Abstract
Behavioral research suggests two cognitive systems are at the foundations of numerical thinking: one for representing 1-3 objects in parallel and one for representing and comparing large, approximate numerical magnitudes. We tested for dissociable neural signatures of these systems in preverbal infants, by recording event-related potentials (ERPs) as 6-7.5 month-old infants (n = 32) viewed dot arrays containing either small (1-3) or large (8-32) sets of objects in a number alternation paradigm. If small and large numbers are represented by the same neural system, then the brain response to the arrays should scale with ratio for both number ranges, a behavioral and brain signature of the approximate numerical magnitude system obtained in animals and in human adults. Contrary to this prediction, a mid-latency positivity (P500) over parietal scalp sites was modulated by the ratio between successive large, but not small, numbers. Conversely, an earlier peaking positivity (P400) over occipital-temporal sites was modulated by the absolute cardinal value of small, but not large, numbers. These results provide evidence for two early developing systems of non-verbal numerical cognition: one that responds to small quantities as individual objects and a second that responds to large quantities as approximate numerical values. These brain signatures are functionally similar to those observed in previous studies of non-symbolic number with adults, suggesting that this dissociation may persist over vast differences in experience and formal training in mathematics.
Introduction
Studies of human infants and adults suggest the presence of two cognitive systems that support non-verbal number representation: a parallel individuation system that represents distinct objects simultaneously and a numerical magnitude system that represents the approximate numerical magnitude of a collection of objects. Behavioral experiments suggest that that these capacities are distinct from one another and continuous across development (Dehaene, 1997; Feigenson, Dehaene, & Spelke, 2004). The comparisons used to support these claims, however, are complicated by the vast differences in methodology and dependent measures used to assess number processing across these ages. In contrast, measures of brain response to number are well documented in adults and can also be obtained in pre-verbal infants, under identical conditions of passive viewing. Such measures may allow more direct comparisons of spontaneous numerical processing across development and begin to shed light on the neural mechanisms that support non-verbal numerical cognition early in life.
Measuring the brain response to number in infants may also provide insight into contrasting signatures between small and large number processing observed behaviorally. In looking time and head-turning paradigms, infants discriminate between arrays of dots, sequences of actions, or sequences of sounds on the basis of number when other non-numerical continuous quantitative variables are controlled (Wood & Spelke, 2005; Xu & Spelke, 2000). Critically, discrimination depends on the ratio of the two numbers, and the critical ratio narrows over development from 1:3 for newborn infants (e.g., 4 vs. 12), to 1:2 at 6 months (e.g., 8 vs. 16), 2:3 at 9 months (e.g., 8 vs. 12), 3:4 for preschool children, and 7:8 for adults (Feigenson et al., 2004; Xu & Spelke, 2000; Izard, Sann, Spelke, & Steri, 2009; Lipton & Spelke, 2003; Halberda & Feigenson, 2008; Van Oeffelen & Vos, 1982). Interestingly, the ratio limit does not appear to apply to small numbers. When infants are presented with small numbers of objects under these same conditions and with the same stimulus controls, they typically fail to discriminate visually between the small numbers even though the ratio is within the limits of large number discrimination (e.g., 1 vs. 2 at 6 months)(Xu, Spelke, & Goddard, 2005). In other experimental paradigms involving looking time, manual search, or locomotor approach, 10 to 14 month-old infants distinguish the number of individual items hidden in two adjacent buckets when comparisons are 1 vs. 2, 1 vs. 3, and 2 vs. 3, but they fail to distinguish between sets of 1 vs. 4 or 2 vs. 4 (Feigenson et al., 2004; Feigenson & Carey, 2005). This set size limit, which is also observed in non-human animals and in adults under some conditions, has been interpreted as a limit on object directed attention, revealing that only 3-4 distinct objects can be individuated in parallel (e.g. Scholl, 2001). Infants’ failures to discriminate small continuously-controlled quantities within the ratio limits of the numerical magnitude system, coupled with their failures to track more than 3 objects in parallel, suggest a different representational system operates over small numbers in these contexts.
One potential explanation for the above pattern of findings is that infants represent small numbers of objects (1-3) as individual items, whereas they represent larger numbers of objects (4 +) as sets with approximate cardinal values. Some behavioral experiments with adults showing contrasting signatures of small and large number processing (Mandler & Shebo, 1982; Revkin, Piazza, Izard, Cohen, & Dehaene, 2008; Trick & Pylyshyn, 1994) support this idea. Other evidence from studies of adults and non-human primates, however, suggests that a single numerical magnitude system operates over all quantities (Brannon & Terrace, 1998; Cantlon & Brannon, 2006; Cordes, Gelman, & Gallistel, 2001; Whalen, Gallistel, & Gelman, 1999). Indeed, most formal models of numerical cognition posit that all numbers are represented by one system (e.g. Gallistel, 1990; Gallistel & Gelman, 2000; Meck & Church, 1983). Solid evidence of numerical magnitude representations for small numbers with infants, however, is scarce. One recent study found that 7 month-old infants discriminated between sets of 2 and 8, even though they failed to discriminate between sets of 2 and 4 (Cordes & Brannon, 2009). The authors suggest that discrimination in the small number range may also be ratio-dependent, but this suggestion does not explain why infants fail to discriminate 1 from 4 when objects appear individually and are occluded (Feigenson & Carey, 2005) or why the ratio limit on discrimination is higher in the small number range, given the invariant 1:2 ratio signature obtained at this age with numbers greater than 4.
Because behavioral studies of numerical processing fail to reveal conclusively whether infants form numerical magnitude representations over small sets of objects, measures of brain response may provide an important tool for distinguishing between competing theories of the nature and origins of numerical cognition. Neuroimaging studies consistently find a ratio-dependent brain response to number in human adults in a distinct region of cortex: the horizontal segment of the intraparietal sulcus (hIPS) (Ansari & Dhital, 2006; Cantlon, Brannon, Carter, & Pelphrey, 2006; Dehaene, Piazza, Pinel, & Cohen, 2003; Piazza, Izard, Pinel, Le Bihan, & Dehaene, 2004). In some experiments with adults using explicitly numerical tasks or presenting number words or symbols, moreover, the ratio-dependent IPS activation occurs throughout the numerical range (Dehaene, 1996; Knops, Thirion, Hubbard, Michel, & Dehaene, 2009; Pinel, Le Clec’ H, van de Moortele, Naccache, Le Bihan, & Dehaene, 1999). Furthermore, single-cell recordings from non-human primates reveal ratio-dependent neuronal tuning functions in the small number range (Nieder & Merten, 2007; Nieder & Miller, 2003). However, the human adults in these studies had decades of practice with symbolic number concepts spanning the full numerical range, and the animals were extensively trained to abstract the set size from small and large arrays of objects for comparison. When humans and trained animals are given an explicitly numerical task, these experiences may obscure the distinct activities of the two systems of number.
Consistent with this possibility, recent ERP studies with human adults, presenting non-symbolic numerical displays under passive viewing conditions, reported contrasting brain signatures of small and large number processing (Hyde & Spelke, 2009). Specifically, the amplitude of an early (~150 msec post-stimulus) negative peak (N1), centered over parietal cortex, was modulated by the absolute number of elements in the display for small numbers, regardless of the direction or magnitude of change from one quantity to the next. In contrast, the amplitude of a later (~250 msec post-stimulus) positive peak, (P2p), also centered over parietal cortex, was modulated by the ratio of change in the large number range, regardless of the absolute number of objects presented. This double dissociation provides support for the two-systems view of numerical cognition in adults, but it does not reveal whether the two systems are products of experience and skill, or reflections of core cognitive capacities.
Studies of pre-verbal infants can probe the nature of number representations before formal experience and training; by using electrophysiological methods, moreover, they can permit a direct comparison to previous studies of the electrophysiological response to number in adults and older children (Berger, Tzur, & Posner, 2006; Dehaene, 1996; Hyde & Spelke, 2009; Libertus, Woldorff, & Brannon, 2007; Temple & Posner, 1998). Although research on the neural signatures of number processing in infants has only begun, we do know that infant ERPs are modulated by changes in number (Izard, Dehaene-Lambertz, & Dehaene, 2008; Libertus, Pruitt, Woldorff, & Brannon, 2009). For example, in a number adaptation paradigm, Izard and colleagues (2008) showed that after adapting infants to a given number, instances of the same number produced more positive amplitude potentials, compared to presentations of a different number, on a mid-latency positive component over posterior parietal sites (~ 800 ms). This response occurred for both large and small numbers, showing that the infant brain response distinguishes number changes from no change throughout the numerical range. Nevertheless, the ratio of change, the signature of approximate number representation, was not included as a factor in this study. It is not clear, therefore, whether the observed response to number reflects activity of the approximate number system or activity of a different change detection mechanism.
Another recent study by Libertus and colleagues (2009) did include ratio as a factor in their design and found evidence of a ratio dependent response to large numbers in the EEG alpha band. However, they did not analyze the effects of numerical ratio on the ERP, and they did not test the response to small numbers. No electrophysiological study of infants, therefore, has compared the ratio effect for changes in the small versus large numerical range.
In contrast to the previous studies, we directly tested for the ratio signature of the approximate number system during both small and large number processing. We measured ERPs in 6-7.5 month-old infants as they viewed arrays of small or large numbers in a number-alternation paradigm (Ansari, Dhital, & Soon, 2006). Three different ratio changes were presented over different blocks to each subject; a different ratio change was repeatedly presented during each block by presenting novel dot arrays that alternated in number. Experiment 1 presented only large number relationships (8/8, 8/16, & 8/32 or 32/32, 32/16, & 32/8). Experiment 2 presented only small number relationships (1/1, 1/2, & 1/3 or 3/3, 3/2, & 3/1) (see Figure 1). We asked whether the infant brain response was influenced by the numerical ratio relationship presented in each block, a behavioral signature of the non-verbal numerical magnitude system. If the two numerical systems found in adults are functional early in development, then the functional pattern of electrophysiological responses to small and large numbers in pre-verbal infants should be similar in nature to those observed in adults (Hyde & Spelke, 2009): infants’ ERPs should be modulated by ratio for large numbers, and by absolute value for small numbers.
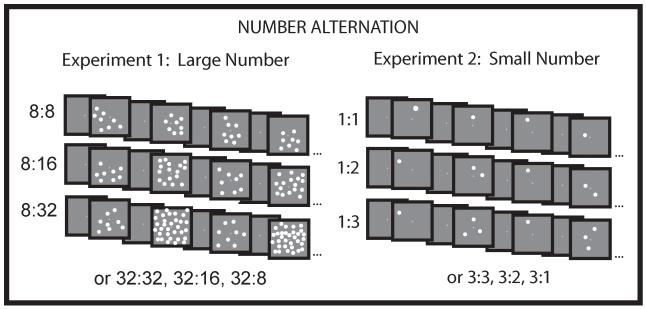
Figure 1.
Schematic depiction of the number alternation conditions presented to infants. Images depict low base conditions for both experiments; the numbers used in the high base conditions are presented underneath the images. Each image was presented for 500 ms and followed by the presentation of a cross in the middle of the screen for an interval that varied randomly in duration from 500-1000 ms.
Method
Participants
A total of 32 6-7.5 month old infants participated in two experiments. All parents provided written informed consent for their children to participate in the study. All infants were recruited from the greater Cambridge, MA community and received a travel reimbursement of 5 dollars, along with a small gift (toy, stuffed animal, t-shirt, bib, etc.) for their participation. An additional 61 infants participated but were excluded from the final analyses for fussiness or failure to complete the experiment (31), excessive artifacts yielding an insufficient number of good trials (28), equipment malfunction (1), or parental interference (1). This attrition rate is comparable with similar infant ERP studies involving both numerical and non-numerical stimuli (Berger, Tzur, & Posner, 2006; Hyde, Jones, Porter, & Flom, 2010; Izard et al., 2008; Libertus et al., 2009; Quinn, Westerlund, & Nelson, 2006).
Design
Infants were shown dot arrays in a number-alternation paradigm (Ansari & Dhital, 2006). Over different blocks, infants saw arrays of dots that alternated in number by one of three different ratios. Because behavioral research provides evidence for a ratio limit of 1:2 at this age (e.g., Xu & Spelke, 2000; Lipton & Spelke, 2003), infants were presented with displays of 8, 16, and 32 dots. In Experiment 1, half of the infants saw a block of images all containing 8 dots (1:1 or no change), a block of images with alternating arrays of 8 and 16 dots (1:2), and a block of images with alternating arrays of 8 and 32 dots (1:4). The remaining infants saw a block of images containing 32 dots (1:1 or no change condition), a block of images of 32 and 16 dots in alternation (1:2), and a block of images of 32 and 8 dots in alternation (1:4). Order of block presentation (1:1, 1:2, 1:4) was counterbalanced across participants (see Figure 1).
Experiment 2 was nearly identical to Experiment 1 except the displays contained small numbers (1,2,3). Because the set size limit on parallel individuation is 3 at this age (e.g., Feigenson et al., 2004; Feigenson & Carey, 2005), infants were presented with displays of 1, 2, or 3 dots. Thus, the contrasting limits on large- and small-number representation required that the two studies test different ratios: the largest ratio change was reduced from 1:4 in Experiment 1 to 1:3 in Experiment 2. Half of the infants saw a block of images all containing 1 (1:1 or no change), a block of images of 1 and 2 dots in alternation (1:2), and a block of images of 1 and 3 dots in alternation (1:3). The remaining infants saw a block of images all with 3 dots (1:1), a block of images of 3 and 2 dots in alternation (2:3), and a block of images that alternated between 3 dots and 1 dot (1:3) (see Figure 1).
Stimuli
Dot arrays in the images varied on individual item size, inter-item spacing, total occupied area and total luminance to rule out the possibility that non-numerical properties of the displays could account for any systematic response In half of the images, the intensive parameters (individual item size and inter-item spacing) of the dot arrays were confounded with number and the extensive parameters (total occupied area and total luminance) were equated; the remainder of the images were equated on the intensive parameters (individual item size and inter-item spacing) and varied on the extensive parameters. A similar logic to this method of controlling for non-numerical parameters is routinely used in behavioral number comparison tasks with adults and children (e.g. Barth, LaMont, Lipton, & Spelke, 2005). Extensively and intensively controlled images were presented with equal frequency in novel, random order for each participant for 500 ms separated by an inter-stimulus interval containing a central fixation cross that varied in duration randomly between 500-1000 ms. Forty images were presented per block; 120 images were presented over the entire experiment.
Data Reduction, Processing, and Analysis
We recorded the ongoing EEG using Geodesic Sensor Net 128 (EGI, Eugene, OR). We monitored the electro-oculogram (EOG) from electrodes above and beside the eyes. Recordings were referenced online to the vertex site and bandpass filtered at .1-100 Hz, while sampling at 250 Hz. Offline, the data were lowpass filtered at 30 Hz, segmented into epochs from 200 ms before stimulus onset to 1200 ms after, and baseline corrected to the average amplitude from 0 to 200 ms before image onset.1 We then subjected the data to artifact detection and rejection through visual inspection. We rejected any epoch containing an eye blink or eye movement in the EOG, off-scale activity (movement artifact, muscle artifact, or electrode drift), or more than 15% (> 19 channels) bad channels. We used spherical spline interpolation to replace bad channels in epochs containing less than 15% total bad channels. We then averaged the remaining trials into experimental conditions for each subject and re-referenced to the average reference. We excluded from the final analysis any subject that retained less than 10 good epochs out of possible 40 in each of three experimental conditions. Finally, we created grand averages for each experimental condition for each participant that included responses to both numbers of the alternation block (i.e. Large number-large ratio change responses were comprised of responses to both 8 and 32)2. On average, each subject contributed 42.81 good epochs (36 % of total presented), 14.27 per ratio change condition. No statistically significant differences in the number of good epochs were observed between ratio change conditions, experiments, or trials retained containing extensively or intensively controlled images.
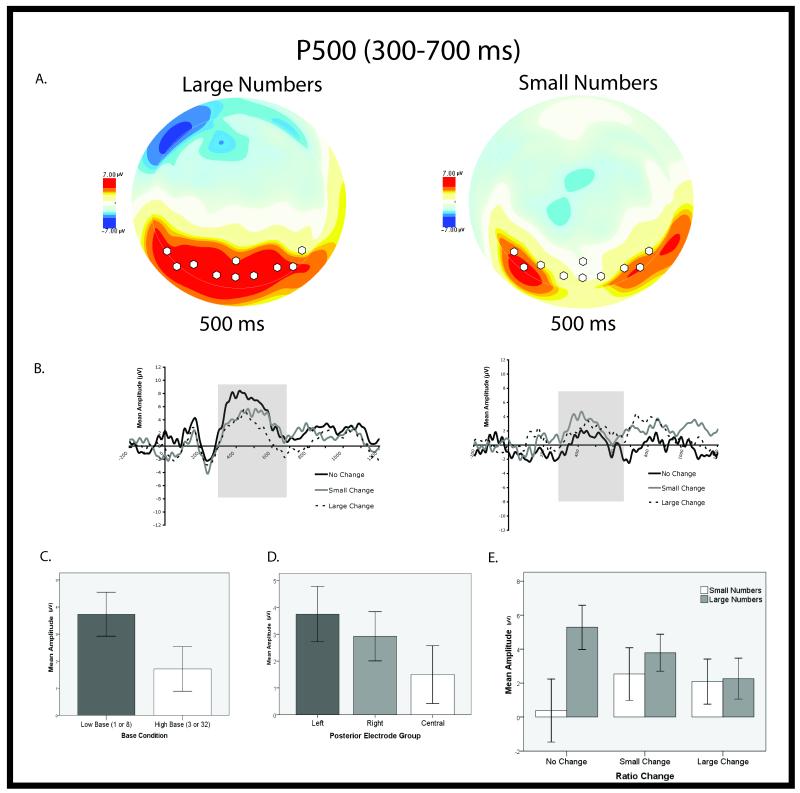
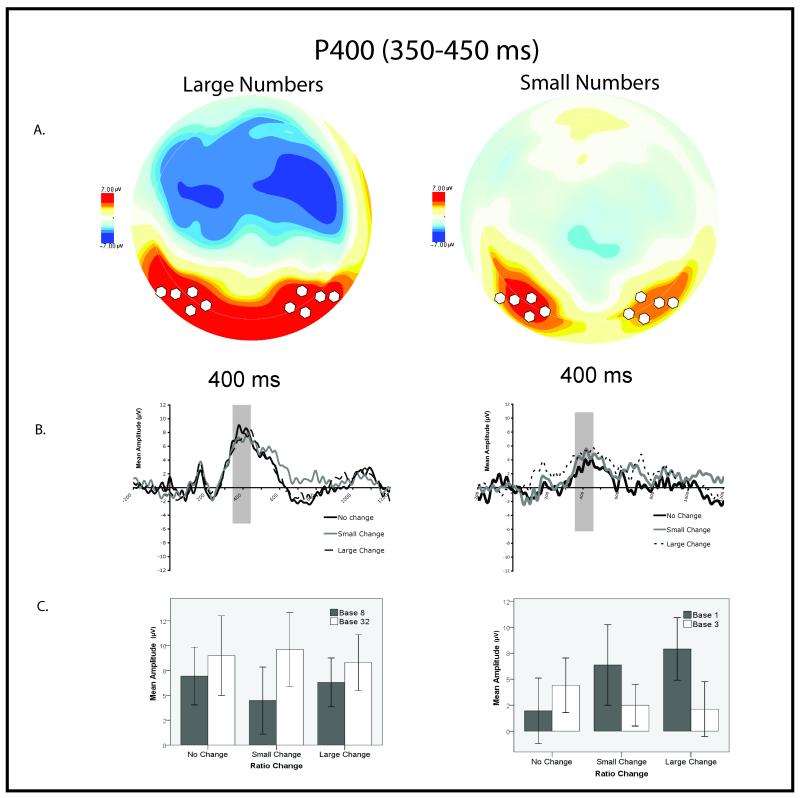
Previous work has shown the major mid-latency posterior components (P2p in adults; P400 in infants) are sensitive to numerical changes (Dehaene, 1996; Hyde & Spelke, 2009; Izard et al., 2008; Libertus et al., 2009; Temple & Posner, 1998). Guided by this work we focused our analysis on the major mid-latency component over posterior sites to test for effects of ratio. Actual time windows and electrode sites of interest were chosen by visual inspection of the grand average waveform (all experimental conditions averaged together)3. Visual inspection revealed the second posterior positivity to have a different latency and scalp topography in the small and large number range (see Figure 2:a and 3:a). Large number processing was better characterized by the P500, defined as the mean amplitude between 300-700 ms over left (EGI sites 59, 65, 66), right (EGI sites 85, 91,92), and central (EGI sites 68,72,73,77) posterior parietal sites (see Figure 2). Small number processing was better characterized by the P400, defined as the mean amplitude between 350-450 ms over left (63, 64, 65, 69, 70) and right (90, 91, 95, 96 100) occipital-temporal sites (see Figure 3). We did not include a central parietal electrode group in the analysis of the P400 because scalp topography suggested the small number response was strongly lateralized to left and right scalp sites in contrast to the P500 scalp electrophysiology which was clearly distributed across the entire posterior scalp including midline channels (see Figures 2:a and 3:a).
Figure 2.
Results of parietal P500 analysis. (A). Overhead view of scalp topography for both experiments at 500 ms. White circles represent electrode grouping used to calculate mean amplitude. (B). Average evoked waveform over posterior parietal sites from −200 before stimulus presentation to 1200 ms after for both experiments. (C). Graph depicting main effect of Base Condition on mean P500 amplitudes. Error bars represent 95% confidence intervals. (D). Graph depicting main effect of Electrode Grouping on mean P500 amplitude. Error bars represent 95% confidence intervals. (E). Graph depicting interaction between Numerical Range and Ratio Change on P500. Error bars represent 95% confidence intervals.
Figure 3.
Results of occipital-temporal P400 analysis. (A). Overhead view of scalp topography for both experiments at 400 ms. White circles represent electrode groupings used to calculate mean amplitude. (B). Average evoked waveform over occipital-temporal sites from −200 before stimulus presentation to 1200 ms after for both experiments. (C.) Graphs depicting Base Condition by Numerical Range x Ratio Change interaction on the P400 amplitude for the large number and the small number experiments separately.
The same analysis was conducted on the parietal P400 and the occipital-temporal P500. Specifically, we analyzed the effects of ratio using a multi-factor ANOVA with between-subjects factors of Number Range (small or large numbers) and Base Condition (high base condition or low base condition) and within-subjects factors of Ratio Change (no change, small change, large change) and Electrode Group. Tests of linear contrast assessed significant interactions between variables where our a priori hypotheses made clear predictions regarding the pattern of modulation. Other incidental interactions were investigated with post-hoc t-tests. We applied Greenhouse-Geisser corrections to all tests that violated the assumption of sphericity (indicated by an * in Tables).
Results
Both large and small number displays evoked mid-latency posterior components (P2). However, the timing and topography of the P2 was slightly different in the two numerical ranges. Large numbers evoked a large, mid-latency positivity over widespread posterior parietal sites, peaking around 500 ms after stimulus onset, that we descriptively termed the P500 (see Figure 2.). In contrast, small numbers evoked a shorter-latency positivity, peaking around 400 ms over left and right occipital-temporal sites, that we descriptively termed the P400 (see Figure 3). We analyzed the effects of Ratio and Numerical Range on each of these components separately.
Parietal P500
A multi-factor ANOVA with between subject variables of Numerical Range (small vs. large numbers), Base Condition (low base number 1 or 8; high base number 3 or 32) and the within-subjects repeated factors of Electrode Group (left, center, and right posterior parietal) and Ratio Change (no change, small ratio change, and large ratio change) was carried out on the mean amplitude of the P500 (300-700 ms) over posterior scalp groupings. Statistical results are presented in Table 1. We observed main effects of Numerical Range, Base Condition, and Electrode Group, and an interaction between Ratio Change and Numerical Range. Overall, low base conditions produced larger amplitude potentials compared to the high base conditions. Post hoc t-tests revealed that over both experiments, the left and right posterior electrode groups evoked greater mean amplitude potentials compared to the center electrode group. Crucially, an analysis of the interaction between Ratio Change and Numerical Range revealed that mean amplitude decreased linearly as ratio increased for large numbers, but not for small numbers (see Figure 2)4. Large number changes evoked a posterior parietal brain response that scaled with ratio change regardless of the absolute cardinal value used, whereas small numbers did not (see Figure 2).
Table 1.
Multi-factor analysis of variance for parietal P500 (300-700 ms)
| Omnibus Analysis | ||||
|---|---|---|---|---|
| Within-subject variables |
Within-subject effects |
Between Subject Variables |
||
| Numerical Range | Base Condition | Range X Base | ||
| - |
F (1,28) = 4.87, p = .03,
η2p = .15 |
F (1,28) = 4.49, p = .04,
η2p = .14 |
F (1,28) = 0.99, p = .32, η2p = ..03 |
|
| Electrode Group |
F (2,56) = 8.52, p < .01,
η2p = .23 |
F (2,56) = 1.37, p = .26, η2p = .05 |
F (2,56) = 2.25, p = .11, η2p = .07 |
F (2,56) = 0.48, p = .62, η2p = .02 |
| Ratio Change | F (2,56) = .56, p = .57, η2p = .02 |
F (2,56) = 3.40, p = .04,
η2p = .11 |
F (2,56) =0.89, p =.41, η2p = .02 |
F (2,56) = 0.63, p = .53, η2p = .02 |
| Elect. Group X Ratio Change |
F (4,112) = .97, p = .45, η2p = .03 |
F (4,112) = .53, p = .71, η2p = .02 |
F (4,112) =1.74, p = .14, η2p = .06 |
F (4,112) =2.16, p = .07, η2p = .07 |
| Post hoc analysis of Ratio Change x Numerical Range | ||||
|
| ||||
| Large Numbers | Post-hoc Linear Contrast: F (1,14) = 6.10, p = .02, η2p = .30 | |||
| Small Numbers | Post-hoc Linear Contrast: F (1,14) = 1.26, p = .28, η2p = .08 | |||
| Post hoc analysis of Electrode Group | ||||
|
| ||||
| Left vs. Center | Paired samples t-test: t (31) = 4.38, p < .01, Cohen’s d = .61 | |||
| Right vs. Center | Paired samples t-test: t (31) = 2.50, p = .01, Cohen’s d = .40 | |||
| Left vs. Right | Paired samples t-test: t (31) = 1.38, p =.18, Cohen’s d = .25 | |||
Because adults’ system of parallel individuation does not robustly extend beyond 3, and infants’ system of large, approximate numerical discrimination does not robustly distinguish between ratios smaller than 1:2, the small and large number experiments necessarily presented numbers that differed by different ratios. To assure that the observed differences did not result from a failure to discriminate all of the ratios used in the small number range (specifically 2:3), we re-analyzed the data after equating the magnitude of ratio change across the small and large number experiments and including only ratios known to be discriminated by the approximate numerical magnitude system. Specifically, we compared the no change condition (1:1 and 3:3) and the large change condition (1:3 and 3:1) in the small number range to the no change condition (8:8 and 32:32) and the average of the small and large ratio change conditions in the large number range (average of 8:16/32:16 and 8:32/32:8). A multi-factor ANOVA with between subject variables of Numerical Range (small vs. large numbers), Base Condition (low base number 1 or 8; high base number 3 or 32) and the within subject repeated factors of Electrode Group (left, center, and right posterior parietal) and Ratio Change (no change versus change) was again carried out on the mean amplitude of the P500 (300-700 ms) over posterior scalp groupings. Statistical results are presented in Table 2. We observed main effects of Electrode Group and Numerical Range and an interaction between Ratio Change and Numerical Range. Post hoc paired samples t-tests revealed that left and right electrode groupings yielded significantly greater mean amplitudes compared to the central posterior scalp grouping and a significant difference between the no-change and change conditions in the large number range, but not the small number range. The P500 was modulated by ratio for large, but not small numbers, even when comparing only ratio changes well within the limits of the approximate numerical magnitude system.
Table 2.
Multi-factor analysis of variance on parietal P500 (300-700 ms) equated for ratio change across number ranges
| Omnibus Analysis | ||||
|---|---|---|---|---|
| Within-subject variables |
Within-subject effects |
Between Subject Variables |
||
| Numerical Range | Base Condition | Range X Base | ||
| - |
F (1,28) = 7.29, p = .01,
η2p = .21 |
F (1,28) = 3.41, p = .07, η2p = .11 |
F (1,28) = 0.60, p = .45, η2p = .02 |
|
| Electrode Group |
F (2,56) = 6.95, p < .01,
η2p = .20 |
F (2,56) = 1.53, p = .22, η2p = .05 |
F (2,56) = 3.00, p = .06, η2p = .10 |
F (2,56) = 0.36, p = .70, η2p = .01 |
| Ratio Change | F (1,28) = 0.90, p = .77, η2p < .01 |
F (1,28) = 4.55, p = .04,
η2p = .14 |
F (1,28) =0.29, p =.60, η2p = .01 |
F (1,28) = 0.65, p = .43, η2p = .02 |
| Elect. Group X Ratio Change |
F (2,56) = 0.84, p = .44, η2p = .03 |
F (2, 56) = 0.50, p = .61, η2p = .02 |
F (2, 56) =2.63, p = .08, η2p = .09 |
F (2, 56) =2.14, p = .13, η2p = .07 |
| Post hoc analysis of Ratio Change x Numerical Range | ||||
|
| ||||
| Large Numbers | Paired samples t-test: t (15) =2.17, p = .04, Cohen’s d =.75 | |||
| Small Numbers | Paired samples t-test: t (15) =−1.14, p = .27, Cohen’s d = −.35 | |||
| Post hoc analysis of Electrode Group | ||||
|
| ||||
| Left vs. Center | Paired samples t-test: t (31) = 4.19, p < .001, Cohen’s d = .53 | |||
| Right vs. Center | Paired samples t-test: t (31) = 2.60 , p = .01, Cohen’s d = .42 | |||
| Left vs. Right | Paired samples t-test: t (31) = .876, p = .38, Cohen’s d = .15 | |||
Occipital-temporal P400
A multi-factor ANOVA with between subject variables of Numerical Range (small vs. large numbers), Base Condition (low base number 1 or 8; high base number 3 or 32) and the within-subject repeated factors of Electrode Group (left and right posterior occipital-temporal) and Ratio Change (no change, small ratio change, and large ratio change) analyzed differences in mean amplitude of the P400 (350-450 ms) over posterior occipital-temporal scalp groupings. Statistical results are presented in Table 3. We observed a significant Numerical Range by Ratio Change by Base Condition interaction. The interaction was further explored by an ANOVA with the factors of Ratio Change (no change, small change, large change) and Base Condition (low base or high base) conducted on each experiment separately. This analysis revealed no significant main effects or interactions for large numbers, but a significant interaction between Ratio Change and Base Condition for small numbers. Linear contrasts revealed amplitude increased with ratio in the base 1 condition but decreased with ratio in the base 3 condition. This interaction implies that the evoked brain response was sensitive to absolute number of objects used in the display, rather than the ratio between the number used, such that the no change condition in the base 1 condition (1:1) produced the smallest relative amplitude response where amplitude scaled positivity with ratio, and the no change in the base 3 condition (3:3) produced the largest relative response where amplitude scaled negatively with ratio (see Figure 3). In other words, experimental conditions only varied by the number paired with the base number within-subjects and P400 amplitude scaled with the cardinal value (1-3) of the “paired number” similarly within the base 1 and the base 3 groups for small numbers. This contrasts with the P500 effects for large numbers where P500 amplitude was dependent on the ratio between the base and paired number.
Table 3.
Multi-factor analysis of variance of occipital-temporal P400 (350-450 ms)
| Omnibus Analysis | ||||
|---|---|---|---|---|
| Within-subject variables |
Within-subject Effects | Between Subject Variables |
||
| Numerical Range | Base Condition | Range X Base | ||
| Electrode Group | F(1,28) = 3.15, p = .08, η2p = .10 |
F(1,28) = 0.29, p = .59, η2p = .01 |
F(1,28) = 0.40, p = .53, η2p = .01 |
F(1,28) = 1.03, p = .31, η2p = .04 |
| Ratio Change | F(1.6, 45.4) = 0.21, p = .76* , η2p < .01 |
F(1.6, 45.4) = 1.10, p = .33*, η2p = .04 |
F(1.6, 45.4) = 2.60, p
.09*, η2p = .09 |
F(1.6, 45.4) = 3.87, p =
.03*, η2p = .12 |
| Elect. Group X Ratio Change |
F(1.8, 51.1) = 2.63, p= .08*, η2p = .09 |
F(1.8, 51.1) = 0.28, p = .76*, η2p = .01 |
F(1.8, 51.1) =0.35, p = .68* , η2p = .01 |
F(1.8, 51.1) = 0.92, p = .40*, η2p = .03 |
| Numerical Range x Base x Ratio Change Interaction | ||||
|
| ||||
| Large Number Range | - | - | F (1,14) = 1.63, p = .22, η2p = .10 |
- |
|
|
||||
| Ratio | F (2,28) = .37, p = .69, η2p = .03 |
- | F (2,28) = 1.31, p =.28, η2p = .09 |
- |
| Small Number Range | - | F (1,14) = 2.18, p = .16, η2p = .13 |
- | |
|
|
||||
| Ratio | F (2,28) = 0.82, p = .45, η2p = .04 |
- |
F (2,28) = 4.33, p = .02,
η2p = .24 |
- |
|
Post hoc linear contrast F (1,14) = 9.19, p < .01,
η2p = .40 |
||||
indicates cases where Greenhouse-Geisser corrections were applied to p values because the assumption of sphericity was violated.
Non-numerical properties
To see if observed ERP effects were affected by the non-numerical properties of the displays, we analyzed the effects of intensively compared to extensively controlled images. Specifically, we split the data into intensively controlled trials (equated for individual item size and inter-item spacing) and extensively controlled trials (equated for total occupied area and total luminance) and compared the ratio change effects observed on the P500 and P400. Similar patterns of P500/P400 response were observed between the ERPs to intensively and extensively controlled images. We observed no significant main effects of Control Type (Intensive versus Extensive) or interactions of Control Type with Ratio, Numerical Range, or Condition (all ps > .18). Thus, the observed pattern of experimental effects were not significantly influenced by whether images varied between conditions on intensive or extensive parameters.
Discussion
These results provide evidence for two early developing representational systems that support non-verbal numerical cognition. Large numbers evoked a mid-latency parietal response around 500 milliseconds that was dependent on the ratio between the numerical pairs presented, irrespective of the absolute values of the numbers shown. Small numbers, in contrast, evoked an earlier peaking occipital-temporal response around 400 ms that was dependent on the cardinal value of items presented in each block of displays within-subjects, irrespective of the ratio change in number from one array to the next.
Our results cannot be explained on the basis of non-numerical stimulus properties because our stimulus controls ensured that these properties held one relationship to number in half of the images and the opposite relationship to number on the other half of the images. Moreover, the same scheme to control for non-numerical parameters was applied equally to large and small numbers yet contrasting patterns of results were observed. Our effects also cannot be explained by differential attention to images in the different ratio conditions. Equal numbers of test trials were retained after artifact rejection, suggesting infants were equally behaviorally attentive to the different experimental conditions. Moreover, the observed effects occurred earlier and had different scalp topographies than attention orienting responses, which typically evoke a large negative central component (Nc) over frontal and fronto-central sites around 700 ms post stimulus onset (de Haan, 2007; Nelson & deRegnier, 1992; Reynolds & Richards, 2005).
Our results add to recent investigations of number processing in the infant brain in three ways. First, our results extend previous work by showing modulation of the ERP by numerical ratio for large numbers. Previous work has shown that infant ERPs are sensitive to a number change compared to no-change, with no-change events eliciting more positive amplitude potentials compared to the change events on a mid-latency component over posterior parietal scalp sites, in accord with the present findings (Izard et al., 2008; Libertus et al., 2009). Ratio modulation of the P500 in our study and by the EEG alpha band in a previous study (Libertus and colleagues 2009) provide further evidence the approximate number system is being engaged by the infant. In addition, our study links the observed infant brain response to the approximate number system by revealing that the P500 in infants is similar in scalp topography, relative timing (mid-latency), and functional modulation to the P2p found in adults: the ERP component functionally linked to number representation in adults and localized to number-related parietal regions of the brain (Dehaene, 1996; Hyde & Spelke, 2009; Pinel et al., 2001).
Previous conclusions about ratio limits have been drawn primarily from behavioral research showing (a) that infants display similar abilities to discriminate between pairs of numbers that differ by the same ratio, regardless of the specific numbers presented (e.g. 8 versus 16 or 16 versus 32), and (b) that older infants succeed at closer ratios than do younger infants (Lipton & Spelke, 2003; Xu & Arriaga, 2007). Our results and those of Libertus and colleagues (2009) extend these previous findings by showing the signature graded electrophysiological response to ratio in the large number range, whereby the amplitude of the P500 further decreases as ratio increases over compatible scalp sites within the same infant or group of infants, providing stronger evidence that infant large number representations are approximate and ratio-dependent.
Second, our results show brain modulation by cardinal value for small numbers, but not for large numbers. These results seemingly contradict those obtained by Izard and colleagues (2008) that showed both large and small number changes modulate a late positivity over frontal and parietal sites. The origin of this contradiction is simply unclear. One possibility is that the two studies are measuring functionally different brain components. While the scalp topography of the large number effects was similar in both cases, our small number effects were more lateralized and ventral compared to those of the previous study. Furthermore, number-related modulation occurred earlier in our data (~400-500 ms) compared to theirs (~ 800 ms). Both timing and topographical differences make it difficult to determine whether the components of interest were in fact the same in both studies.
Another possibility is that differences in stimulus complexity could have contributed to the differing findings of the two studies. The parallel individuation system is traditionally characterized by its capacity limit (Feigenson et al., 2004). Previous work in vision has shown that the complexity of visual stimuli determines, in part, the capacity of the visual system to represent, track, and retain items in parallel (e.g. Alvarez & Cavanagh, 2004). For example, adults are able to simultaneously represent, track, and remember more objects when the objects are simple than when they are complex. It is possible that the complex cartoon figures used in the study of Izard et al. (2008) limited infants’ ability to simultaneously represent all the objects as individuals, a requisite of representation through parallel individuation. If object individuation failed in their study, then approximate number representations may have been elicited by default for infants, as they are for adults (Hyde & Wood, under review). In contrast, the visually simple dots in our study may have more easily engaged the parallel individuation system, leading to the distinct brain signature for processing of small numbers. That is, objects may be represented by parallel individuation only insofar as the capacity of the system is not surpassed, total number of objects being one constraint on capacity and item complexity being another.
Third, our studies allow a comparison of the electrophysiological response obtained in infants to that obtained with adults in previous research. As mentioned above, our findings with infants show striking similarity to those of recent studies of numerical cognition in adults, whose spontaneous cognitive processing of number was assessed while passively viewing numerical arrays with electrophysiological or hemodynamic measures (Ansari et al., 2006; Hyde & Spelke, 2009; Piazza et al., 2004). Specifically, recent adult ERP results show the same dissociating patterns of modulation (decreasing amplitude response with increasing ratio for large numbers; modulation by cardinal value for small numbers) to those observed with infants in the current studies (Hyde & Spelke, 2009). It should be noted that such similarities are rare; in many cases, infants’ ERPs differ vastly from those of adults (Dehaene-Lambertz & Dehaene, 1994; Leppanen, Moulson, Vogel-Farley, & Nelson, 2007). The observed similarities in functional properties of number processing suggest that core numerical representations are functional and distinct early in the first year of life.
Nevertheless, two main differences were observed between adults in previous studies and infants in the present research. First, the ratio-dependent component (P500) and the absolute value-dependent component (P400) occurred about 250 ms later for the infants than the corresponding P2p (~250 ms) and N1 (~150 ms) in adults. This difference may reflect the greater speed of neural processing of number in adults compared to infants. This speculation is supported by chronometric behavioral studies of infant numerical cognition, providing evidence for a developmental decrease in the time it takes to encode number from a given array of objects over the first year of life (Wood & Spelke, 2005).
Second, the polarity of the cardinal value modulation was positive for infants but negative for adults (Hyde & Spelke, 2009). It should be noted that developmental changes in polarity, timing, and topography are common in developmental studies of scalp electrophysiology. For this reason, comparisons of the pattern of response patterns to experimental manipulations, such as those tested here, are better suited for making inferences about the functional equivalence of the neural processes underlying the electrophysiology of different age groups than are comparisons of response properties such as polarity (DeBoer, Scott, & Nelson, 2004; Dehaene-Lambertz & Dehaene, 1994). Nevertheless, based on these differences, claims of continuity in the neural systems of number are only tentative and future studies should attempt to determine whether the functional similarities observed between infants and adults arise from the same brain systems. Ongoing work using methods with higher spatial resolution such as Near-infrared Spectroscopy (NIRS) with infants and fRMI with adults may serve to localize these contrasting responses.
In summary, our results provide evidence for dissociable neural responses to small and large numbers, suggesting the presence of two cognitive systems of non-verbal numerical cognition that operate from early in the first year of life. More broadly, our results provide a neurophysiological basis of core numerical intuitions that will allow researchers to ask more in-depth theoretical and applied questions regarding the nature and development of numerical thinking. For instance, recent evidence suggests core numerical intuitions are involved in learning to count (Condry & Spelke, 2008). Furthermore, mathematical achievement has been found to be related to core numerical abilities (Halberda et al., 2008). Our results may provide a metric by which researchers can measure core numerical abilities in infants and potentially identify children likely to be at-risk for developmental difficulties with mathematics.
Acknowledgements
We would like to acknowledge Christina Zhou, Yen-Ling Kao, Ben Smith, Nick Moseley, Clark Van Den Berghe, and Andy Peters for their assistance in data collection. We would also like to thank Susan Carey for her helpful feedback and enthusiasm during this project. This project was generously funded by a grant from National Institute of Health (NIH-HD23103) to E.S.S.
Footnotes
We chose this particular segmentation routine (−200-1200 ms) because of a previous study reporting ERP modulation by number as late as 1200 milliseconds (Izard, Dehaene-Lambertz, & Dehaene, 2008).
The ERPs for each experimental condition are made up of the average response to both the “base” and the “paired” number for each block (e.g. responses to 1 and 2 during the small number, small ratio change block). We included both numbers to maximize the number of trials per experimental condition contributing to the average. An analysis of the “paired” number trials alone was not possible because of the insufficient number of trials per condition left after eliminating “base” trials from the average.
This method of using the grand average waveform to choose electrode sites and time windows of interest is widely used and accepted in infant ERP experiments (e.g. recent papers using similar method: Elsabbagh, Volein, Csibra, Holmboe, Garwood, Tucker, Krljes, Baron-Cohen, Bolton, Charman, Baird, & Johnson, 2009; Grossmann, Gliga, Johnson, & Mareschal, 2009; Kushnerenko, Teinonen, Volein, & Csibra, 2008; Scott & Nelson, 2006). Furthermore, because sites and time windows are chosen based on the average of the experimental conditions, this method is not biased towards a particular pattern of component modulation.
Some waveform fluctuation was observed in the baseline period before stimulus presentation (see Figure 2, b for example). Early baseline differences may be attributable to stimulus overlap from the preceding trial given our presentation rate and & jitter was, in some instances, not particularly long for infant studies. Normally, stimulus overlap is avoided because experimenters wish to isolate responses to different experimental conditions presented close in time to one another. Such overlap effects are not problematic, however, in the present experiments because our block design presented the same numerical relationship repeatedly and the different experimental blocks were separated by a substantial rest period (5-10 seconds). This means that any systematic overlap in the ERPs was contributed by the desired experimental condition and therefore likely reflects continual processing of the desired numerical relationship. Nonetheless, the baseline differences alone can not account for the observed effects as they are not significant and they do not pattern with the ratio modulation observed for the P500 or the P400 crucial to our claims.
References
- Ansari D, Dhital B. Age-related changes in the activation of the intraparietal sulcus during non-symbolic magnitude processing: an event-related fMRI study. Journal of Cognitive Neuroscience. 2006;18:1820–28. doi: 10.1162/jocn.2006.18.11.1820. [DOI] [PubMed] [Google Scholar]
- Ansari D, Dhital B, Soon CS. Parametric effects of numerical distance on the intraparietal sulcus during passive viewing of rapid numerosity changes. Brain Research. 2006;1067:181–188. doi: 10.1016/j.brainres.2005.10.083. [DOI] [PubMed] [Google Scholar]
- Barth H, La Mont K, Lipton J, Spelke E. Abstract number and arithmetic in preschool children. Proceedings of the National Academy of Sciences. 2005;102:14116–14121. doi: 10.1073/pnas.0505512102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A, Tzur G, Posner MI. Infant brains detect arithmetic errors. Proceedings of the National Academy of Sciences USA. 2006;103:12649–12653. doi: 10.1073/pnas.0605350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon EM, Terrace HS. Ordering of the numerosities 1-9 by monkeys. Science. 1998;282:746–749. doi: 10.1126/science.282.5389.746. [DOI] [PubMed] [Google Scholar]
- Cantlon JF, Brannon EM, Carter EJ, Pelphrey KA. Functional imaging of numerical processing in adults and 4-y-old children. PLOS Biology. 2006;4(5):e125. doi: 10.1371/journal.pbio.0040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantlon JF, Brannon EM. Shared system for ordering small and large numbers in monkeys and humans. Psychological Science. 2006;17:401–406. doi: 10.1111/j.1467-9280.2006.01719.x. [DOI] [PubMed] [Google Scholar]
- Condry KF, Spelke ES. The development of language and abstract concepts: The case of natural number. Journal of Experimental Psychology: General. 2008;137(1):22–38. doi: 10.1037/0096-3445.137.1.22. [DOI] [PubMed] [Google Scholar]
- Cordes S, Brannon EM. Crossing the divide: Infants discriminate small from large numerosities. Developmental Psychology. 2009 doi: 10.1037/a0015666. To appear in. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes S, Gelman R, Gallistel CR. Variability signatures distinguish verbal from nonverbal counting in both large and small numbers. Psychological Bulletin and Review. 2001;8:698–707. doi: 10.3758/bf03196206. [DOI] [PubMed] [Google Scholar]
- DeBoer T, Scott L, Nelson CA. Event-related potentials in developmental populations. In: Handy T, editor. Event-related potentials: A methods handbook. MIT Press; Cambridge, MA: 2004. pp. 263–297. [Google Scholar]
- de Haan M. Visual attention and recognition memory in infancy. In: de Haan M, editor. Infant EEG and event-related potentials. Psychology Press; Hove UK: 2007. pp. 101–143. [Google Scholar]
- Dehaene-Lambertz G, Dehaene S. Speed and neural correlates of syllable discrimination in infants. Nature. 1994;370:292–295. doi: 10.1038/370292a0. [DOI] [PubMed] [Google Scholar]
- Dehaene S. The number sense. Oxford University Press; New York: 1997. [Google Scholar]
- Dehaene S, Piazza M, Pinel P, Cohen L. Three parietal circuits for number processing. Cognitive Neuropsychology. 2003;20:487–506. doi: 10.1080/02643290244000239. [DOI] [PubMed] [Google Scholar]
- Dehaene S. The organization of brain activations in number comparison: Event-related potentials and the additive-factors method. Journal of Cognitive Neuroscience. 1996;8:47–68. doi: 10.1162/jocn.1996.8.1.47. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Volein A, Csibra G, Holmboe K, Garwood H, Tucker L, Krljes S, Baron-Cohen S, Bolton P, Charman T, Baird G, Johnson MH. Neural correlates of eye gaze processing in the infant broader autism phenotype. Biological Psychiatry. 2009;65:31–38. doi: 10.1016/j.biopsych.2008.09.034. [DOI] [PubMed] [Google Scholar]
- Feigenson L, Carey S. On the limits of infants’ quantification of small object arrays. Cognition. 2005;97:295–313. doi: 10.1016/j.cognition.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Feigenson L, Dehaene S, Spelke ES. Core systems of number. Trends in Cognitive Sciences. 2004;8:307–314. doi: 10.1016/j.tics.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Gallistel CR. The organization of learning. Bradford Books/MIT Press; Cambridge, MA: 1990. [Google Scholar]
- Gallistel CR, Gelman R. Non-verbal numerical cognition: From reals to integers. Trends in Cognitive Sciences. 2000;4:59–65. doi: 10.1016/s1364-6613(99)01424-2. [DOI] [PubMed] [Google Scholar]
- Grossmann T, Gliga T, Johnson MH, Mareschal D. The neural basis of perceptual category learning in human infants. Journal of Cognitive Neuroscience. 2009;21:2276–2286. doi: 10.1162/jocn.2009.21188. [DOI] [PubMed] [Google Scholar]
- Halberda J, Feigenson L. Developmental change in the acuity of the “Number Sense”: The approximate number system in 3-, 4-, 5-, 6-year-olds and adults. Developmental Psychology. 2008;44(5):1457–1465. doi: 10.1037/a0012682. [DOI] [PubMed] [Google Scholar]
- Halberda J, Mazzocco M, Feigenson L. Individual differences in nonverbal estimation ability predict maths achievement. Nature. 2008;455:665–669. doi: 10.1038/nature07246. [DOI] [PubMed] [Google Scholar]
- Hyde DC, Spelke ES. All numbers are not equal: An electrophysiological investigation of large and small number representations. Journal of Cognitive Neuroscience. 2009;21:1039–1053. doi: 10.1162/jocn.2009.21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde DC, Jones BL, Porter CL, Flom R. Visual stimulation enhances auditory processing in 6-month old infants and adults. Developmental Psychobiology. doi: 10.1002/dev.20417. in press. To appear in. [DOI] [PubMed] [Google Scholar]
- Hyde DC, Wood JN. Attention determines the nature of non-verbal number representation. under review. [DOI] [PubMed] [Google Scholar]
- Izard V, Dehaene-Lambertz G, Dehaene S. Distinct cerebral pathways for object identity and number in 3-month-old infants. PLOS Biology. 2008;6/e11:1–11. doi: 10.1371/journal.pbio.0060011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izard V, Sann C, Spelke ES, Steri A. Newborn infants perceive abstract numbers. Proceedings of the National Academy of Sciences of the U.S.A. 2009;106(25):10382–10385. doi: 10.1073/pnas.0812142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnerenko E, Teinonen T, Volein A, Csibra G. Electrophysiological evidence of illusory audiovisual speech integration in human infants. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11442–11445. doi: 10.1073/pnas.0804275105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knops A, Thirion B, Hubbard EM, Michel V, Dehaene S. Recruitment of an area involved in eye movements during mental arithmetic. Science. 2009;324(5934):1583–1585. doi: 10.1126/science.1171599. [DOI] [PubMed] [Google Scholar]
- LeCorre M, Carey S. One, two, three, four, nothing more: An investigation of the conceptual sources of the verbal counting principles. Cognition. 2007;105:394–438. doi: 10.1016/j.cognition.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppanen JM, Moulson MC, Vogel-Farley VK, Nelson CA. An ERP study of emotional face processing in the adult and infant brain. Child Development. 2007;78:232–245. doi: 10.1111/j.1467-8624.2007.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libertus ME, Woldorff MG, Brannon EM. Electrophysiological evidence for notation independence in numerical processing. Behavioral and Brain Functions. 2007;3(1) doi: 10.1186/1744-9081-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libertus ME, Pruitt LB, Woldorff MG, Brannon EM. Induced alpha-band occillations reflect ratio-dependent number discrimination in the infant brain. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2008.21162. in press. To appear in. [DOI] [PubMed] [Google Scholar]
- Lipton JS, Spelke ES. Origins of number sense: Large number discrimination in human infants. Psychological Science. 2003;14:396–401. doi: 10.1111/1467-9280.01453. [DOI] [PubMed] [Google Scholar]
- Mandler G, Shebo BJ. Subitizing: An analysis of its component processes. Journal of Experimental Psychology: General. 1982;111:1–21. doi: 10.1037//0096-3445.111.1.1. [DOI] [PubMed] [Google Scholar]
- McCrink K, Wynn K. Ratio abstraction by 6-month-old infants. Psychological Science. 2007;18:740–746. doi: 10.1111/j.1467-9280.2007.01969.x. [DOI] [PubMed] [Google Scholar]
- Meck WH, Church RM. A mode control model of counting and timing processes. Journal of Experimental Psychology: Animal Behavior and Process. 1983;9:320–334. [PubMed] [Google Scholar]
- Nelson CA, deRegnier RA. Neural correlates of attention and memory in the first year of life. Developmental Neuropsychology. 1992;8:119–134. [Google Scholar]
- Nieder A, Merten K. A labeled-line code for small and large numerosities in the monkey prefrontal cortex. Journal of Neuroscience. 2007;27:5986–5993. doi: 10.1523/JNEUROSCI.1056-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieder A, Miller EK. Coding of cognitive magnitude: Compressed scaling of numerical information in the primate prefrontal cortex. Neuron. 2003;37:149–157. doi: 10.1016/s0896-6273(02)01144-3. [DOI] [PubMed] [Google Scholar]
- Piazza M, Izard V, Pinel P, Le Bihan D, Dehaene S. Tuning curves for approximate numerosity in the human intraparietal sulcus. Neuron. 2004;44:547–555. doi: 10.1016/j.neuron.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Pinel P, Le Clec’H G, van de Moortele P, Naccache L, Le Bihan D, Dehaene S. Event-related fMRI analysis of the cerebral circuit for number comparison. NeuroReport. 1999;10:1473–1479. doi: 10.1097/00001756-199905140-00015. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Westerlund A, Nelson CA. Neural markers of categorization in 6-month old infants. Psychological Science. 2006;17:59–66. doi: 10.1111/j.1467-9280.2005.01665.x. [DOI] [PubMed] [Google Scholar]
- Revkin SK, Piazza M, Izard V, Cohen L, Dehaene S. Does subitizing reflect numerical estimation? Psychological Science. 2008;19:607–614. doi: 10.1111/j.1467-9280.2008.02130.x. [DOI] [PubMed] [Google Scholar]
- Reynolds GD, Richards JE. Familiarization, attention, and recognition memory in infancy: An ERP and cortical source localization study. Developmental Psychology. 2005;41:598–615. doi: 10.1037/0012-1649.41.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl BJ. Objects and attention: The state of the art. Cognition. 2001;80(1/2):1–46. doi: 10.1016/s0010-0277(00)00152-9. [DOI] [PubMed] [Google Scholar]
- Scott LS, Nelson CA. Featural and Configural Face Processing in Adults and Infants: A Behavioral and Electrophysiological Investigation. Perception. 2006;35(8):1107–1128. doi: 10.1068/p5493. [DOI] [PubMed] [Google Scholar]
- Temple E, Posner MI. Brain mechanisms of quantity are similar in 5-year-old children and adults. Proceedings of the National Academy of Sciences, USA. 1998;95:7836–7841. doi: 10.1073/pnas.95.13.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trick LM, Pylyshyn ZW. Why are small and large numbers enumerated differently? A limited capacity preattentive stage in vision. Psychological Review. 1994;101:80–102. doi: 10.1037/0033-295x.101.1.80. [DOI] [PubMed] [Google Scholar]
- Van Oeffelen M, Vos P. A probabilistic model for the discrimination of visual number. Perception and Psychophysics. 1982;32:163–170. doi: 10.3758/bf03204275. [DOI] [PubMed] [Google Scholar]
- Whalen J, Gallistel CR, Gelman R. Nonverbal counting in humans: The psychophysics of number representation. Psychological Science. 1999;10:130–137. [Google Scholar]
- Wood JN, Spelke ES. Chronometric studies of numerical cognition in five-month-old infants. Cognition. 2005;97(1):23–39. doi: 10.1016/j.cognition.2004.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Arriaga RI. Number discrimination in 10-month-old infants. British Journal of Developmental Psychology. 2007;25:103–108. [Google Scholar]
- Xu F, Spelke E, Goddard S. Number sense in human infants. Developmental Science. 2005;8:88–101. doi: 10.1111/j.1467-7687.2005.00395.x. [DOI] [PubMed] [Google Scholar]
- Xu F, Spelke ES. Large number discrimination in 6-month-old infants. Cognition. 2000;74:B1–B11. doi: 10.1016/s0010-0277(99)00066-9. [DOI] [PubMed] [Google Scholar]