary decision-making task with clearly defined probabilities and outcomes for assessing individual differences in risk-taking behavior in adolescents [13]. The task, Wheel of Fortune (WOF), was developed based on prospect theory of weighing probabilities during decision-making [11]. The WOF is unique in testing both reward and punishment contexts separately [14,15]. Previous investigations of the WOF showed sensitivity to psychopathology, including bipolar disorder [13], depression [16], and post-traumatic stress disorder [17]. Neuroimaging studies found that it recruits the expected neural circuits associated with rewards and decision-making [16,18–20]. The study goals were: (1) to assess whether performance on WOF is influenced by sociodemographic variables; (2) whether performance on the task correlates with other psychological constructs associated with motivational factors and risky behavior; and (3) whether performance on WOF is associated with “real-world” risk-taking behavior and substance-related problems.
Methods
Sample
The participants included 82 adolescents who were recruited as controls for studying the onset and clinical course of substance-related disorders in adolescents. They were recruited from local schools and churches, and through advertisements in local newspapers. They were between 12–21 years and had no lifetime history of psychiatric illness. They were medically healthy (determined by physical examination and laboratory investigations).
Diagnostic evaluation
Symptoms of major psychiatric disorders were assessed using the Schedule for Affective Disorders and Schizophrenia for School-Age Children - the Present and Lifetime Version (K-SADS-PL). The K-SADS-PL is a semi-structured interview designed to ascertain present and lifetime history of psychiatric illness according to DSM-IV criteria [21]. Inter-rater and test-retest reliability have been established, as well as convergent and discriminate validity [21]. The K-SADS-PL was administered separately to the parent and adolescent, and both were re-interviewed to resolve discrepancies. Summary scores were tabulated based on the information obtained from both informants.
The Family History-Research Diagnostic Criteria (FH-RDC), a semi-structured interview, was used for the evaluation of psychiatric disorders in family members [22]. A parent was interviewed regarding life-time psychiatric disorders in all first-degree relatives of the adolescent subject (including the self, spouse and all offspring). The FH-RDC is sensitive for obtaining information from knowledgeable relatives [23].
Decision-making task
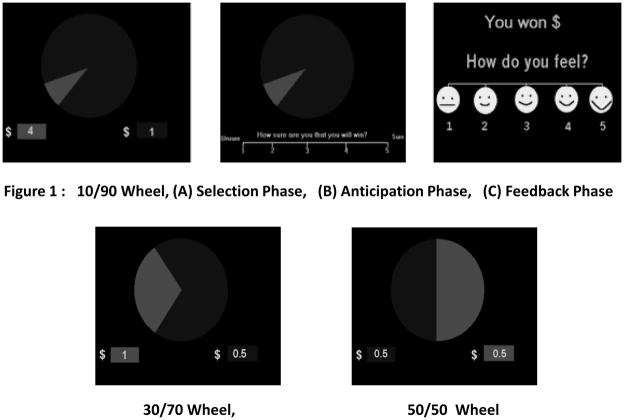
The WOF is a computerized, two-choice, decision-making task involving probabilistic monetary outcomes [13]. It consists of winning and losing sub-tasks. Each sub-task takes approximately 20 minutes to complete and includes three phases: (a) a selection phase during which two choices are presented and the participant is asked to select between them; (b) an anticipation phase before the outcome is presented; and (3) a feedback phase when the outcome is presented (see Figure 1). Because this report is focused on decision-making under probabilistic conditions, data from only the selection phase are presented.
Figure 1.
The Wheel of Fortune (WOF) Task comprises of 3 phases: (1) selection phase; (2) anticipation phase; and (3) feedback phase. The selection phase has 3 types of wheels with varying probabilities and rewards/losses.
On each sub-task, three types of wheels are shown in random order. A total of 62 wheels (amounting to 62 trials) are presented (10/90 wheel = 22 trials; 30/70 wheel = 16 trials; 50/50 wheel = 24 trials). Each wheel has two options, divided into proportions of 10/90; 30/70 or 50/50 (see figure 1). Each option is paired with a dollar amount (10/90 condition: $4.00/$0.50; 30/70 condition: $1.00/$0.50; two types of 50/50 condition: $0.50/$0.50 or $4.00/$4.00).
On each trial, participants were asked to choose the option on which they thought an imaginary pointer would land if the wheel was actually spun. Selection was made by pressing ‘1’ key for the left side of the pie and ‘2’ key for the right side. If the computer selected the same option, then the participants earned or lost the associated money, and if the computer selected the other option they did not earn or lose any money. The task was programmed to generate the outcomes according to the probabilities of the selected options. In effect, on each trial of the winning task, a decision-making challenge was presented by asking the participants to choose between a high reward ($4.00 or $1.00) with a low probability (10% or 30%, respectively), or a low reward ($0.50) with a high probability (90% or 70%). Similarly, on the losing task the participants were asked to choose between suffering a smaller loss with high probability, or a higher loss with low probability. Participants started with no money on the winning sub-task, and they could earn a maximum of $75.00. On the losing sub-task, they started with an endowment of $75.00 and kept the remaining balance at the end.
Relevant sociodemographic, behavioral and psychological variables
Information was obtained on age, gender and ethnicity/race. Pubertal status was assessed with the Tanner Staging Method [24, 25]. Socioeconomic status (SES) was assessed with the Hollingshead Scale [26]. Scaled scores from the Vocabulary and Block Design subtests of the Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV) [27], or Wechsler Adult Intelligence Scale-Third Edition (WAIS-III) [28], were used to estimate Intelligence Quotient (IQ).
Responses on six domains from the General Health Questionnaire that relate to the participant’s “real-world” risk-taking behavior were used to tabulate a composite score [29]. The items included alcohol, nicotine and/or drug use, being a member of a gang, carrying a weapon, getting into physical fights, not wearing a helmet while riding a bicycle/motorcycle and/or a seatbelt when riding in a car, and unprotected sex.
The Drug Use Screening Inventory (DUSI) was used to assess substance-related problems. The DUSI is a self-report designed to assess the severity of alcohol/drug abuse and related psychiatric and psychosocial problems, and it has good psychometric properties [30]. It consists of 149 ‘yes/no’ questions in 10 domains, including drug involvement, behavior, health, psychiatric, social competency, family system, school performance/adjustment, work adjustment, peer relationships, and leisure and recreation. A rating above 0.3 on the overall-problem density score is considered as substance-related problem.
The Behavioral Inhibition System (BIS) and the Behavioral Activation System (BAS) scales were used to assess motivational systems. The BIS/BAS is a 24-item self-report measure rated on a 4-point scale developed on Gray’s theory of brain functions and behavior [31]. The BIS is sensitive to signals of punishment, non-reward and novelty. The BAS is believed to control appetitive motivation and is sensitive to signals of reward. The BAS has three sub-scales: drive pertains to the persistent pursuit of desired goals; fun-seeking reflects a spontaneous desire for new rewards; and reward-responsiveness focuses on positive responses to the anticipation of reward. The Positive and Negative Affectivity Scale (PANAS) was used to assess two distinctive trait measures of mood states that potentially affect decision-making [32]. The Eysenck Impulsivity Scale was used to measure impulsivity [33]. It is comprised of seven questions on a 5-point Likert scale. The Junior Temperament and Character Inventory (JTCI), a 108-item, ‘true/false’ scale, was used to obtain information on temperament/personality traits [34]. The JTCI consists of seven temperament factors including novelty-seeking, reward-dependence, harm-avoidance, persistence, self-directedness, cooperativeness, and self-transcendence.
Primary outcome variables
The primary outcome variables included the rate of selection of low-probability (10% and 30%) reward and loss on the WOF task. The low-probability (particularly 10%) choice is considered risky on the winning sub-task since there is very low probability of gaining a reward, whereas it is the safest option on the losing sub-task (only 10% probability of a loss). The 50/50 wheel is random since there is equal chance of winning/losing. Real-world risk-taking behavior and substance-related problems served as secondary outcome variables.
Statistical analysis
Descriptive statistics were derived for the full sample. For group comparisons, the chi-square was used for categorical variables, and Student’s t test or analysis of variance with repeated measures for continuous variables. The Spearman rank-order correlation was utilized for associations between variables. Regression procedures were employed for the prediction of risk-taking behavior and substance-related problems. Alpha was set at .05.
Results
Sample characteristics
Sociodemographic, behavioral and psychological parameters of the sample are described in Table 1. The scores showed a good range of inter-individual variability, and the mean scores of behavioral and psychological variables were within normal ranges. Of 82 participants, 14 (17.1%) had a first-degree relative with history of a psychiatric disorder, and 6/77 (7.8%) had substance-related problems based on the DUSI.
Table 1.
Demographic, behavioral and psychosocial variables in the sample
| Demographic Characteristics | |
| age (years)1 | 16.2 ± 2.7 (12 – 21) |
| Gender2 | |
| male | 42 (51.2) |
| female | 40 (48.8) |
| Pubertal status2 | |
| Tanner stage 2 | 2 (2.4) |
| Tanner stage 3 | 4 (4.9) |
| Tanner stage 4 | 20 (24.4) |
| Tanner stage 5 | 56 (68.3) |
| Race2 | |
| Caucasian | 42 (51.2) |
| Non-Caucasian (African, Asian & Mexican Americans) | 40 (48.8) |
| socioeconomic status1,3 | 46.5 ± 12.1 (13 – 67) |
| Behavioral measures | |
| IQ1 | 106.1 ± 18.2 (70 – 141) |
| Real-world risk-taking behavior1 | 1.7 ± 1.5 (0 – 6) |
| Drug Use Screening Inventory – problem density1 | 0.1 ± 0.1 (0.0 – 0.5) |
| Psychological variables | |
| Behavioral inhibition system1 | 18.1 ± 3.1 (11 – 23) |
| Behavioral activation system – drive1 | 11.0 ± 2.6 (5 – 16) |
| Behavioral activation system – fun-seeking1 | 12.3 ± 2.3 (6 – 16) |
| Behavioral activation system – reward responsiveness1 | 17.3 ± 2.2 (10 – 20) |
| Positive affectivity1 | 34.6 ± 8.0 (14 – 50) |
| Negative affectivity1 | 15.8 ± 3.8 (11 – 25) |
| Impulsivity1 | 13.8 ± 5.9 (7 – 35) |
| Novelty-seeking1 | 7.7 ± 3.4 (1 – 17) |
| Reward dependence1 | 5.8 ± 1.9 (1 – 9) |
| Harm avoidance1 | 4.7 ± 2.8 (0 – 11) |
| Persistence1 | 3.2 ± 1.4 (0 – 5) |
| Self-directedness1 | 16.3 ± 2.5 (11 – 20) |
| Cooperativeness1 | 13.0 ± 2.8 (4 – 17) |
| Self-transcendence1 | 3.3 ± 1.4 (0 – 5) |
Values are presented as means and standard deviations along with ranges (in parentheses)
Values are presented as raw values and percentages (in parentheses);
Hollingshead four-factor socioeconomic scale (a higher score represents higher socioeconomic status)
Performance on WOF
Two participants had incomplete data on the losing sub-task. Information on the selection of 10% and 30% probability rewards/losses, reaction times and the total amount gained/lost is presented in Table 2. On the winning sub-task, the participants selected the lowest probability (10%) choice less frequently than the highest probability (90%) choice. In contrast, they selected the lowest probability choice more frequently on the losing sub-task. As the probability increased (e.g., from 10% to 30%), the low-probability choice was selected more frequently on the winning sub-task. There was significant variability in the frequency of low-probability choice across individuals. Among 82 participants that completed the winning subtask, 28 (34.1%) selected 10% probability choice >50% of the time. On the losing sub-task, 13/80 (16.3%) participants selected the 10% probability choice <50% of the time. The low-probability choice on the winning and losing sub-tasks did not correlate significantly (r = −.15).
Table 2.
Performance on the Wheel of Fortune in the full sample, and stratified by age, gender and ethnicity
| Winning sub-task | Losing sub-task | |
|---|---|---|
| Full Sample | ||
| 10% choice on 10/90wheel (%) | 41.4 ± 29.3 (0 – 100) | 79.6 ± 26.7 (0 – 100) |
| 30% choice on 30/70 wheel (%) | 57.9 ± 27.5 (0 – 100) | 68.7 ± 25.3 (12.5 – 100) |
| 10% & 30% choice on all gambles (%) | 48.3 ± 25.0 (0 – 100) | 75.0 ± 23.2 (5.3 – 100) |
| Amount won/lost (USD) | 41.6 ± 7.1 (23.0 – 62.0) | 42.5 ± 8.2 (22.0 – 62.5) |
| Reaction time (10% choice) (sec) | 2.5 ± 0.7 (1.3 – 4.0) | 2.0 ± 0.6 (1.1 – 3.7) |
| Reaction time (30% choice) (sec) | 2.4 ± 0.6 (1.2 – 4.0 | 2.3 ± 0.7 (0.9 – 4.0) |
| Reaction time (70% choice) (sec) | 2.3 ± 0.6 (1.1 – 3.7) | 2.7 ± 0.8 (1.2 – 4.0) |
| Reaction time (90% choice) (sec) | 2.3 ± 0.6 (1.0 – 3.9) | 2.6 ± 0.9 (1.3 – 4.0) |
| Age | ||
| 10% choice on 10/90wheel (%) | ||
| younger (≤ 16 years; n = 41) | 45.0 ± 29.2 | 77.0 ± 26.0 |
| older (> 16 years; n = 41) | 37.7 ± 29.4 | 82.0 ± 27.5 |
| 30% choice on 30/70 wheel (%) | ||
| younger (≤ 16 years; n = 41) | 56.3 ± 28.7 | 64.1 ± 27.1 |
| older (> 16 years; n = 41) | 59.6 ± 26.6 | 73.0 ± 23.0 |
| 10% & 30% choice on all gambles (%) | ||
| younger (≤ 16 years; n = 41) | 49.7 ± 26.3 | 71.6 ± 23.1 |
| older (> 16 years; n = 41) | 46.9 ± 23.8 | 78.2 ± 23.1 |
| Amount won/lost (USD) | ||
| younger (≤ 16 years; n = 41) | 40.9 ± 6.2 | 43.7 ± 8.6 |
| older (> 16 years; n = 41) | 42.3 ± 8.0 | 41.3 ± 7.8 |
| Gender | ||
| 10% choice on 10/90wheel (%) | ||
| males (n = 42) | 40.5 ± 27.8 | 84.9 ± 20.7 |
| females (n = 40) | 42.3 ± 31.1 | 73.8 ± 31.4 |
| 30% choice on 30/70 wheel (%) | ||
| males (n = 42) | 56.1 ± 24.4 | 69.9 ± 24.7 |
| females (n = 40) | 59.8 ± 30.7 | 67.3 ± 26.2 |
| 10% & 30% choice on all gambles (%) | ||
| males (n = 42) | 47.1 ± 22.7 | 78.6 ± 18.9 |
| females (n = 40) | 49.7 ± 27.4 | 71.0 ± 26.9 |
| Amount won/lost (USD) | ||
| males (n = 42) | 41.4 ± 7.1 | 44.5 ± 7.6* |
| females (n = 40) | 41.8 ± 7.3 | 40.3 ± 8.4 |
| Pubertal status | ||
| 10% choice on 10/90wheel (%) | ||
| lower (II–IV; n = 26) | 45.6 ± 30.2 | 73.8 ± 26.5 |
| higher (V; n = 56) | 39.4 ± 29.0 | 82.2 ± 26.7 |
| 30% choice on 30/70 wheel (%) | ||
| lower (II–IV; n = 26) | 53.8 ± 29.3 | 61.5 ± 27.4 |
| higher (V; n = 56) | 59.8 ± 26.7 | 71.9 ± 23.9 |
| 10% & 30% choice on all gambles (%) | ||
| lower (II–IV; n = 26) | 49.1 ± 27.5 | 68.6 ± 23.4 |
| higher (V; n = 56) | 48.0 ± 24.0 | 77.9 ± 22.7 |
| Amount won/lost (USD) | ||
| lower (II–IV; n = 26) | 42.2 ± 6.4 | 43.6 ± 7.9 |
| higher (V; n = 56) | 41.3 ± 7.5 | 42.0 ± 8.4 |
| Ethnicity/Race | ||
| 10% choice on 10/90wheel (%) | ||
| Caucasians (n = 42) | 42.2 ± 28.9 | 82.4 ± 25.4 |
| non-Caucasians (n = 40) | 40.0 ± 30.9 | 74.5 ± 29.1 |
| 30% choice on 30/70 wheel (%) | ||
| Caucasians (n = 42) | 56.3 ± 29.2 | 69.5 ± 25.9 |
| non-Caucasians (n = 40) | 61.0 ± 25.0 | 67.0 ± 25.1 |
| 10% & 30% choice on all gambles (%) | ||
| Caucasians (n = 42) | 48.1 ± 26.3 | 77.0 ± 22.4 |
| non-Caucasians (n = 40) | 48.9 ± 23.5 | 71.3 ± 24.8 |
| Amount won/lost (USD) | ||
| Caucasians (n = 42) | 42.4 ± 7.8 | 41.3 ± 8.5 |
| non-Caucasians (n = 40) | 40.2 ± 5.9 | 44.7 ± 7.5 |
Values are means and standard deviations
For the losing task, the total samples comprised 80 participants, 39 younger adolescents, 40 males, and 41 Caucasians and 39 non-Caucasians.
t = 2.32, p = .02
On the winning sub-task, the reaction time for 10% probability choice was significantly longer than other choices (F3,68 = 5.26, p ≤ .005). On the losing sub-task, the reaction time for 10% probability choice was significantly faster than other choices (F3,48 = 9.95, p ≤.0001).
Influence of sociodemographic characteristics on task performance
Selections of 10% and 30% probability on winning and losing sub-tasks, stratified by age (≤ 16 years = younger), gender, pubertal status (Tanner stage II–IV = lower) and ethnicity/race are presented in Table 2. Younger and older adolescents were comparable on reward selection or avoidance of losses. Females lost less money during the losing sub-task. Pubertal status and ethnicity/race did not affect performance. IQ did not influence performance (r = .17 and .02 on winning and losing sub-tasks, respectively). Adolescents from higher SES selected the risky choice more frequently on the winning sub-task than those from lower SES (r = .22, p ≤.05), but SES did not affect performance on the losing sub-task (r = .16).
Relationship between low-probability choice and psychological measures
Older adolescents had lower scores on novelty-seeking but age did not affect other psychological measures. Gender, ethnicity/race, SES, IQ and Tanner stage did not influence the psychological measures. Correlation coefficients between low-probability choice and psychological variables are depicted in Table 3. Participants who made the low-probability choice more frequently on the winning sub-task (risky choice) reported significantly higher scores on drive and fun-seeking scales of the BAS and reward-dependence scale of the JTCI. In contrast, participants who made the low-probability choice more frequently on the losing subtask (optimal choice) scored higher on positive-affectivity scale.
Table 3.
Spearman’s correlation co-efficients depicting the association of low-probability choices on the Wheel of Fortune with behavioral and psychological measures
| Low-probability (winning task) | Low-probability (losing task) | |
|---|---|---|
| Behavioral inhibition system | .14 | −.02 |
| BAS drive | .36** | −.13 |
| BAS fun-seeking | .42*** | −.05 |
| BAS reward responsiveness | .17 | .15 |
| Positive affectivity | −.01 | .30* |
| Negative affectivity | .05 | .15 |
| Eysenck impulsiveness | .14 | −.10 |
| JTCI/novelty-seeking | .11 | −.05 |
| JTCI/reward dependence | .31* | −.08 |
| JTCI/harm avoidance | −.20 | −.06 |
| JTCI/persistence | −.17 | .10 |
| JTCI/self-directedness | −.13 | .08 |
| JTCI/cooperativeness | −.10 | .10 |
| JTCI/self-transcendence | .01 | .12 |
BAS = Behavioral activating system; JTCI = the Junior Temperament and Character Inventory
p ≤.01;
p ≤ .005;
p ≤.0001
Prediction of real-world risk-taking behavior and substance-related problems
Older participants had a higher score the risk-taking scale (r = .24, p ≤.05). No other sociodemographic or psychological factor affected risk-taking behavior. With the exception of SES (r = −.24, p ≤.05), sociodemographic variables did not affect substance-related problems. Substance-related problems correlated negatively with positive-affectivity (r = −.34, p ≤.005), reward-dependence (r = −.31, p ≤.01), self-directedness (r = −.35, p ≤.005) and cooperativeness (r = −.28, p ≤.05), but positively correlated with negative-affectivity (r = .34, p ≤.05), impulsivity (r = .43, p ≤.0001) and novelty-seeking (r = .38 p ≤.001). Higher frequency of risk-taking behavior was associated with more severe substance-related problems (r = .39, p ≤.0001).
After controlling for age and SES, low-probability choice on the winning sub-task predicted risk-taking behavior (β = 0.02, SE = 0.01, CI = 0.01–0.03, p ≤.001). The low-probability choice contributed 13% of the variance to risk-taking behavior (F1,75 = 10.74, p ≤.001). Low-probability choice on the losing sub-task did not predict risk-taking behavior.
After controlling for relevant sociodemographic and psychological factors, low-probability choice on the winning sub-task predicted substance-related problems (β = 0.03, SE = 0.01, CI = 0.01–0.50, p ≤.005). The low-probability choice contributed 19% of the variance to substance-related problems (F1,61 = 10.92, p ≤ .005). On categorical classification, participants who made a high-risk selection had higher prevalence of substance-related problems than those who make a low-risk selection (24.0% vs. 0%, p ≤.001). Low-probability choice on the losing sub-task did not have predictive power in determining substance-related problems.
In order to assess whether the discriminative power of WOF is limited to risky choice (on 10/90 and 30/70 wheels), relationship between 50/50 wheel selections and risk-taking behavior (or substance-related problems) was examined. 50/50 wheel selections did not discriminate these behaviors.
Discussion
The findings indicated that adolescents made optimal choices on a laboratory task when selecting between prospects with clearly-defined probabilities and outcomes, and especially when the differences between prospects were large (e.g., 10/90 trials). On both sub-tasks, choosing the 90% gamble on 10/90 trials is regarded as a preference for certainty and choosing 10% gamble is regarded as taking a chance. On the winning sub-task, approximately 60% of adolescents chose to settle for a small but more certain reward rather than choosing a large, unlikely reward. On the losing sub-task, approximately 80% of adolescents chose the gamble of a large loss rather than settling for the certainty of a small loss. This pattern of responses is termed risk-seeking for losses and risk-avoidant for gains, and studies in adults reported this pattern of behavior [11]. The participants also took significantly longer time to select a high-risk choice than a low-risk choice on the winning sub-task, suggesting that they deliberated before making a more risky choice.
Although the mean response level indicated optimal performance, there was substantial inter-individual variability. Approximately, one-third of the participants made a high-risk choice in the context of earning a reward. Performance in the laboratory was associated with risk-taking behavior and substance-related problems, even after accounting for relevant sociodemographic and psychological variables. This suggests that WOF makes a distinct contribution in predicting high-risk behavior. Risky choice of rewards was associated with other psychological constructs of reward-seeking.
Risk-taking behavior can be conceptualized as a two-dimensional trait [35]. The first trait, reward drive, reflects differences in sensitivity to incentive motivation and engagement in appetitive behavior upon detection of reward cues. The second trait, rash impulsiveness, reflects individual differences in the ability to modify or inhibit pre-potent behaviors in light of potential negative consequences. Risky choice in the context of graining rewards in this sample was associated with the first trait since it correlated with reward-dependent behaviors but not with impulsive or novelty-seeking traits [36,37]. These results suggest that heightened sensitivity of the brain approach system (e.g., dorsolateral prefrontal cortex, ventral striatum, and dopamine) contributes to the increased prevalence of reward-related risky behaviors in adolescents [18,38]. In neuroimaging studies, greater activation was observed in the dorsal part of the anterior cingulate cortex and ventrolateral portion of the prefrontal cortex while making risky selections on the WOF in adolescents [19,39]. Reduced activity in these areas correlated with greater risk-taking performance. However, not all adolescents have a propensity to take risks and individual differences in the mesolimbic dopaminergic circuitry, especially increased activity in the nucleus accumbens, modulate sensitivity to rewarding outcomes [18,36,39].
Although individual differences in risky selections on the WOF were associated with risk-taking behavior and substance-related problems, the prevalence of substance-related problems was low probably because this was a selected group at extremely low-risk for psychopathology. A longitudinal follow-up of this sample will determine if the risk-taking behavior and/or substance-related problems are self-limited or persistent. Although reward drive is likely to pose a risk for adolescent substance use, particularly in the context of peer influences, it can also be adaptive in certain situations, such as social potency and entrepreneurship [37]. Understanding the possible mechanisms through which reward drive exerts positive outcomes potentially can create a paradigm shift in therapeutic interventions. For example, the traditional interventions for substance-related problems focus on techniques aimed at increasing restraint and avoiding high-risk situations. Some interventions, such as contingency management and motivational interviewing/enhancement, capitalize on the adolescent’s reward-sensitivity by redirecting this trait towards healthier goals.
In contrast to the findings on IGT [9,10], age did not correlate with risk preferences. Improved performance with age on the IGT could reflect an improvement in the ability to process available options rather than a change in risk-preference. Gender also did not influence risk preferences. There is a general impression of males being more risk-seeking but such differences have narrowed recently [40]. Adolescents from higher SES selected a risky reward more frequently than those from lower SES, suggesting that their economic advantage offers the choice to take greater monetary risks. The low-probability choice on the winning and losing sub-tasks did not correlate, highlighting that reward and punishment are processed by distinct neural networks [14,15].
Limitations
Several methodological issues should be considered in interpreting these results. Given the modest sample size and large number of variables explored, the results should be considered preliminary until they are replicated in larger samples. The sample was selected based on strict inclusion/exclusion criteria, and the findings might not be applicable to adolescents in the community. Given this limitation, the relationship between risky decision-making and risk-taking behaviors is noteworthy. The task does not model real-world risky decision-making because participants do not experience real monetary losses (since they play with the house money). It is possible that risk-takers have difficulty in setting a limited budget for losses. However, risk-taking behavior occurred in the context of gaining rewards where everyone starts with $0 balance, and not losses. Another limitation is that risk-taking behavior, substance-related problems and other psychological measures were based on self-reports. Parent/peer-reports might be helpful to increase the validity of self-reports. Also, the risk-taking behavior scale was not validated. The General Health Questionnaire is used widely in pediatric practice and research [30], and the items overlap with the Youth Risk Behavior Survey [40]. Finally, the contribution of decision-making strategy in the laboratory to risk-taking behavior and substance-related problems was only modest, and the other psychosocial factors that potentially influence decision-making (e.g., competence, perceptions of control, social norms, and peer/social influences) were not assessed [3, 4].
Conclusions
The WOF is a useful laboratory task to study risk-taking behavior associated with potential rewards in adolescents. These results should be confirmed in larger samples of adolescents in the community in addition to including youth with various types of psychopathology in whom there is a high prevalence of risk-taking behavior.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (DA14037, DA15131, DA17804, DA17805, MH62464, MH68391 and RR00633), and the Sarah M. and Charles E. Seay Endowed Chair in Child Psychiatry at UT Southwestern Medical Center. The authors are grateful to Dallas and Fort Worth Independent School Districts for their support in recruiting research participants and for providing office space for assessments at the Youth and Family Resource Centers.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- 2.Arnett J. Reckless behavior in adolescence: a developmental perspective. Dev Rev. 1992;12:339–373. [Google Scholar]
- 3.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobeh Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 4.Reyna VF, Rivers SE. Current theories of risk and rational decision-making. Dev Rev. 2008;28:1–11. doi: 10.1016/j.dr.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benthin A, Slovic P, Severson H. A psychometric study of adolescent risk perception. J Adolesc. 1993;16:153–168. doi: 10.1006/jado.1993.1014. [DOI] [PubMed] [Google Scholar]
- 6.Boyer TW. The development of risk-taking: A multi-perspective review. Developmental Review. 2006;26:291–345. [Google Scholar]
- 7.Bechara A, Dolan S, Denburg N, et al. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- 8.Buelow MT, Suhr JA. Construct validity of the Iowa Gambling Task. Psychol Rev. 2009;19:102–114. doi: 10.1007/s11065-009-9083-4. [DOI] [PubMed] [Google Scholar]
- 9.Blair RJ, Colledge E, Mitchell DG. Somatic markers and response reversal: is there orbitofrontal cortex dysfunction in boys with psychopathic tendencies? J Abnorm Child Psychol. 2001;29:499–511. doi: 10.1023/a:1012277125119. [DOI] [PubMed] [Google Scholar]
- 10.Overman WH, Frassrand K, Ansel S, et al. Performance on the IOWA card task by adolescents and adults. Neuropsychologia. 2004;42:1838–1851. doi: 10.1016/j.neuropsychologia.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Kahneman D, Tversky A. Prospect Theory: an analysis of decision under risk. Econometrica. 1979;47:263–291. [Google Scholar]
- 12.Lejuez CW, Read JP, Kahler CW, et al. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J Exp Psychol Appl. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- 13.Ernst M, Dickstein DP, Munson S, et al. Reward-related processes in pediatric bipolar disorder: a pilot study. J Affect Disord. 2004;82 (Suppl 1):S89–S101. doi: 10.1016/j.jad.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 14.Wrase J, Kahnt T, Schlagenhauf F, et al. Different neural systems adjust motor behavior in response to reward and punishment. Neuroimage. 2007;36:1253–1262. doi: 10.1016/j.neuroimage.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Yacubian J, Glascher J, Schroeder K, et al. Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. J Neurosci. 2006;26:9530–9537. doi: 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smoski MJ, Felder J, Bizzell J, et al. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord. 2009;118:69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guyer AE, Kaufman J, Hodgdon HB, et al. Behavioral alterations in reward system function: the role of childhood maltreatment and psychopathology. J Am Acad Child Adolesc Psychiatry. 2006;45:1059–1067. doi: 10.1097/01.chi.0000227882.50404.11. [DOI] [PubMed] [Google Scholar]
- 18.Ernst M, Nelson EE, Jazbec S, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 19.Eshel N, Nelson EE, Blair RJ, et al. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith BW, Mitchell DG, Hardin MG, et al. Neural substrates of reward magnitude, probability, and risk during a wheel of fortune decision-making task. Neuroimage. 2009;44:600–609. doi: 10.1016/j.neuroimage.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Andreasen NC, Endicott J, Spitzer RL, et al. The family history method using diagnostic criteria. Reliability and validity. Arch Genl Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- 23.Thompson WD, Orvaschel H, Prusoff BA, et al. An evaluation of the family history method for ascertaining psychiatric disorders. Arch Gen Psychiatry. 1982;39:53–58. doi: 10.1001/archpsyc.1982.04290010031006. [DOI] [PubMed] [Google Scholar]
- 24.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Childhood. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Childhood. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale University Department of Sociology; 1975. [Google Scholar]
- 27.Wechsler D. WISC-IV: Administration and Scoring Manual. 4. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- 28.Wechsler D. WAIS-III Wechsler Adult Intelligence Scale. 3. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 29.Goldberg DP, Blackwell B. Psychiatric illness in general practice. A detailed study using a new method of case identification. Br Med J. 1970;1:439–443. doi: 10.1136/bmj.2.5707.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarter RE, Laird SB, Kabene M, et al. Drug abuse severity in adolescents is associated with magnitude of deviation in temperament traits. Br J Addict. 1990;85:1501–1504. doi: 10.1111/j.1360-0443.1990.tb01636.x. [DOI] [PubMed] [Google Scholar]
- 31.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Pers Soc Psychol. 1994;67:319–333. [Google Scholar]
- 32.Watson D, Clark LA, Tellegan A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 33.Eysenck SB, Eysenck HJ. Impulsiveness and venturesomeness: their position in a dimensional system of personality description. Psychol Rep. 1978;43:1247–1255. doi: 10.2466/pr0.1978.43.3f.1247. [DOI] [PubMed] [Google Scholar]
- 34.Cloninger CR. The Temperament and Character Inventory (TCI): a guide to its development and use. Center for the Psychobiology of Personality, Washington University, ; Louis, Missouri: 1994. [Google Scholar]
- 35.Dawe S, Loxton NJ. The role of impulsivity in the development o fsubstance use and eating disorders. Neurosci Biobeh Rev. 2004;28:343–351. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Galvan A, Hare T, Voss H, et al. Risk-taking and the adolescent brain: who is at risk? Dev Sci. 2007;10:F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- 37.Gullo MJ, Dawe S. Impulsivity and adolescent substance use: rashly dismissed as “all- bad”? Neurosci Biobeh Rev. 2008;32:1507–1518. doi: 10.1016/j.neubiorev.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neurosci Biobehav Rev. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shad MU, Bidesi AS, Chen L-A, et al. Individual differences in the neurobiology of decision-making in adolescents. Submitted for publication. [Google Scholar]
- 40.Eaton DK, Kann L, Kinchen S, et al. Youth risk behavior surveillance--United States, 2007. MMWR Surveill Summ. 2008;57:1–131. [PubMed] [Google Scholar]